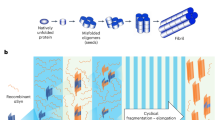
Abstract
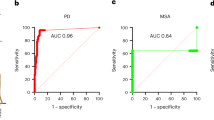
Synucleinopathies are neurodegenerative diseases that are associated with the misfolding and aggregation of α-synuclein, including Parkinson’s disease, dementia with Lewy bodies and multiple system atrophy1. Clinically, it is challenging to differentiate Parkinson’s disease and multiple system atrophy, especially at the early stages of disease2. Aggregates of α-synuclein in distinct synucleinopathies have been proposed to represent different conformational strains of α-synuclein that can self-propagate and spread from cell to cell3,4,5,6. Protein misfolding cyclic amplification (PMCA) is a technique that has previously been used to detect α-synuclein aggregates in samples of cerebrospinal fluid with high sensitivity and specificity7,8. Here we show that the α-synuclein-PMCA assay can discriminate between samples of cerebrospinal fluid from patients diagnosed with Parkinson’s disease and samples from patients with multiple system atrophy, with an overall sensitivity of 95.4%. We used a combination of biochemical, biophysical and biological methods to analyse the product of α-synuclein-PMCA, and found that the characteristics of the α-synuclein aggregates in the cerebrospinal fluid could be used to readily distinguish between Parkinson’s disease and multiple system atrophy. We also found that the properties of aggregates that were amplified from the cerebrospinal fluid were similar to those of aggregates that were amplified from the brain. These findings suggest that α-synuclein aggregates that are associated with Parkinson’s disease and multiple system atrophy correspond to different conformational strains of α-synuclein, which can be amplified and detected by α-synuclein-PMCA. Our results may help to improve our understanding of the mechanism of α-synuclein misfolding and the structures of the aggregates that are implicated in different synucleinopathies, and may also enable the development of a biochemical assay to discriminate between Parkinson’s disease and multiple system atrophy.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
References
Goedert, M., Jakes, R. & Spillantini, M. G. The synucleinopathies: twenty years on. J. Parkinsons Dis. 7, S51–S69 (2017).
Wenning, G. K. et al. What clinical features are most useful to distinguish definite multiple system atrophy from Parkinson’s disease? J. Neurol. Neurosurg. Psychiatry 68, 434–440 (2000).
Melki, R. Role of different alpha-synuclein strains in synucleinopathies, similarities with other neurodegenerative diseases. J. Parkinsons Dis. 5, 217–227 (2015).
Prusiner, S. B. et al. Evidence for α-synuclein prions causing multiple system atrophy in humans with parkinsonism. Proc. Natl Acad. Sci. USA 112, E5308–E5317 (2015).
Peng, C. et al. Cellular milieu imparts distinct pathological α-synuclein strains in α-synucleinopathies. Nature 557, 558–563 (2018).
Tarutani, A., Arai, T., Murayama, S., Hisanaga, S. I. & Hasegawa, M. Potent prion-like behaviors of pathogenic α-synuclein and evaluation of inactivation methods. Acta Neuropathol. Commun. 6, 29 (2018).
Shahnawaz, M. et al. Development of a biochemical diagnosis of Parkinson disease by detection of α-synuclein misfolded aggregates in cerebrospinal fluid. JAMA Neurol. 74, 163–172 (2017).
Kang, U. J. et al. Comparative study of cerebrospinal fluid α-synuclein seeding aggregation assays for diagnosis of Parkinson’s disease. Mov. Disord. 34, 536–544 (2019).
Wood, S. J. et al. α-synuclein fibrillogenesis is nucleation-dependent. Implications for the pathogenesis of Parkinson’s disease. J. Biol. Chem. 274, 19509–19512 (1999).
Volles, M. J. & Lansbury, P. T. Jr. Zeroing in on the pathogenic form of α-synuclein and its mechanism of neurotoxicity in Parkinson’s disease. Biochemistry 42, 7871–7878 (2003).
El-Agnaf, O. M. et al. Detection of oligomeric forms of α-synuclein protein in human plasma as a potential biomarker for Parkinson’s disease. FASEB J. 20, 419–425 (2006).
Tokuda, T. et al. Detection of elevated levels of α-synuclein oligomers in CSF from patients with Parkinson disease. Neurology 75, 1766–1770 (2010).
Herva, M. E. et al. Anti-amyloid compounds inhibit α-synuclein aggregation induced by protein misfolding cyclic amplification (PMCA). J. Biol. Chem. 289, 11897–11905 (2014).
Jung, B. C. et al. Amplification of distinct α-synuclein fibril conformers through protein misfolding cyclic amplification. Exp. Mol. Med. 49, e314 (2017).
Groveman, B. R. et al. Rapid and ultra-sensitive quantitation of disease-associated α-synuclein seeds in brain and cerebrospinal fluid by αSyn RT-QuIC. Acta Neuropathol. Commun. 6, 7 (2018).
Fairfoul, G. et al. Alpha-synuclein RT-QuIC in the CSF of patients with alpha-synucleinopathies. Ann. Clin. Transl. Neurol. 3, 812–818 (2016).
Naiki, H., Higuchi, K., Hosokawa, M. & Takeda, T. Fluorometric determination of amyloid fibrils in vitro using the fluorescent dye, thioflavin T1. Anal. Biochem. 177, 244–249 (1989).
Sjöqvist, J. et al. Toward a molecular understanding of the detection of amyloid proteins with flexible conjugated oligothiophenes. J. Phys. Chem. A 118, 9820–9827 (2014).
Klingstedt, T. & Nilsson, K. P. Luminescent conjugated poly- and oligo-thiophenes: optical ligands for spectral assignment of a plethora of protein aggregates. Biochem. Soc. Trans. 40, 704–710 (2012).
Rasmussen, J. et al. Amyloid polymorphisms constitute distinct clouds of conformational variants in different etiological subtypes of Alzheimer’s disease. Proc. Natl Acad. Sci. USA 114, 13018–13023 (2017).
Sigurdson, C. J. et al. Prion strain discrimination using luminescent conjugated polymers. Nat. Methods 4, 1023–1030 (2007).
Bessen, R. A. & Marsh, R. F. Biochemical and physical properties of the prion protein from two strains of the transmissible mink encephalopathy agent. J. Virol. 66, 2096–2101 (1992).
Tuttle, M. D. et al. Solid-state NMR structure of a pathogenic fibril of full-length human α-synuclein. Nat. Struct. Mol. Biol. 23, 409–415 (2016).
Li, Y. et al. Amyloid fibril structure of α-synuclein determined by cryo-electron microscopy. Cell Res. 28, 897–903 (2018).
Guerrero-Ferreira, R. et al. Cryo-EM structure of alpha-synuclein fibrils. eLife 7, e36402 (2018).
Grazia Spillantini, M. et al. Filamentous α-synuclein inclusions link multiple system atrophy with Parkinson’s disease and dementia with Lewy bodies. Neurosci. Lett. 251, 205–208 (1998).
Crowther, R. A., Daniel, S. E. & Goedert, M. Characterisation of isolated α-synuclein filaments from substantia nigra of Parkinson’s disease brain. Neurosci. Lett. 292, 128–130 (2000).
Armijo, E. et al. Increased susceptibility to Aβ toxicity in neuronal cultures derived from familial Alzheimer’s disease (PSEN1-A246E) induced pluripotent stem cells. Neurosci. Lett. 639, 74–81 (2017).
Soto, C. & Pritzkow, S. Protein misfolding, aggregation, and conformational strains in neurodegenerative diseases. Nat. Neurosci. 21, 1332–1340 (2018).
Olanow, C. W. & Prusiner, S. B. Is Parkinson’s disease a prion disorder? Proc. Natl Acad. Sci. USA 106, 12571–12572 (2009).
Tolosa, E., Wenning, G. & Poewe, W. The diagnosis of Parkinson's disease. Lancet Neurol. 5, 75–86 (2006).
Roostaee, A., Beaudoin, S., Staskevicius, A. & Roucou, X. Aggregation and neurotoxicity of recombinant α-synuclein aggregates initiated by dimerization. Mol. Neurodegener. 8, 5 (2013).
Åslund, A. et al. Novel pentameric thiophene derivatives for in vitro and in vivo optical imaging of a plethora of protein aggregates in cerebral amyloidoses. ACS Chem. Biol. 4, 673–684 (2009).
Klingstedt, T. et al. Distinct spacing between anionic groups: an essential chemical determinant for achieving thiophene-based ligands to distinguish β-amyloid or tau polymorphic aggregates. Chemistry 21, 9072–9082 (2015).
Shirani, H. et al. Synthesis of thiophene-based optical ligands that selectively detect tau pathology in Alzheimer’s disease. Chemistry 23, 17127–17135 (2017).
Shirani, H. et al. A palette of fluorescent thiophene-based ligands for the identification of protein aggregates. Chemistry 21, 15133–15137 (2015).
Mastronarde, D. N. Automated electron microscope tomography using robust prediction of specimen movements. J. Struct. Biol. 152, 36–51 (2005).
Zheng, S. Q. et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331–332 (2017).
Mastronarde, D. N. & Held, S. R. Automated tilt series alignment and tomographic reconstruction in IMOD. J. Struct. Biol. 197, 102–113 (2017).
Ludtke, S. J., Baldwin, P. R. & Chiu, W. EMAN: semiautomated software for high-resolution single-particle reconstructions. J. Struct. Biol. 128, 82–97 (1999).
Pettersen, E. F. et al. UCSF Chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Acknowledgements
This study was funded in part by grants from the Michael J. Fox Foundation for Parkinson’s disease (to C.S. and S.P.); NIH (R01AG055053, R01AG061069) and Department of Defense (to C.S.); NIH (P01NS44233, U54NS065736, K23NS075141, R01 FD004789, R01 NS092625), Department of Defense and Mayo Funds (to P.A.L.); RO1 NS094535 (to A.-L.T.); and the Swedish Research Council (2016-00748 to H.S. and K.P.R.N.). We are grateful to the Banner Sun Health Research Institute Brain and Body Donation Program of Sun City, Arizona for the provision of brain tissue. We also thank N. P. Rocha for providing CSF samples, I. Moreno-Gonzalez for helping with the preparation and characterization of brain homogenate and T. Eckland for editing the manuscript.
Author information
Authors and Affiliations
Contributions
C.S. and M.S. conceived and designed the experiments and analysed the data, with important contributions from A.M. and S.P. for some of the experiments; M.S. performed all PMCA assays, analysed data and prepared figures; A.M. performed FTIR assays, analysed data and prepared figures; S.P. performed assays with thiophene-based ligands, analysed data and prepared figures; N.M. performed all protease-resistance and epitope-mapping experiments, prepared figures and performed the sedimentation studies; P.R. purified the recombinant α-syn for the experiments; X.L. and B.H. performed cryo-ET, constructed models and measured pitch lengths; X.L. discovered the key difference in the pitch length between PD and MSA fibrils; C.S. and A.M. analysed the cryo-ET data and prepared figures; A.M., G.W. and A.L.-T. performed circular dichroism spectroscopy, analysed data and prepared figures; M.S. and A.M. performed cytotoxicity assays, analysed data and prepared figures; H.S. and K.P.R.N. provided thiophene-based ligands and experimental support for their use; A.S., W.S. and P.A.L. provided most of the CSF samples and clinical data; C.S. wrote the manuscript with input from all co-authors.
Corresponding author
Ethics declarations
Competing interests
C.S. and M.S. are inventors on patent applications (US20160077111, WO2016040905, EP3191599A1, US20160077112 and WO2016040907) for the use of PMCA technology for high-sensitive detection of α-syn aggregates in patients affected by synucleinopathies. These applications were filed by the University of Texas Health Science Center at Houston and Amprion Inc. C.S. is an inventor on several patents related to PMCA technology and is a Founder, Chief Scientific Officer and Member of the Board of Directors of Amprion Inc, a biotechnology company that focuses on the commercial use of PMCA (RT-QuIC) for high-sensitivity detection of misfolded protein aggregates that are implicated in a variety of neurodegenerative diseases. The University of Texas Health Science Center at Houston owns some patent applications related to the PMCA (RT-QuIC) technology that have been licensed to Amprion Inc.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Kinetics of α-syn aggregation in the presence of CSF from patients with PD, patients with MSA or healthy control individuals.
a–c, Individual α-syn aggregation curves are shown in the presence of CSF samples (40 μl) from all study participants, including healthy controls (a; n = 56), patients with MSA (b; n = 75) and patients with PD (c; n = 94). The α-syn-PMCA assay was started by adding α-syn monomers (1 mg ml−1) and ThT (5 μM) to 100 mM PIPES, pH 6.5 containing 500 mM NaCl. The plate was incubated at 37 °C with intermittent shaking for 1 min every 30 min at 500 rpm. The extent of aggregation was monitored using a fluorometer to measure ThT fluorescence, with an excitation of 435 nm and emission of 485 nm. The colours represent the expected aggregation curves for patients with PD (red), patients with MSA (blue) and healthy controls (black), regardless of clinical diagnosis.
Extended Data Fig. 2 Serial propagation of α-syn aggregates derived from patients with MSA and patients with PD.
For serial propagation of α-syn aggregates, an aliquot of the final product of the first α-syn-PMCA reaction (starting from CSF samples) was diluted 100-fold into a solution containing fresh α-syn monomers (1 mg ml−1). A second round of amplification was done in the same buffer (100 mM PIPES, pH 6.5 containing 500 mM NaCl) at 37 °C with intermittent shaking for 1 min every 30 min at 500 rpm. The extent of aggregation was monitored by the increase in ThT fluorescence. The maximum fluorescence value at the plateau of aggregation was recorded and plotted in the graph as the second round of amplification (R2). Similarly, the third and fourth rounds of amplification (R3 and R4) were performed by diluting the product 100-fold on amplification each time into fresh α-syn monomer substrate and repeating the α-syn-PMCA assay. The results shown are from one patient with PD and one patient with MSA. The experiment was carried out in duplicate, each dot represents an individual technical replicate and data are mean ± s.e.m.
Extended Data Fig. 3 Analyses of the quantity of α-syn aggregates after amplification from patients with MSA and patients with PD by sedimentation assay.
Aggregates of α-syn that were obtained after two rounds of α-syn-PMCA amplification (starting from CSF samples from patients with MSA (n = 43) and patients with PD (n = 43)) were centrifuged at 20,000g for 30 min. a, The resultant pellets were separated on a 12% Bis-Tris gel, and protein bands were visualized by silver staining as per the manufacturer’s protocol. Molecular weight markers (kDa) are indicated on the left of the gel. b, Resuspended pellets (2 µl) were spotted onto nitrocellulose membranes and air-dried for 30 min at room temperature. After blocking with 5% w/v non-fat dry milk at room temperature for 2 h, membranes were probed with an anti-α-syn antibody (BD Bioscience, 1:2,000) and anti-rabbit HRP-conjugated secondary antibodies (1:5,000). The blots were visualized using enhanced chemiluminescence and a western blotting detection kit. The dot blot shows each of the 86 samples (n = 43, PD; n = 43, MSA) and a positive control using non-aggregated α-syn monomer (dotted box). The results are representative of two independent experiments with similar results. c, Protein concentration in the supernatants was determined by a BCA assay kit as per the manufacturer’s instructions. Each dot represents an individual sample (n = 43, PD; n = 43, MSA) in each disease group and data are mean ± s.e.m.
Extended Data Fig. 4 Proteinase K digestion profiles of α-syn aggregates derived from samples of CSF from patients with PD and patients with MSA.
This is the same experiment as Fig. 2a–c, showing proteinase K digestion profiles of other representative samples from patients with PD (n = 3) and patients with MSA (n = 3). The amplified product from the second round of α-syn-PMCA in samples of CSF from patients with MSA or patients with PD was incubated either without (–) or in the presence of increasing concentrations of proteinase K (0.001, 0.01, 0.1 and 1 mg ml−1) at 37 °C for 1 h. Proteins were separated on a 12% Bis-Tris gel and immunoblotted with the same antibodies as in Fig. 2 (SC N-19 (top), BD anti-α-syn clone 42 (middle) and SC 211 (bottom)). Each blot represents an individual sample. Molecular weight markers (kDa) are indicated on the left of the blot.
Extended Data Fig. 5 Proteinase K digestion profiles of α-syn aggregates derived from samples of CSF from all 43 patients with PD and 43 patients with MSA.
This is the same experiment as Fig. 2d, showing proteinase K digestion profiles of all 86 (n = 43, PD; n = 43, MSA) biologically independent samples analysed. Aliquots of the product of the second round of the α-syn-PMCA assay were treated with proteinase K (1 mg ml−1) at 37 °C for 1 h. Proteins were separated on a 12% Bis-Tris gel and immunoblotted with the BD anti-α-syn clone 42 antibody. Molecular weight markers (kDa) are indicated on the left of the blot. The third blot on the top row is the same as that shown in Fig. 2d.
Extended Data Fig. 6 Proteinase K digestion profiles of α-syn aggregates after several rounds of α-syn-PMCA.
This is the same experiment as Fig. 2e, showing the results obtained with samples from different patients with PD (n = 3) and patients with MSA (n = 3). The first round corresponds to direct amplification from the CSF of the patients. For the second round of amplification, aggregates produced in the first round were diluted 100-fold into fresh α-syn monomer substrate and a new round of α-syn-PMCA was performed. The assay was then repeated for the third and fourth rounds using amplified α-syn aggregates (1%) from the previous round. Amplified aggregates were treated with proteinase K (1 mg ml−1) for 1 h and proteins were separated on a 12% Bis-Tris gel and immunoblotted with the BD anti-α-syn clone 42 antibody. Molecular weight markers (kDa) are indicated on the left of the blot.
Extended Data Fig. 7 Electron microscopy images of PD-associated fibrils and MSA-associated fibrils.
Representative images of fibrils produced after two rounds of α-syn-PMCA in samples from different patients with PD (n = 3) and patients with MSA (n = 3). The negative-stained fibrils were imaged with a 300 kV electron microscope. Scale bar, 10 nm (applies to all of the images).
Extended Data Fig. 8 Reaction scheme for the chemical synthesis of HS-199.
HS-199 was synthesized by mixing 0.462 mM methyl 5′-bromo-[2,2′-bithiophene]-5-carboxylate with 0.508 mM (5-formylthiophen-2-yl)boronic acid, K2CO3 (1.39 mmol) in 1,4-dioxane/methanol (8: 2, 8 mL/mM, degassed) and PEPPS-IPr (2 mol %).
Supplementary information
Supplementary Figures
Main and extended data figures: uncropped blots with size marker indications.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shahnawaz, M., Mukherjee, A., Pritzkow, S. et al. Discriminating α-synuclein strains in Parkinson’s disease and multiple system atrophy. Nature 578, 273–277 (2020). https://doi.org/10.1038/s41586-020-1984-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-020-1984-7
This article is cited by
-
Parkinson’s disease-derived α-synuclein assemblies combined with chronic-type inflammatory cues promote a neurotoxic microglial phenotype
Journal of Neuroinflammation (2024)
-
In vitro modulation of mTOR and mGlur5 influence α-synuclein accumulation
Molecular Brain (2024)
-
Brain clearance of protein aggregates: a close-up on astrocytes
Molecular Neurodegeneration (2024)
-
Biases in α-synuclein immuno-quantitation: a core problem for basic and ancillary studies of Parkinson’s disease and multiple system atrophy
Translational Neurodegeneration (2024)
-
CSF α-synuclein seed amplification kinetic profiles are associated with cognitive decline in Parkinson’s disease
npj Parkinson's Disease (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.