Abstract
Overfishing is the primary cause of marine defaunation, yet declines in and increasing extinction risks of individual species are difficult to measure, particularly for the largest predators found in the high seas1,2,3. Here we calculate two well-established indicators to track progress towards Aichi Biodiversity Targets and Sustainable Development Goals4,5: the Living Planet Index (a measure of changes in abundance aggregated from 57 abundance time-series datasets for 18 oceanic shark and ray species) and the Red List Index (a measure of change in extinction risk calculated for all 31 oceanic species of sharks and rays). We find that, since 1970, the global abundance of oceanic sharks and rays has declined by 71% owing to an 18-fold increase in relative fishing pressure. This depletion has increased the global extinction risk to the point at which three-quarters of the species comprising this functionally important assemblage are threatened with extinction. Strict prohibitions and precautionary science-based catch limits are urgently needed to avert population collapse6,7, avoid the disruption of ecological functions and promote species recovery8,9.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Data are available on https://www.sharkipedia.org/ and at https://doi.org/10.5281/zenodo.4135325. Source data are provided with this paper.
References
Dulvy, N. K. et al. You can swim but you can’t hide: the global status and conservation of oceanic pelagic sharks and rays. Aquat. Conserv. 18, 459–482 (2008).
Webb, T. J. & Mindel, B. L. Global patterns of extinction risk in marine and non-marine systems. Curr. Biol. 25, 506–511 (2015).
McCauley, D. J. et al. Marine defaunation: animal loss in the global ocean. Science 347, 1255641 (2015).
Tittensor, D. P. et al. A mid-term analysis of progress toward international biodiversity targets. Science 346, 241–244 (2014).
Butchart, S. H. et al. Global biodiversity: indicators of recent declines. Science 328, 1164–1168 (2010).
Davidson, L. N., Krawchuk, M. A. & Dulvy, N. K. Why have global shark and ray landings declined: improved management or overfishing? Fish Fish. 17, 438–458 (2016).
Dulvy, N. K. et al. Challenges and priorities in shark and ray conservation. Curr. Biol. 27, R565–R572 (2017).
Simpfendorfer, C. A. & Dulvy, N. K. Bright spots of sustainable shark fishing. Curr. Biol. 27, R97–R98 (2017).
Sumaila, U. R. et al. Benefits of rebuilding global marine fisheries outweigh costs. PLoS ONE 7, e40542 (2012).
Brooks, T. M. et al. Harnessing biodiversity and conservation knowledge products to track the Aichi Targets and Sustainable Development Goals. Biodiversity (Nepean) 16, 157–174 (2015).
FAO. The State of World Fisheries and Aquaculture 2016. Contributing to Food Security and Nutrition for All (FAO, 2016).
Hicks, C. C. et al. Harnessing global fisheries to tackle micronutrient deficiencies. Nature 574, 95–98 (2019).
Pereira, H. M., Navarro, L. M. & Martins, I. S. Global biodiversity change: the bad, the good, and the unknown. Annu. Rev. Environ. Resour. 37, 25–50 (2012).
Stein, R. W. et al. Global priorities for conserving the evolutionary history of sharks, rays and chimaeras. Nat. Ecol. Evol. 2, 288–298 (2018).
Pimiento, C. et al. Functional diversity of marine megafauna in the Anthropocene. Sci. Adv. 6, eaay7650 (2020).
Dulvy, N. K. et al. Extinction risk and conservation of the world’s sharks and rays. eLife 3, e00590 (2014).
Stuart, S. N. et al. Status and trends of amphibian declines and extinctions worldwide. Science 306, 1783–1786 (2004).
Hoffmann, M. et al. The impact of conservation on the status of the world’s vertebrates. Science 330, 1503–1509 (2010).
Pardo, S. A., Kindsvater, H. K., Reynolds, J. D. & Dulvy, N. K. Maximum intrinsic rate of population increase in sharks, rays, and chimaeras: the importance of survival to maturity. Can. J. Fish. Aquat. Sci. 73, 1159–1163 (2016).
McClenachan, L., Cooper, A. B. & Dulvy, N. K. Rethinking trade-driven extinction risk in marine and terrestrial megafauna. Curr. Biol. 26, 1640–1646 (2016).
Clarke, S. C. et al. Global estimates of shark catches using trade records from commercial markets. Ecol. Lett. 9, 1115–1126 (2006).
Brander, K. Disappearance of common skate Raia batis from Irish Sea. Nature 290, 48–49 (1981).
Manire, C. A. & Gruber, S. H. Many sharks may be headed toward extinction. Conserv. Biol. 4, 10–11 (1990).
Southeast Data, Assessment, and Review (SEDAR). Update Assessment to SEDAR 21, HMS Dusky Shark http://sedarweb.org/docs/suar/Dusky_update_report_2016.pdf (SEDAR, 2016).
International Commission for the Conservation of Atlantic Tunas. Report of the 2019 ICCAT Shortfin Mako Shark Stock Assessment Update Meeting. (ICCAT, 2019).
Dudley, S. F. & Simpfendorfer, C. A. Population status of 14 shark species caught in the protective gillnets off KwaZulu-Natal beaches, South Africa, 1978–2003. Mar. Freshw. Res. 57, 225–240 (2006).
Roff, G., Brown, C. J., Priest, M. A. & Mumby, P. J. Decline of coastal apex shark populations over the past half century. Commun. Biol. 1, 223 (2018).
Jiao, Y., Cortés, E., Andrews, K. & Guo, F. Poor‐data and data‐poor species stock assessment using a Bayesian hierarchical approach. Ecol. Appl. 21, 2691–2708 (2011).
Cortés, E. et al. Ecological risk assessment of pelagic sharks caught in Atlantic pelagic longline fisheries. Aquat. Living Resour. 23, 25–34 (2010).
Loh, J. et al. The Living Planet Index: using species population time series to track trends in biodiversity. Phil. Trans. R. Soc. B 360, 289–295 (2005).
Butchart, S. H. et al. Improvements to the Red List Index. PLoS ONE 2, e140 (2007).
Winker, H., Carvalho, F. & Kapur, M. JABBA: Just Another Bayesian Biomass Assessment. Fish. Res. 204, 275–288 (2018).
Sherley, R. B. et al. Estimating IUCN Red List population reduction: JARA—a decision‐support tool applied to pelagic sharks. Conserv. Lett. 13, e12688 (2020).
Punt, A. E. & Smith, A. D. in Conservation of Exploited Species (ed. Reynolds, J. D.) 41–66 (Cambridge Univ. Press, 2001).
Marler, P. N. & Marler, T. E. An assessment of Red List data for the Cycadales. Trop. Conserv. Sci. 8, 1114–1125 (2015).
Anticamara, J. A., Watson, R., Gelchu, A. & Pauly, D. Global fishing effort (1950–2010): trends, gaps, and implications. Fish. Res. 107, 131–136 (2011).
Vannuccini, S. Shark Utilization, Marketing, and Trade (FAO, 1999).
Salafsky, N. et al. A standard lexicon for biodiversity conservation: unified classifications of threats and actions. Conserv. Biol. 22, 897–911 (2008).
Juan-Jordá, M. J., Mosqueira, I., Cooper, A. B., Freire, J. & Dulvy, N. K. Global population trajectories of tunas and their relatives. Proc. Natl Acad. Sci. USA 108, 20650–20655 (2011).
Lawson, J. M. & Fordham, F. Realizing the Potential of the Convention on Migratory Species to Conserve Elasmobranchs https://www.cms.int/sites/default/files/publication/CMS-SAI-76pp-FINAL-5DEC-HIGH%20%281%29-min.pdf (Shark Advocates International, 2018).
Juan‐Jordá, M. J., Murua, H., Arrizabalaga, H., Dulvy, N. K. & Restrepo, V. Report card on ecosystem‐based fisheries management in tuna regional fisheries management organizations. Fish Fish. 19, 321–339 (2018).
Gilman, E., Passfield, K. & Nakamura, K. Performance of regional fisheries management organizations: ecosystem‐based governance of bycatch and discards. Fish Fish. 15, 327–351 (2014).
Curtis, T. H. et al. Seasonal distribution and historic trends in abundance of white sharks, Carcharodon carcharias, in the western North Atlantic Ocean. PLoS ONE 9, e99240 (2014).
Queiroz, N. et al. Global spatial risk assessment of sharks under the footprint of fisheries. Nature 572, 461–466 (2019).
Peterson, C. D. et al. Preliminary recovery of coastal sharks in the south‐east United States. Fish Fish. 18, 845–859 (2017).
Jennings, S. Reporting and advising on the effects of fishing. Fish Fish. 8, 269–276 (2007).
Kitchell, J. F., Essington, T. E., Boggs, C. H., Schindler, D. E. & Walters, C. J. The role of sharks and longline fisheries in a pelagic ecosystem of the central Pacific. Ecosystems (N. Y.) 5, 202–216 (2002).
Polovina, J. J., Abecassis, M., Howell, E. A. & Woodworth, P. Increases in the relative abundance of mid-trophic level fishes concurrent with declines in apex predators in the subtropical North Pacific, 1996–2006. Fish Bull. 107, 523–531 (2009).
Jabado, R. W. et al. Troubled waters: threats and extinction risk of the sharks, rays and chimaeras of the Arabian Sea and adjacent waters. Fish Fish. 19, 1043–1062 (2018).
Costello, C. et al. Global fishery prospects under contrasting management regimes. Proc. Natl Acad. Sci. USA 113, 5125–5129 (2016).
Tremblay-Boyer, L., Carvalho, F., Neubauer, P. & Pilling, G. M. Stock Assessment for Oceanic Whitetip Shark in the Western and Central Pacific Ocean. Scientific Committee Fifteenth Regular Session Report. No. WCPFC-SC15-2019/SA-WP-06 (WCPFC, 2019).
Cailliet, G. M. & Goldman, K. J. in Biology of Sharks and their Relatives (eds Carrier, J. C., Musick, J. A. & Heithaus, M. R.) 404–453 (CRC, 2004).
Cailliet, G. M. Perspectives on elasmobranch life‐history studies: a focus on age validation and relevance to fishery management. J. Fish Biol. 87, 1271–1292 (2015).
Harry, A. V. Evidence for systemic age underestimation in shark and ray ageing studies. Fish Fish. 19, 185–200 (2018).
IUCN Standards and Petitions Subcommittee. Guidelines for using the IUCN Red List Categories and Criteria. Version 13 (IUCN, 2017).
Pacifici, M. et al. Generation length for mammals. Nat. Conserv. 5, 89–94 (2013).
Winker, H., Pacoureau, N. & Sherley, R. B. JARA: ‘Just Another Red-List Assessment’. Preprint at https://doi.org/10.1101/672899 (2020).
Gelman, A. & Rubin, D. B. Inference from iterative simulation using multiple sequences. Stat. Sci. 7, 457–472 (1992).
Conn, P. B., Johnson, D. S., Williams, P. J., Melin, S. R. & Hooten, M. B. A guide to Bayesian model checking for ecologists. Ecol. Monogr. 88, 526–542 (2018).
Gelman, A. et al. Bayesian Data Analysis (CRC, 2013).
R Core Team. R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2018).
Su, Y.-S. & Yajima, M. R2jags: using R to run ‘JAGS’. R package version 0.5-7, Vol. 34 https://cran.r-project.org/web/packages/R2jags/index.html (2015).
Plummer, M. JAGS version 4.3.0 User Manual (2017).
Pauly, D., Zeller, D. & Palomares, M. L. D. Sea Around Us Concepts, Design and Data. http://www.seaaroundus.org/ (2015).
IUCN. The IUCN Red List of Threatened Species. Version 2019-2 http://www.iucnredlist.org (IUCN, 2019).
Takeuchi, Y., Tremblay-Boyer, L., Pilling, G. M. & Hampton, J. Assessment of Blue Shark in the Southwestern Pacific. Scientific Committee Twelfth Regular Session Report. No. WCPFC-SC12-2016/SA-WP-08 REV1 (WCPFC, 2016).
Acknowledgements
We thank all members of the IUCN SSC SSG and other experts who contributed to the data collation and, in particular, A. Aires-da-Silva, F. Carvalho, J. Cheok, S. Clarke, R. Coelho, E. Cortés, T. Driggers, C. Dudgeon, M. Hoffmann, Y. Jiao, T. Kashiwagi, A. Kock, C. Lowe, J. Rice, L. Tremblay-Boyer, W. J. VanderWright and S. Wintner. The scientific results and conclusions, as well as any views or opinions expressed herein, are those of the author(s) and do not necessarily reflect those of institutions or data providers. This project was funded by the Shark Conservation Fund, a philanthropic collaborative pooling expertise and resources to meet the threats facing the world’s sharks and rays. The Shark Conservation Fund is a project of Rockefeller Philanthropy Advisors. This work was funded by the Shark Conservation Fund as part of the Global Shark Trends Project to N.K.D. and C.A.S., and US National Science Foundation grant DEB-1556779 to H.K.K. P.M.K. was supported by the Marine Biodiversity Hub, a collaborative partnership supported through funding from the Australian Government’s National Environmental Science Program. N.K.D. was supported by Natural Science and Engineering Research Council Discovery and Accelerator Awards and the Canada Research Chairs Program.
Author information
Authors and Affiliations
Contributions
C.L.R., P.M.K., R.A.P. and N.K.D. organized and led the workshop investigation of data quality and facilitated the 2018 Red List assessments. N.P., H.K.K. and N.K.D. conceptualized the analysis. J.S.Y., C.L.R., H.K.K., R.B.S., N.P. and N.K.D. compiled and curated the time-series data. J.K.C., A.D.M. and H.W. provided additional time-series data. N.P., R.B.S. and H.W. conducted the statistical analysis. N.P., H.K.K. and N.K.D. visualized the data and wrote the first draft. N.K.D. and H.K.K. acquired the funding. All authors discussed the time-series data, analysis and results, and contributed to writing the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature thanks Paul Conn, Johann Mourier, Nuno Queiroz and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
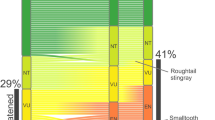
Extended Data Fig. 1 Hierarchical building of the global LPI and RLI.
LC, least concern; NT, near threatened; VU, vulnerable; EN, endangered; CR, critically endangered; EX, extinct.
Extended Data Fig. 2 Calculation of the LPI.
a, Schematic example of constructing the observed (black) and projected (blue) LPI. First, year-to-year rates of change (yyrc) (dt) are averaged between species in the same region (for example, in region 1 (R1), species A with \({d}_{{{\rm{A}}}_{t}}\) and species B with \({d}_{{{\rm{B}}}_{t}}\) averaged in \({d}_{{{\rm{R}}1}_{t}}\)). In a second step, yyrc are averaged between regions 1, 2 and 3 to give the global yyrc. The observed LPI builds on the yyrc calculated from the estimated abundance index from the state–space population model. The projected LPI builds on the yyrc calculated from the estimated and projected abundance index from the state-space population model. Projections are from the last data point to 2020. b, Global LPI for oceanic sharks and rays estimated from 1970 to 2018 in black and extrapolated to 2020 in blue. The black and the thick blue lines denote, respectively, the mean of the estimated and extrapolated LPI. The white and thin blues lines denote, respectively, the 95% credible intervals of the estimated and extrapolated LPI and the grey lines denote each iteration of the estimated LPI. c, The annual percentage change was calculated from the posteriors of the estimated LPI (grey) and extrapolated LPI (blue) around the final-assessment year relative to the posteriors for 1970. Vertical bars for the 1970–2018 period denote the median of the estimated and extrapolated LPI.
Extended Data Fig. 3 Global and species-specific LPI for oceanic sharks and rays from 1970 to 2018.
Global original LPI is the mean black line. Faint grey lines show the effect of excluding all data for a single species at a time and recalculating the mean global LPI for all other species. No means from jackknife species trends fall outside the 95% credible interval from the run with all of the datasets included, suggesting that our selection of species did not unduly influence the overall LPI result.
Extended Data Fig. 4 Time-series output for Carcharhinidae.
a–d, Observed (black or empty points and stars indicate different time-series) and modelled (black line) abundance indices for silky shark (Carcharhinus falciformis) (a), oceanic whitetip shark (C. longimanus) (b), dusky shark (C. obscurus) (c) and blue shark (Prionace glauca) (d) obtained from the state–space population model. The thick black line denotes the mean of the estimated abundance index and the shaded regions denote 95% credible intervals.
Extended Data Fig. 5 Time-series output for Sphyrnidae.
a–c, Observed (black or empty points and stars indicate different time-series) and modelled (black line) abundance indices for scalloped hammerhead (S. lewini) (a), great hammerhead (S. mokarran) (b) and smooth hammerhead (S. zygaena) (c) obtained from the state–space population model. The thick black line denotes the mean of the estimated abundance index and the shaded regions denote 95% credible intervals.
Extended Data Fig. 6 Time-series output for Alopiidae.
a–c, Observed (points) and modelled (black line) abundance indices for pelagic thresher (A. pelagicus) (a), bigeye thresher (Alopias superciliosus) (b) and common thresher (Alopias vulpinus) (c) obtained from the state–space population model. The thick black line denotes the mean of the estimated abundance index and the shaded regions denote 95% credible intervals.
Extended Data Fig. 7 Time-series output for Lamnidae.
a–d, Observed (black or empty points and stars indicate different time-series) and modelled (black line) abundance indices for white shark (C. carcharias) (a), shortfin mako (I. oxyrinchus) (b), longfin mako (I. paucus) (c) and porbeagle (L. nasus) (d) obtained from the state–space population model. The thick black line denotes the mean of the estimated abundance index and the shaded regions denote 95% credible intervals.
Extended Data Fig. 8 Time-series output for Dasyatidae and Mobulidae.
a–d, Observed (points) and modelled (black line) abundance indices for pelagic stingray (P. violacea) (a), reef manta ray (M. alfredi) (b), giant manta ray (Mobula birostris) (c) and shortfin devilray (M. kuhlii) (d) obtained from the state–space population model. The thick black line denotes the mean of the estimated abundance index and the shaded regions denote 95% credible intervals.
Extended Data Fig. 9 Stock assessments for oceanic sharks.
a, Oceanic shark stock status—over time—being at levels of biomass or abundance above MSY (green lines) or below MSY (red lines). Data were obtained from refs. 24,25,28,51, and refs. 81–84,93,94,96,97 in the Supplementary Information. Dotted lines indicate that a stock is above or below the biomass or abundance levels producing MSY following the last stock assessment value. b, Number of published stock assessments for oceanic sharks and rays over time. c, Presentation of 14 stocks of oceanic sharks (no available stock assessments for oceanic rays), status (biomass or abundance over value at MSY) versus pressure (F/FMSY) in a Kobe plot style, for the last year with available data. Circles represent the unique values of each species if only one stock exists and represent the mean of the values of the different stocks (diamonds) when the species has multiple stocks. The plot is divided into four panels: the red panel (top left), with four stocks and three species, corresponds to stocks that are being overfished and where overfishing is occurring; the orange panel (top right), with one stock and one species, corresponds to stocks that are not overfished but where overfishing is occurring; the yellow panel (bottom left), with four stocks and three species, corresponds to stocks that are overfished but where overfishing is not occurring; and the green panel (bottom right), with five stocks and one species, corresponds to stocks that are not overfished and where overfishing is not occurring.
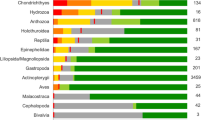
Extended Data Fig. 10 Percentage of reported threat categories in the 31 oceanic shark IUCN Red List assessments.
‘Biological resource use’ and, more specifically, ‘fishing and harvesting aquatic resources’ is the major reported threat.
Supplementary information
Supplementary Information
This file contains Supplementary Methods 1 and 2, Supplementary Tables 1-3, Supplementary Discussion 1-3 and Supplementary References. The Supplementary Methods 1 explains the method used to correct fishing effort, using technological efficiency, into an effective fishing effort. The Supplementary Methods 2 describes the nine encountered situations details when selecting generation time of species and describes the quality of data. Supplementary Table 1 describes all the time-series analysed and provides the ecological information used in the analyses. Supplementary Table 2 describes all the available Red List status available for oceanic sharks and rays and the ones used in the analyses. Supplementary Table 3 describes the type of trajectories and gives the source of the 15 stock assessment outputs of 8 species used in the analysis. The Supplementary Discussion 2 discusses the reasons why the Living Planet Index analysis for oceanic sharks and rays is conservative (true abundance trend index values are likely to be lower and the calculated percent declines worse than estimated here). The Supplementary Discussion 2 discusses the declines in devil rays’ time-series in Mozambique and the context suggesting that these declines may have occurred in other Indian Ocean countries. The Supplementary Discussion 3 discusses some additional management details of the regional fishery management organizations focused on tunas, relative to oceanic shark species.
Supplementary Data 1
Table of the LPI per oceanic shark and ray species per year: median (Credible Interval 95%).
Supplementary Data 2
Table of compiled time-series of mean oceanic shark stock assessments biomass/abundance trajectories relative to maximum sustainable yield per year.
Rights and permissions
About this article
Cite this article
Pacoureau, N., Rigby, C.L., Kyne, P.M. et al. Half a century of global decline in oceanic sharks and rays. Nature 589, 567–571 (2021). https://doi.org/10.1038/s41586-020-03173-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-020-03173-9
This article is cited by
-
Targeting ocean conservation outcomes through threat reduction
npj Ocean Sustainability (2024)
-
Warming waters lead to increased habitat suitability for juvenile bull sharks (Carcharhinus leucas)
Scientific Reports (2024)
-
Expanded vertical niche for two species of pelagic sharks: depth range extension for the dusky shark Carcharhinus obscurus and novel twilight zone occurrence by the silky shark Carcharhinus falciformis
Environmental Biology of Fishes (2024)
-
A review of the life history and ecology of euryhaline and estuarine sharks and rays
Reviews in Fish Biology and Fisheries (2024)
-
A tangled web: global review of fishing interactions with rhino rays
Reviews in Fish Biology and Fisheries (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.