Abstract
Haematopoietic stem cells (HSCs) reside in specialized microenvironments in the bone marrow—often referred to as ‘niches’—that represent complex regulatory milieux influenced by multiple cellular constituents, including nerves1,2. Although sympathetic nerves are known to regulate the HSC niche3,4,5,6, the contribution of nociceptive neurons in the bone marrow remains unclear. Here we show that nociceptive nerves are required for enforced HSC mobilization and that they collaborate with sympathetic nerves to maintain HSCs in the bone marrow. Nociceptor neurons drive granulocyte colony-stimulating factor (G-CSF)-induced HSC mobilization via the secretion of calcitonin gene-related peptide (CGRP). Unlike sympathetic nerves, which regulate HSCs indirectly via the niche3,4,6, CGRP acts directly on HSCs via receptor activity modifying protein 1 (RAMP1) and the calcitonin receptor-like receptor (CALCRL) to promote egress by activating the Gαs/adenylyl cyclase/cAMP pathway. The ingestion of food containing capsaicin—a natural component of chili peppers that can trigger the activation of nociceptive neurons—significantly enhanced HSC mobilization in mice. Targeting the nociceptive nervous system could therefore represent a strategy to improve the yield of HSCs for stem cell-based therapeutic agents.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
RNA sequencing data have been deposited in the Gene Expression Omnibus under accession number GSE156449. All other data are available from the corresponding author upon reasonable request. Source data are provided with this paper.
References
Gao, X., Xu, C., Asada, N. & Frenette, P. S. The hematopoietic stem cell niche: from embryo to adult. Development 145, dev139691 (2018).
Hoggatt, J., Kfoury, Y. & Scadden, D. T. Hematopoietic stem cell niche in health and disease. Annu. Rev. Pathol. 11, 555–581 (2016).
Katayama, Y. et al. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell 124, 407–421 (2006).
Lucas, D. et al. Chemotherapy-induced bone marrow nerve injury impairs hematopoietic regeneration. Nat. Med. 19, 695–703 (2013).
Maryanovich, M. et al. Adrenergic nerve degeneration in bone marrow drives aging of the hematopoietic stem cell niche. Nat. Med. 24, 782–791 (2018).
Méndez-Ferrer, S. et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature 466, 829–834 (2010).
Bensinger, W., DiPersio, J. F. & McCarty, J. M. Improving stem cell mobilization strategies: future directions. Bone Marrow Transplant. 43, 181–195 (2009).
Ordovas-Montanes, J. et al. The regulation of immunological processes by peripheral neurons in homeostasis and disease. Trends Immunol. 36, 578–604 (2015).
Pavlov, V. A., Chavan, S. S. & Tracey, K. J. Molecular and functional neuroscience in immunity. Annu. Rev. Immunol. 36, 783–812 (2018).
Pinho-Ribeiro, F. A., Verri, W. A. & Chiu, I. M. Nociceptor sensory neuron–immune interactions in pain and inflammation. Trends Immunol. 38, 5–19 (2017).
Tsunokuma, N. et al. Depletion of neural crest-derived cells leads to reduction in plasma noradrenaline and alters B lymphopoiesis. J. Immunol. 198, 156–169 (2017).
Suekane, A. et al. CGRP−CRLR/RAMP1 signal is important for stress-induced hematopoiesis. Sci. Rep. 9, 429 (2019).
Chow, A. et al. Bone marrow CD169+ macrophages promote the retention of hematopoietic stem and progenitor cells in the mesenchymal stem cell niche. J. Exp. Med. 208, 261–271 (2011).
Christopher, M. J., Rao, M., Liu, F., Woloszynek, J. R. & Link, D. C. Expression of the G-CSF receptor in monocytic cells is sufficient to mediate hematopoietic progenitor mobilization by G-CSF in mice. J. Exp. Med. 208, 251–260 (2011).
Winkler, I. G. et al. Bone marrow macrophages maintain hematopoietic stem cell (HSC) niches and their depletion mobilizes HSCs. Blood 116, 4815–4828 (2010).
Broxmeyer, H. E. et al. Rapid mobilization of murine and human hematopoietic stem and progenitor cells with AMD3100, a CXCR4 antagonist. J. Exp. Med. 201, 1307–1318 (2005).
Lucas, D. et al. Norepinephrine reuptake inhibition promotes mobilization in mice: potential impact to rescue low stem cell yields. Blood 119, 3962–3965 (2012).
Shastri, A. et al. Stimulation of adrenergic activity by desipramine enhances hematopoietic stem and progenitor cell mobilization along with G-CSF in multiple myeloma: A pilot study. Am. J. Hematol. 92, 1047–1051 (2017).
Ford, C. D., Green, W., Warenski, S. & Petersen, F. B. Effect of prior chemotherapy on hematopoietic stem cell mobilization. Bone Marrow Transplant. 33, 901–905 (2004).
Adams, G. B. et al. Haematopoietic stem cells depend on Gαs-mediated signalling to engraft bone marrow. Nature 459, 103–107 (2009).
Hagedorn, E. J., Durand, E. M., Fast, E. M. & Zon, L. I. Getting more for your marrow: boosting hematopoietic stem cell numbers with PGE2. Exp Cell Res. 329, 220–226 (2014).
Hoggatt, J. et al. Differential stem- and progenitor-cell trafficking by prostaglandin E2. Nature 495, 365–369 (2013).
Sands, W. A. & Palmer, T. M. Regulating gene transcription in response to cyclic AMP elevation. Cell. Signal. 20, 460–466 (2008).
Pulsipher, M. A. et al. Adverse events among 2408 unrelated donors of peripheral blood stem cells: results of a prospective trial from the National Marrow Donor Program. Blood 113, 3604–3611 (2009).
Schweizerhof, M. et al. Hematopoietic colony-stimulating factors mediate tumor–nerve interactions and bone cancer pain. Nat. Med. 15, 802–807 (2009).
Charles, A. & Pozo-Rosich, P. Targeting calcitonin gene-related peptide: a new era in migraine therapy. Lancet 394, 1765–1774 (2019).
Cui, M., Gosu, V., Basith, S., Hong, S. & Choi, S. Polymodal transient receptor potential vanilloid type 1 nocisensor: structure, modulators, and therapeutic applications. Adv. Protein Chem. Struct. Biol. 104, 81–125 (2016).
Asada, N. et al. Differential cytokine contributions of perivascular haematopoietic stem cell niches. Nat. Cell Biol. 19, 214–223 (2017).
Li, M. et al. Deficiency of RAMP1 attenuates antigen-induced airway hyperresponsiveness in mice. PLoS ONE 9, e102356 (2014).
Fritz-Six, K. L., Dunworth, W. P., Li, M. & Caron, K. M. Adrenomedullin signaling is necessary for murine lymphatic vascular development. J. Clin. Invest. 118, 40–50 (2008).
Acknowledgements
We thank J. Wood for providing Nav1.8-Cre mice, C. Prophete for technical assistance, A. Birbrair and N. Asada for advice with the initial experiments, and D. Sun from the Human Stem Cell FACS and Xenotransplantation Facility for assistance with cell sorting. This work was supported by grants from the National Institutes of Health (U01DK116312, R01DK056638, R01DK112976 and R01HL069438) to P.S.F. H.L. is the recipient of a F32 Ruth L. Kirschstein Postdoctoral Individual National Research Service Award (HL142243-01).
Author information
Authors and Affiliations
Contributions
X.G. designed the study, performed most of the experiments, analysed data and wrote the manuscript. D.Z. advised on experiment design, helped with flow cytometry analysis, sorting and bone marrow transplantations, and provided input on the manuscript. C.X. participated in study design and performed experiments. H.L. advised on experiment design and helped with experiments. K.M.C. provided Ramp1−/− and Calcrlfl/fl mice and commented on the manuscript. P.S.F. designed and supervised the study, interpreted data and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
P.S.F. serves as consultant for Pfizer, has received research funding from Ironwood Pharmaceuticals and is a shareholder of Cygnal Therapeutics. The rest of the authors declare no competing interests.
Additional information
Peer review information Nature thanks Iannis Aifantis, Toshio Suda and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
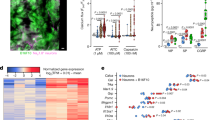
Extended Data Fig. 1 Characterization of nociceptive and sympathetic innervation in the bone marrow.
a, b, Representative confocal z-stack projection montages of C57BL/6 mouse femur stained for CGRP (nociceptive nerves), TH (sympathetic nerves), and TUBB3 (all peripheral nerves), CD31+CD144+ double-positive vasculature. Scale bar, 100 μm. Femoral sensory and sympathetic innervation is quantified by the total length of all CGRP+, TH+ or TUBB+ nerve fibres divided by the bone marrow (BM) area. n = 3 mice. c, Schematic illustration of the pharmacological denervation experiment using RTX and 6OHDA. d, e, Representative images of confocal z-stack projections from femurs of control, RTX-, 6OHDA- and dual-denervated mice stained for CGRP+ nociceptive nerve fibres, TH+ sympathetic nerve fibres, CD31+CD144+ blood vessels and DAPI. Scale bar, 100 μm. Femoral sensory and sympathetic innervation is quantified by the total length of CGRP+ or TH+ nerves divided by total bone marrow area. Data from n = 4, 5, 4, 4 mice, respectively. Data are mean ± s.e.m.; significance was assessed using a one-way ANOVA.
Extended Data Fig. 2 Nociceptive or sympathetic nerves are dispensable for HSC maintenance, whereas the depletion of both systems expands poorly functional HSCs.
a, b, Bone marrow cellularity and absolute numbers of B cells (B220+), T cells (CD3e+) and myeloid cells (Mac-1+) per femur from control, RTX, 6OHDA, or dual-denervated mice. n = 10, 8, 8, 11 (a) and 10, 9, 7, 10 (b) mice, respectively. c, Representative FACS plots showing the gating strategy for Lin−Sca-1+Kit+CD150+CD48− HSCs. The same gating strategy was used throughout. d, Absolute number of HSCs and LSK cells (Lin−Sca-1+Kit+) per femur from control, RTX, 6OHDA or dual-denervated mice. n = 10, 8, 8, 11 mice, respectively. e, Peripheral blood chimerism (CD45.2+) in CD45.1-recipient mice transplanted with 0.5 × 106 CD45.1 competitor bone marrow cells mixed with 0.5 × 106 donor bone marrow cells (CD45.2) from control, RTX, 6OHDA or dual-denervated mice at the indicated time points post-transplantation. n = 4, 4, 4, 5 mice, respectively. f, Bone marrow chimerism (CD45.2+) 20 weeks after transplantation. g, Experimental design to determine the homing efficiency of HSCs and LSK cells (CD45.2) from control- or dual-denervated mice to the bone marrow of recipients (CD45.1). h, Homing efficiency of donor CD45.2 HSCs and LSK cells detected in the recipient bone marrow. n = 4, 5 mice, respectively. Data are mean ± s.e.m.; significance was assessed using a two-tailed unpaired Student’s t-test (h) or one-way ANOVA (a, b, d–f). For box plots, the box spans from the 25th to 75th percentiles and the centre line was plotted at the median. Whiskers represent minimum to maximum range.
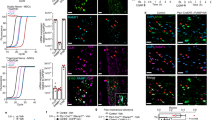
Extended Data Fig. 3 Expansion of phenotypic HSCs after dual sympathetic and nociceptive denervation is normalized by administration of CGRP or a β-adrenergic agonist.
a, Representative confocal z-stack projections from femurs of Nav1.8-Cre+; iTdTomato+ mice stained for CD31+CD144+ vasculature and CGRP+ nociceptive nerves. Nociceptive innervation quantified by the total length of all Nav1.8+ (red), CGRP+ (green) and Nav1.8+CGRP+ double positive (yellow) nerves divided by the bone marrow area. n = 4 mice. b, Schematic illustration of the dual denervation experiment with Nav1.8-Cre; iDTA mice. c, d, Representative images of confocal z-stack projections from femurs of Nav1.8-Cre+;iTdTomato+;iDTA− or Nav1.8-Cre+;iTdTomato+;iDTA+ mice stained for blood vessels (blue). Quantification of TdTomato+ nociceptive nerves in the femurs. n = 3 mice per group. e, f, Absolute numbers of HSCs (Lin−Sca-1+Kit+CD150+CD48−), B cells (B220+), T cells (CD3e+) and myeloid cells (Mac-1+) per femur from Nav1.8-Cre−;iDTA+ or Nav1.8-Cre+;iDTA+ mice with or without 6OHDA treatment. Each dot represents data from individual mice. n = 8, 7, 3, 8 (e) and 6, 6, 4, 7 (f) mice, respectively. g, Absolute numbers of HSCs, B cells and myeloid cells per femur from control and dual-denervated mice implanted with osmotic pumps containing saline as control, CGRP, isoproterenol or substance P. n = 14, 9, 5, 5, 5 mice respectively. Data are mean ± s.e.m. Significance was assessed using a two-tailed unpaired Student’s t-test (d) or one-way ANOVA (e–g). For box plots, the box spans from the 25th to 75th percentiles and the centre line was plotted at the median. Whiskers represent minimum to maximum range.
Extended Data Fig. 4 G-CSF-induced HSPC mobilization is impaired in mice that lack nociceptor neurons.
a, White blood cell counts and absolute numbers of LSK cells per ml of peripheral blood after G-CSF mobilization in vehicle-treated control and RTX-treated mice. n = 11, 13 mice, respectively. b, Spleen weight and the absolute numbers of LSK cells in the spleen after G-CSF mobilization in vehicle-treated control and RTX-treated mice. n = 11 mice per group. c, Bone marrow cellularity and the absolute numbers of LSK cells in the bone marrow after G-CSF-induced mobilization in vehicle-treated control and RTX-treated mice. n = 11, 13 mice, respectively. d, Cell cycle analysis of bone marrow HSCs (Lin−Sca-1+Kit+CD150+CD48−) from control or RTX-treated mice determined by FACS using anti-Ki67 and Hoechst 33342 staining. n = 6, 4 mice, respectively. e, White blood cell counts and absolute numbers of LSK cells per ml of peripheral blood after G-CSF-induced mobilization in Nav1.8-Cre−;iDTA+ and Nav1.8-Cre+;iDTA+ mice. n = 4, 5 mice, respectively. f, Bone marrow cellularity and the absolute numbers of LSK cells in the bone marrow after G-CSF-induced mobilization in Nav1.8-Cre−;iDTA+ and Nav1.8-Cre+;iDTA+ mice. n = 4, 5 mice, respectively. g, Peripheral blood B-cell (B220+CD45.2+), blood T-cell (CD3e+CD45.2+), and myeloid-cell (Mac-1+CD45.2+) donor chimerism in CD45.1-recipient mice transplanted with mobilized blood (CD45.2) derived from saline or RTX-treated mice mixed with CD45.1 competitor bone marrow cells at the indicated time points post-transplantation. n = 9, 8 mice, respectively. h, Total bone marrow chimerism (CD45.2+) and bone marrow HSC chimerism (Lin−Sca-1+Kit+CD150+CD48−CD45.2+) 20 weeks after transplantation. n = 9, 8 mice, respectively. Data are mean ± s.e.m. Significance was assessed using a two-tailed unpaired Student’s t-test. For box plots, the box spans from the 25th to 75th percentiles and the centre line was plotted at the median. Whiskers represent minimum to maximum range.
Extended Data Fig. 5 The neuropeptide CGRP, but not substance P, promotes G-CSF-induced HSC mobilization.
a, Absolute numbers of HSCs (Lin−Sca-1+Kit+CD150+CD48−) and LSK cells (Lin−Sca-1+Kit+) per ml of peripheral blood after G-CSF-induced mobilization in C57BL/6 mice implanted with osmotic pumps containing saline or substance P. n = 5 mice per group. b, Bone marrow cellularity and the absolute numbers of LSK cells and HSCs in the bone marrow after G-CSF administration in C57BL/6 mice implanted with osmotic pumps containing saline or substance P. n = 5 mice per group. c, Bone marrow cellularity and the absolute numbers of HSCs per femur from mice described in Fig. 2a. n = 18, 9, 7, 7 mice, respectively. d, Left, experimental design to determine the effect of CGRP on HSC mobilization of 6OHDA-denervated mice. Right, absolute number of HSCs per ml of peripheral blood after G-CSF administration in saline-or 6OHDA- treated C57BL/6 mice implanted with osmotic pumps containing saline or CGRP. n = 6 mice per group. Data are mean ± s.e.m.; significance was assessed using a two-tailed unpaired Student’s t-test.
Extended Data Fig. 6 Ramp1-deficient mice exhibit no haematopoietic defect at steady state.
a, Ramp1 mRNA expression levels determined by quantitative PCR in total bone marrow cells derived from Ramp1+/+ or Ramp1−/− mice. n = 5 biological samples. b, c, White blood cell counts (b) and the absolute numbers of B cells (B220+), T cells (CD3e+) and myeloid cells (Mac-1+) (c) per ml of peripheral blood from Ramp1+/+ or Ramp1−/− mice at steady state. n = 5 mice per group. d, e, Bone marrow cellularity (d) and the absolute numbers of LSK cells, B cells (B220+), T cells (CD3e+) and myeloid cells (Mac-1+) (e) per femur from Ramp1+/+ or Ramp1−/− mice at steady state. n = 5 mice per group. f, Left, experimental design to determine the homing efficiency of HSCs (Lin−Sca-1+Kit+CD150+CD48−) and LSK cells from Ramp1+/+ and Ramp1−/− mice (CD45.2) to the bone marrow of lethally irradiated recipients (CD45.1). Right, the percentage of donor CD45.2+ HSCs and LSK cells detected in the recipient bone marrow. n = 5 mice per group. g, Bone marrow cellularity and the absolute number of HSCs in the bone marrow. n = 3, 4, 3, 3 mice, respectively. Data are mean ± s.e.m. Significance was assessed using a two-tailed unpaired Student’s t-test (a–f) or one-way ANOVA (g).
Extended Data Fig. 7 Nociceptive-nerve-deficient mice exhibit no alteration of HSC niche components after G-CSF treatment.
a, Absolute numbers of mesenchymal stem cells (MSCs) (CD45−Ter119−CD31−CD51+PDGFRα+), endothelial cells (ECs) (CD45−Ter119−CD31high) and macrophages (Gr-1−F4/80+CD115intSSCint/low) per femur from saline- or RTX-treated mice after G-CSF treatment. n = 4, 4 (left and middle), 6, 8 (right) mice, respectively. b, qPCR quantification of Cxcl12 mRNA levels in total bone marrow cells, sorted MSCs and ECs from saline- or RTX-treated mice after G-CSF treatment. n = 5 mice per group. c, Levels of CXCL12 in bone marrow extracellular fluid (BMEF) measured by ELISA. n = 3, 5, 4 (left), 3, 4, 5 (middle), 4, 5 (right) mice. d, Mean fluorescence intensities (MFI) in the expression of CXCR4, VLA4 and CD44 on HSCs (Lin−Sca-1+Kit+CD150+CD48−). n = 4 mice per group. Data are mean ± s.e.m. Significance was assessed using a two-tailed unpaired Student’s t-test (a, b, d) or one-way ANOVA (c).
Extended Data Fig. 8 Transcriptome analysis by RNA sequencing of HSCs from Ramp1+/+ and Ramp1−/− mice.
a, Heat map shows differentially expressed genes between wild-type- and Ramp1−/−-sorted HSCs (adjusted P value <0.05). b, Gene set enrichment analyses showing upregulated and downregulated pathways in Ramp1−/− HSCs compared to wild-type HSCs (P < 0.01, n = 3 biological replicates per group). c, Gene set enrichment analyses showing significant alterations of gene sets involved in the Gαs/adenylyl cyclase/cAMP pathway. d, Schematic illustration of the dual stimulation experiment. e, Absolute number of HSCs (Lin−Sca-1+Kit+CD150+CD48−) in the mobilized peripheral blood of mice treated with control saline, desipramine (DES), CGRP, or both DES and CGRP. n = 9, 4, 8, 4 mice, respectively. Data are mean ± s.e.m. Significance was assessed using a one-way ANOVA.
Extended Data Fig. 9 Ingestion of spicy food enhances HSC mobilization.
a, Scoville heat scale for chili peppers. 100 ppm = 100 mg kg−1. b, Three pellets of standard chow (brown) and three pellets of spicy chow (red) were provided to two mice at 18:00 on day 1. Sixteen hours later (10:00 on day 2), two of the three standard pellets had been consumed whereas the spicy food pellets remained untouched. c, Daily food intake (left) and the body weight (right) of mice fed with standard diet or capsaicin-containing diet. n = 5 (left), 4 mice (right) per group. d, CGRP levels in the BMEF from mice fed with control diet or capsaicin diet. n = 9 mice per group. e, Left, absolute numbers of HSCs (Lin−Sca-1+Kit+CD150+CD48−) in the bone marrow after G-CSF-induced mobilization in mice fed with standard or capsaicin-containing chow. n = 6, 7 mice, respectively. Right, cell cycle analysis of bone marrow HSCs was determined by FACS using anti-Ki67 and Hoechst 33342 staining. n = 6 mice. f, White blood cell (WBC) counts (left) and absolute numbers of LSK cells (right) per ml of peripheral blood after G-CSF-induced mobilization in mice fed with standard diet or capsaicin-containing diet. n = 6, 7 mice, respectively. g, i, Peripheral total blood donor chimerism (CD45.2+) in CD45.1-recipient mice transplanted with 0.5 × 106 CD45.1 competitor bone marrow cells and 250 HSCs sorted from mobilized blood (g) or bone marrow (i) from mice fed with standard or capsaicin chow. n = 9, 8 (g), 8, 8 (i) mice, respectively. h, j, Peripheral blood B cell (B220+CD45.2+), T cell (CD3e+CD45.2+) and myeloid cell (Mac-1+CD45.2+) donor chimerism in CD45.1-recipient mice transplanted with 250 blood HSCs (h) or bone marrow HSCs (j) with competitor bone marrow cells at 16 weeks post-transplantation. n = 9, 8 (h), 8, 8 (j) mice, respectively. k, Experimental design to determine the effects of CGRP administration on HSC competitiveness. l, Peripheral total blood donor chimerism (CD45.2+) in CD45.1-recipient mice transplanted with 0.5 × 106 CD45.1 competitor bone marrow cells and 250 HSCs sorted from mobilized blood from PBS- or CGRP-treated mice. n = 7, 9 mice, respectively. m, Peripheral blood B cell, T cell and myeloid cell donor chimerism in CD45.1-recipient mice transplanted with 250 blood HSCs with competitor bone marrow cells at 16 weeks post-transplantation. n = 7, 9 mice, respectively. Data are mean ± s.e.m. Two-tailed unpaired Student’s t-test. For box plots, the box spans from the 25th to 75th percentiles and the centre line was plotted at the median. Whiskers represent minimum to maximum range.
Extended Data Fig. 10 Nociceptor-mediated HSC mobilization.
Schematic representation showing that nociceptive-nerve-derived CGRP, but not substance P, acts via CGRP receptors on HSCs to enhance their mobilization via a cAMP-mediated signalling pathway.
Supplementary information
Source data
Rights and permissions
About this article
Cite this article
Gao, X., Zhang, D., Xu, C. et al. Nociceptive nerves regulate haematopoietic stem cell mobilization. Nature 589, 591–596 (2021). https://doi.org/10.1038/s41586-020-03057-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-020-03057-y
This article is cited by
-
Somatosensory cortex and central amygdala regulate neuropathic pain-mediated peripheral immune response via vagal projections to the spleen
Nature Neuroscience (2024)
-
Platelet-derived circulating soluble P-selectin is sufficient to induce hematopoietic stem cell mobilization
Stem Cell Research & Therapy (2023)
-
IgG immune complex-induced acute lung injury is ameliorated by cAMP via down-regulation of C/EBP- and AP-1-mediated transcriptions
Journal of Inflammation (2023)
-
Leptin receptor+ cells promote bone marrow innervation and regeneration by synthesizing nerve growth factor
Nature Cell Biology (2023)
-
Sensory nerve niche regulates mesenchymal stem cell homeostasis via FGF/mTOR/autophagy axis
Nature Communications (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.