Abstract
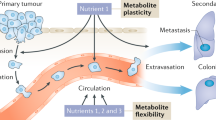
Metastasis requires cancer cells to undergo metabolic changes that are poorly understood1,2,3. Here we show that metabolic differences among melanoma cells confer differences in metastatic potential as a result of differences in the function of the MCT1 transporter. In vivo isotope tracing analysis in patient-derived xenografts revealed differences in nutrient handling between efficiently and inefficiently metastasizing melanomas, with circulating lactate being a more prominent source of tumour lactate in efficient metastasizers. Efficient metastasizers had higher levels of MCT1, and inhibition of MCT1 reduced lactate uptake. MCT1 inhibition had little effect on the growth of primary subcutaneous tumours, but resulted in depletion of circulating melanoma cells and reduced the metastatic disease burden in patient-derived xenografts and in mouse melanomas. In addition, inhibition of MCT1 suppressed the oxidative pentose phosphate pathway and increased levels of reactive oxygen species. Antioxidants blocked the effects of MCT1 inhibition on metastasis. MCT1high and MCT1−/low cells from the same melanomas had similar capacities to form subcutaneous tumours, but MCT1high cells formed more metastases after intravenous injection. Metabolic differences among cancer cells thus confer differences in metastatic potential as metastasizing cells depend on MCT1 to manage oxidative stress.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Vanharanta, S. & Massagué, J. Origins of metastatic traits. Cancer Cell 24, 410–421 (2013).
Le Gal, K. et al. Antioxidants can increase melanoma metastasis in mice. Sci. Transl. Med. 7, 308re8 (2015).
Piskounova, E. et al. Oxidative stress inhibits distant metastasis by human melanoma cells. Nature 527, 186–191 (2015).
Luo, M. et al. Targeting breast cancer stem cell state equilibrium through modulation of redox signaling. Cell Metab. 28, 69–86 (2018).
Kfoury, A. et al. AMPK promotes survival of c-Myc-positive melanoma cells by suppressing oxidative stress. EMBO J. 37, e97673 (2018).
Xu, I. M. et al. Transketolase counteracts oxidative stress to drive cancer development. Proc. Natl Acad. Sci. USA 113, E725–E734 (2016).
Fan, J. et al. Quantitative flux analysis reveals folate-dependent NADPH production. Nature 510, 298–302 (2014).
Lewis, C. A. et al. Tracing compartmentalized NADPH metabolism in the cytosol and mitochondria of mammalian cells. Mol. Cell 55, 253–263 (2014).
Harris, I. S. et al. Glutathione and thioredoxin antioxidant pathways synergize to drive cancer initiation and progression. Cancer Cell 27, 211–222 (2015).
Sayin, V. I. et al. Antioxidants accelerate lung cancer progression in mice. Sci. Transl. Med. 6, 221ra15 (2014).
DeNicola, G. M. et al. Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature 475, 106–109 (2011).
Schafer, Z. T. et al. Antioxidant and oncogene rescue of metabolic defects caused by loss of matrix attachment. Nature 461, 109–113 (2009).
Reczek, C. R. & Chandel, N. S. The two faces of reactive oxygen species in cancer. Ann. Rev. Cancer Biol. 1, 79–98 (2017).
Vander Heiden, M. G. & DeBerardinis, R. J. Understanding the intersections between metabolism and cancer biology. Cell 168, 657–669 (2017).
Sonveaux, P. et al. Targeting lactate-fueled respiration selectively kills hypoxic tumor cells in mice. J. Clin. Invest. 118, 3930–3942 (2008).
Feron, O. Pyruvate into lactate and back: from the Warburg effect to symbiotic energy fuel exchange in cancer cells. Radiother. Oncol. 92, 329–333 (2009).
Faubert, B. et al. Lactate metabolism in human lung tumors. Cell 171, 358–371.e9 (2017).
Hui, S. et al. Glucose feeds the TCA cycle via circulating lactate. Nature 551, 115–118 (2017).
Halestrap, A. P. Monocarboxylic acid transport. Compr. Physiol. 3, 1611–1643 (2013).
Hong, C. S. et al. MCT1 modulates cancer cell pyruvate export and growth of tumors that co-express MCT1 and MCT4. Cell Reports 14, 1590–1601 (2016).
Doherty, J. R. et al. Blocking lactate export by inhibiting the Myc target MCT1 disables glycolysis and glutathione synthesis. Cancer Res. 74, 908–920 (2014).
Izumi, H. et al. Monocarboxylate transporters 1 and 4 are involved in the invasion activity of human lung cancer cells. Cancer Sci. 102, 1007–1013 (2011).
Payen, V. L. et al. Monocarboxylate transporter MCT1 promotes tumor metastasis independently of its activity as a lactate transporter. Cancer Res. 77, 5591–5601 (2017).
Quintana, E. et al. Human melanoma metastasis in NSG mice correlates with clinical outcome in patients. Sci. Transl. Med. 4, 159ra149 (2012).
Chen, Y. J. et al. Lactate metabolism is associated with mammalian mitochondria. Nat. Chem. Biol. 12, 937–943 (2016).
Kirk, P. et al. CD147 is tightly associated with lactate transporters MCT1 and MCT4 and facilitates their cell surface expression. EMBO J. 19, 3896–3904 (2000).
Pinheiro, C. et al. The metabolic microenvironment of melanomas: prognostic value of MCT1 and MCT4. Cell Cycle 15, 1462–1470 (2016).
Curtis, N. J. et al. Pre-clinical pharmacology of AZD3965, a selective inhibitor of MCT1: DLBCL, NHL and Burkitt’s lymphoma anti-tumor activity. Oncotarget 8, 69219–69236 (2017).
Polański, R. et al. Activity of the monocarboxylate transporter 1 inhibitor AZD3965 in small cell lung cancer. Clin. Cancer Res. 20, 926–937 (2014).
Meeth, K., Wang, J. X., Micevic, G., Damsky, W. & Bosenberg, M. W. The YUMM lines: a series of congenic mouse melanoma cell lines with defined genetic alterations. Pigment Cell Melanoma Res. 29, 590–597 (2016).
Guan, X., Rodriguez-Cruz, V. & Morris, M. E. Cellular uptake of MCT1 inhibitors AR-C155858 and AZD3965 and their effects on MCT-mediated transport of L-lactate in murine 4T1 breast tumor cancer cells. AAPS J. 21, 13 (2019).
Baek, G. et al. MCT4 defines a glycolytic subtype of pancreatic cancer with poor prognosis and unique metabolic dependencies. Cell Reports 9, 2233–2249 (2014).
Jang, C., Chen, L. & Rabinowitz, J. D. Metabolomics and isotope tracing. Cell 173, 822–837 (2018).
Kirkman, H. N. & Hendrickson, E. M. Glucose 6-phosphate dehydrogenase from human erythrocytes. II. Subactive states of the enzyme from normal persons. J. Biol. Chem. 237, 2371–2376 (1962).
Andrés, V., Carreras, J. & Cussó, R. Regulation of muscle phosphofructokinase by physiological concentrations of bisphosphorylated hexoses: effect of alkalinization. Biochem. Biophys. Res. Commun. 172, 328–334 (1990).
Ran, F. A. et al. Genome engineering using the CRISPR–Cas9 system. Nat. Protocols 8, 2281–2308 (2013).
Shi, X. et al. The abundance of metabolites related to protein methylation correlates with the metastatic capacity of human melanoma xenografts. Sci. Adv. 3, eaao5268 (2017).
Marin-Valencia, I. et al. Analysis of tumor metabolism reveals mitochondrial glucose oxidation in genetically diverse human glioblastomas in the mouse brain in vivo. Cell Metab. 15, 827–837 (2012).
Yang, C. et al. Glutamine oxidation maintains the TCA cycle and cell survival during impaired mitochondrial pyruvate transport. Mol. Cell 56, 414–424 (2014).
Tu, B. P. et al. Cyclic changes in metabolic state during the life of a yeast cell. Proc. Natl Acad. Sci. USA 104, 16886–16891 (2007).
Su, X., Lu, W. & Rabinowitz, J. D. Metabolite spectral accuracy on Orbitraps. Anal. Chem. 89, 5940–5948 (2017).
Matsuyama, S., Llopis, J., Deveraux, Q. L., Tsien, R. Y. & Reed, J. C. Changes in intramitochondrial and cytosolic pH: early events that modulate caspase activation during apoptosis. Nat. Cell Biol. 2, 318–325 (2000).
Noguchi, K., Gel, Y., Brunner, E. & Konietschke, F. nparLD: an R software package for the nonparametric analysis of longitudinal data in factorial experiments. J. Stat. Softw. 50, 1–23 (2012).
Acknowledgements
S.J.M. is a Howard Hughes Medical Institute (HHMI) Investigator, the Mary McDermott Cook Chair in Pediatric Genetics, the Kathryn and Gene Bishop Distinguished Chair in Pediatric Research, the director of the Hamon Laboratory for Stem Cells and Cancer, and a Cancer Prevention and Research Institute of Texas Scholar. R.J.D. is an HHMI Investigator, the Robert L. Moody, Sr. Faculty Scholar at UT Southwestern and Joel B. Steinberg, M.D. Chair in Pediatrics. The research was supported by the Cancer Prevention and Research Institute of Texas (RP170114 and RP180778), the National Institutes of Health (R35 CA220449; U01 CA228608) and the Robert A. Welch Foundation (I-1733). A.T. was supported by the Else Kröner-Forschungskolleg and the Leopoldina Fellowship Program (LPDS 2016-16) of the German National Academy of Sciences. B.F. was supported by a postdoctoral fellowship from the Canadian Institutes of Health Research (MFE 140911). B.S. and A.S. were supported by Ruth L. Kirschstein National Research Service Award Postdoctoral Fellowships from the National Heart, Lung, and Blood Institute (F32 HL139016-01) and the National Institute of Child Health and Human Development (F32 HD096786-01). We thank A. Gross for mouse colony management as well as N. Loof and the Moody Foundation Flow Cytometry Facility.
Author information
Authors and Affiliations
Contributions
A.T., R.J.D. and S.J.M. conceived the project, and designed and interpreted experiments. A.T. performed most of the experiments. B.F., A.S., W.G. and R.J.D. participated in the design, analysis and interpretation of isotope tracing and metabolomics experiments. B.F., T.P.M. and R.J.D. developed methods for metabolomics and isotope tracing in vivo. V.R. and J.M.U. helped A.T. to perform the in vivo tumorigenesis assays. J.M.U. performed the melanoma cell migration experiments in culture. B.S., Z.G. and S.Y.K. helped to design the CRISPR gene-targeting and MCT1 overexpression constructs, and generated the constructs. D.S. and T.V. helped with the assessment and interpretation of MCT1 expression patterns in patient specimens. T.P.M. and M.M. performed all of the mass spectrometric analysis of metabolomic and isotope tracing specimens. M.M.M. performed the immunofluorescence analysis of MCT1 expression. Z.Z. performed statistical analyses. A.T., B.F., R.J.D. and S.J.M. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
R.J.D. is an advisor for Agios Pharmaceuticals. S.J.M. is an advisor for Frequency Therapeutics and Protein Fluidics
Additional information
Peer review information Nature thanks John Cleveland, Markus Ralser and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Plasma enrichment of isotopically labelled metabolites after infusion into xenografted mice.
Related to Fig. 1. a, Summary of the melanomas used in this study and their spontaneous metastatic behaviour after subcutaneous transplantation into NSG mice. Melanomas were characterized as inefficient or efficient metastasizers. Before subcutaneous tumours grew to 2.5 cm in diameter (when the mice were killed per approved protocol), inefficient metastasizers rarely formed macrometastases or micrometastases beyond the lung, whereas efficient metastasizers commonly formed macrometastases as well as micrometastases in several organs (data reflect results from one to five independent experiments per melanoma). Some of these data have been published previously24. b–g, Isotope tracing was performed in NSG mice subcutaneously xenografted with efficiently metastasizing melanomas from four patients (M405, M481, M487 and UT10) and inefficiently metastasizing melanomas from nine patients (M715, UM17, UM22, UM43, UM47, M498, M528, M597 and M610). The number of tumours or mice analysed per treatment is indicated. b, Glutamine m + 5 as a fraction of total plasma glutamine in mice infused with [U-13C]glutamine (14 independent experiments). c, Isotope enrichment in subcutaneous tumours after [U-13C]glutamine infusion (14 independent experiments). d, Glucose m + 6 as a fraction of total plasma glucose in mice infused with [U-13C]glucose (20 independent experiments). e, Plasma glucose and lactate concentrations before and after infusion. f, Lactate m + 3 as a fraction of total plasma lactate in mice infused with [U-13C]lactate (23 independent experiments). g, Lactate m + 1 as a fraction of total plasma lactate in mice infused with [2-2H]lactate (three independent experiments). h, Expected isotope labelling after [2-2H]lactate infusion. i, Western blot analysis of LDHA and LDHB in subcutaneous tumours from NSG mice xenografted with efficiently (M405, M481 and UT10) or inefficiently (UM17, UM43 and UM47) metastasizing melanomas (representative of four independent experiments). Data are mean ± s.d. Statistical significance was assessed using Mann–Whitney tests (c) and t-tests at 180 or 300 min when tumours were obtained for analysis (b, d, f, g) or paired t-tests (e).
Extended Data Fig. 2 Efficient metastasizers express higher levels of MCT1 than inefficient metastasizers.
Related to Fig. 2. a, Quantification of MCT1 relative to actin bands from the western blot in Fig. 2a comparing efficient and inefficient metastasizers. b, Quantification of MCT4 relative to actin bands from the western blot in Fig. 2c comparing efficient and inefficient metastasizers. c, d, Quantification of mean fluorescence intensities for MCT1 staining in the flow cytometry plots comparing efficient (Fig. 2e) and inefficient (Fig. 2d) metastasizers. HCC15 cells and MCT1-deficient HCC15 cells were positive and negative controls (c). e, f, Immunofluorescence staining for MCT1 (green) in sections from subcutaneous tumours from inefficiently (e, UM47) or efficiently (f, M405) metastasizing melanomas. An adjacent section was stained with an antibody against the melanoma marker S100b (green). Images are representative of three independent experiments per melanoma. g, h, Immunofluorescence staining for MCT1 (green) in sections from subcutaneous tumours from inefficient (g; M498, M610 and M597) and efficient (h; M481, UT10 and M405) metastasizers. In each case, an adjacent section was stained with an antibody against S100b (green). Images are representative of results from two independent experiments per melanoma. i, j, Although efficient metastasizers often exhibited cell-surface staining (j), inefficient metastasizers typically exhibited diffuse cytoplasmic staining (i). Images are representative of results from two independent experiments per melanoma. Data are mean ± s.d. Statistical significance was assessed using Student’s t-tests (a, b, d).
Extended Data Fig. 3 MCT1 inhibition impairs metastasis without altering MCT1, CD147, CD98 or β1-integrin expression levels.
Related to Fig. 2. a–c, Western blot analysis of MCT1 (a), MCT4 (b) and CD147 (c) in subcutaneous tumours versus metastatic liver, kidney and pancreas nodules from NSG mice transplanted with three melanomas. d–g, Flow cytometry histograms of anti-MCT1 (d, e) or anti-CD147 (f, g) staining in melanoma cells from subcutaneous tumours or metastatic nodules from mice transplanted with M405 (d, f) or M481 (e, g) melanomas. h–o, Flow cytometry histograms and mean fluorescence intensities of anti-MCT1 (h, i), anti-CD147 (j, k), anti-CD98 (l, m) or anti-β1-integrin (n, o) staining in melanoma cells from subcutaneous tumours treated with DMSO (control; black) or AZD3965 (MCT1 inhibitor; blue). The number of tumours or mice analysed in each treatment is indicated (two to three experiments). In all flow cytometric analyses, human melanoma cells were distinguished from mouse cells based on positivity for HLA-ABC and DsRed and negativity for mouse CD31, CD45 and Ter119 staining (see Extended Data Fig. 9e, f for gating strategy). p–u, Western blot analysis of IKKα (p–r) and IKKβ (s–u) in subcutaneous tumours from NSG mice treated with DMSO or AZD3965. Data are mean ± s.d. Statistical significance was assessed with two-way ANOVA (i, k, m, o).
Extended Data Fig. 4 MCT1 inhibition with AZD3965 impairs metastasis without altering markers of epithelial-to-mesenchymal transition.
Related to Fig. 2. a, b, Flow cytometry histograms of anti-E-cadherin (a) and anti-N-cadherin (b) staining in melanoma cells from subcutaneous tumours of mice treated with DMSO (control) or AZD3965. Human keratinocytes were included as a control in each case as they are known to include subpopulations of E-cadherin- and N-cadherin-positive cells. In xenografts, human melanoma cells were distinguished from mouse cells based on positivity for HLA-ABC and DsRed and negativity for mouse CD31, CD45 and Ter119 staining (see Extended Data Fig. 9e, f for gating strategy). Data are representative of two to three mice analysed in two independent experiments. c, Western blot analysis of vimentin in subcutaneous tumours from NSG mice treated with DMSO or AZD3965.
Extended Data Fig. 5 Representative images of the bioluminescence analysis of visceral organs to determine metastatic disease burden at end point.
Extended Data Fig. 6 shRNA-mediated knockdown of MCT1 inhibits melanoma metastasis in vivo.
Related to Fig. 2. a, b, Western blot analysis of MCT1 (a) or MCT4 (b) in subcutaneous tumours from mice xenografted with efficiently metastasizing melanomas transfected with scrambled control shRNA or with two different shRNAs (1 and 2) against MCT1 (a) or MCT4 (b). Wild-type HCC15 cells were used as a positive control for MCT1 (WT, a) and MCT4 (WT, b) and MCT1-deficient (KO, a) or MCT4-deficient (KO, b) HCC15 cells were used as a negative control (representative of two independent experiments). c–e, Growth of subcutaneous tumours (c) in mice transplanted with melanomas transfected with scrambled control shRNA or shRNAs (sh1 and sh2) against MCT1. The number of mice analysed in each treatment is indicated in (one experiment per melanoma). The frequency of circulating melanoma cells in the blood (d) and metastatic disease burden based on bioluminescence imaging (e) in the same mice were determined. f, Western blot analysis of MCT1 in subcutaneous tumours transfected with scrambled control shRNA or shRNAs against MCT1, with (MCT1-OE) or without an shRNA-insensitive MCT1 cDNA. g, h, Growth of subcutaneous tumours (g) and metastatic disease burden at end point (h) in mice transplanted with melanomas transfected with scrambled control shRNA or shRNAs against MCT1 and an shRNA-insensitive MCT1 cDNA. i, Fold change in mean fluorescence intensity for CellRox DeepRed staining (ROS) in xenografted melanoma cells with scrambled control shRNA or shRNAs against MCT1 treated with AZD3965 or DMSO. Data are mean ± s.d.. Statistical significance was assessed using nparLD followed by Benjamini–Hochberg’s multiple comparisons adjustment (c), log2-transformed one-way ANOVAs with Holm–Sidak’s multiple comparisons adjustment (d, e, h), mixed-effects analysis followed by Dunnett’s multiple comparisons adjustment (g) or log2-transformed two-way ANOVA with Sidak’s multiple comparisons adjustment (i).
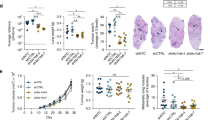
Extended Data Fig. 7 CRISPR deletion of MCT1 from mouse melanoma cells impairs metastasis, whereas MCT1 overexpression in patient-derived xenografts increases metastasis.
Related to Fig. 2. a, Western blot analysis of MCT1 in wild-type parental YUMM1.7 melanoma cells as well as two lines from which MCT1 had been deleted using CRISPR (KO #1 and #2). b–d, Growth of subcutaneous tumours (b), total metastatic disease burden at end point by bioluminescence imaging of visceral organs (c) and CellRox DeepRed staining of subcutaneous tumour cells (d). The number of mice analysed in each treatment is indicated (one experiment; note that one mouse died in the KO #2 treatment before end-point analysis). e, Western blot analysis of MCT1 in an inefficiently metastasizing melanoma (UM47) expressing MCT1 cDNA. f, g, Growth of subcutaneous tumours (f) and total metastatic disease burden at end point by bioluminescence imaging of visceral organs (g) from mice transplanted with these melanomas (one experiment; note that two mice died in the control treatment before end-point analysis). Data are mean ± s.d. Statistical significance was assessed using one-way ANOVA followed by Dunnett’s multiple comparison adjustment (b, day 25) or log2-transformed one-way ANOVAs followed by Dunnett’s multiple comparisons adjustment (c, d), t-test (f, day 90) or log2-transformed t-test (g).
Extended Data Fig. 8 MCT1 inhibition does not impair the migration of melanoma cells in culture but appears to reduce metastatic disease burden by killing metastasizing melanoma cells in vivo.
Related to Fig. 2. a, Migration in transwell invasion assays of three melanomas treated with DMSO (control) or AZD3965 (MCT1 inhibitor), including representative images (left) and counts (right) of the cells that migrated across the insert after 24 h (one experiment with two to three replicate cultures per melanoma). b, c, Effect of acute treatment with AZD3965 (7 days) on the diameter of subcutaneous tumours, the frequency of circulating melanoma cells in the blood and metastatic disease burden in mice with established M481 (b) or M405 (c) melanomas. Treatment was initiated when the subcutaneous tumours reached 2 cm in diameter (one experiment per melanoma with three mice per treatment). d, Efficiently metastasizing melanoma cells (M405) were subcutaneously transplanted into mice and allowed to spontaneously metastasize; then the primary tumours were resected to prolong survival and to allow the metastatic tumours that had formed before primary tumour resection to grow larger. Mice were treated with AZD3965 for the duration of the experiment, only before primary tumour resection, or only after primary tumour resection. e, Analysis of total metastatic disease burden at end point showing that metastatic disease burden was reduced when AZD3965 treatment was performed before primary tumour resection, during the time when melanoma cells were spontaneously metastasizing, but before metastatic tumours were established. The number of mice per treatment is shown (two independent experiments). Data are mean ± s.d. Statistical significance was assessed using two-way ANOVA followed by Dunnett’s multiple comparison’s adjustment (a), t-tests (b, c) or Kruskal–Wallis test followed by Dunn’s multiple comparison’s adjustment (e).
Extended Data Fig. 9 Increased MCT1 expression in melanomas is associated with significantly worse patient survival.
Related to Fig. 2. a–d, Kaplan–Meier overall survival curves of patients with melanoma stratified based on expression levels of MCT1 (a), MCT2 (b), MCT4 (c) and CD147 (d) within tumour specimens. Data are from the SKCM cohort in TCGA (https://portal.gdc.cancer.gov/projects/TCGA-SKCM). Each panel compares the top third of patients with the highest expression levels versus the bottom third of patients with the lowest expression levels. Ticks represent censored values. e, f, Flow cytometry plots showing the gating strategies used to identify human melanoma cells in subcutaneous tumours (e) or the blood (f) of xenografted mice. Cells were gated on forward versus side scatter (FSC-A versus SSC-A) to exclude red blood cells and clumps of cells. Human melanoma cells were selected by including cells that stained positively for DsRed (stably expressed in all melanoma lines) and HLA and excluding cells that stained positively for the mouse haematopoietic and endothelial markers CD45, CD31 or Ter119. Statistical significance of the differences in overall survival (a–d) was assessed using the Mantel–Cox log-rank test.
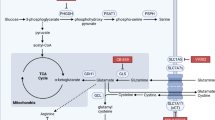
Extended Data Fig. 10 MCT1 inhibition reduces the levels of PPP, but not glycolytic, metabolites.
Related to Figs. 3–5. a, The GSH to GSSG ratios in melanoma cells from mice treated with AZD3965 or DMSO (two independent experiments per melanoma). b, Quantitative analysis of NADPH and NADP+ in melanoma cells from mice treated with AZD3965 or DMSO (one or two experiments per melanoma). Liver cells were included as a control, with a high NADPH/NADP+ ratio. c, Expected isotope-labelled species after infusion of [1,2-13C]glucose. d, Glucose m + 2 as a fraction of total plasma glucose in mice xenografted with efficiently metastasizing melanomas (M405, M481 and UT10), treated with DMSO or AZD3965 and infused with [1,2-13C]glucose. e, Glucose m + 6 as a fraction of total plasma glucose in mice infused with [U-13C]glucose. The number of mice per treatment is indicated (two independent experiments). f–i, LC–MS measurement of the levels of glycolytic (f, h) and oxidative PPP (g, i) metabolites in subcutaneous tumour cells from mice xenografted with melanomas treated with DMSO (control) or AZD3965 (MCT1 inhibitor) for 7 days. j, Flow cytometrically isolated MCT1high or MCT1−/low melanoma cells were subcutaneously transplanted into NSG mice, using 10 or 100 cells per injection. All injections formed tumours. Rate of growth of the tumours initiated with 10-cell injections. Data are mean ± s.d. Statistical significance was assessed using t-tests (a), repeated-measures two-way ANOVAs (b), t-test (e, 180 min), log2-transformed two-way ANOVAs (f, h), log2-transformed t-tests (g, M405 and UT10), Mann–Whitney test (g, M481 and i, M481), Welch’s t-tests (i, M405 and UT10) or using nparLD test (d, j).
Supplementary information
Supplementary Figure
Supplementary Figure 1: uncropped scans with size marker indications.
Rights and permissions
About this article
Cite this article
Tasdogan, A., Faubert, B., Ramesh, V. et al. Metabolic heterogeneity confers differences in melanoma metastatic potential. Nature 577, 115–120 (2020). https://doi.org/10.1038/s41586-019-1847-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-019-1847-2
This article is cited by
-
Multi-omics analysis reveals NNMT as a master metabolic regulator of metastasis in esophageal squamous cell carcinoma
npj Precision Oncology (2024)
-
The pleiotropic functions of reactive oxygen species in cancer
Nature Cancer (2024)
-
Immunosurveillance encounters cancer metabolism
EMBO Reports (2024)
-
The role of mitochondria in the resistance of melanoma to PD-1 inhibitors
Journal of Translational Medicine (2023)
-
Functional heterogeneity of MCT1 and MCT4 in metabolic reprogramming affects osteosarcoma growth and metastasis
Journal of Orthopaedic Surgery and Research (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.