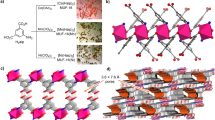
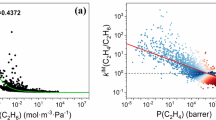
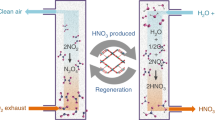
Abstract
Limiting the increase of CO2 in the atmosphere is one of the largest challenges of our generation1. Because carbon capture and storage is one of the few viable technologies that can mitigate current CO2 emissions2, much effort is focused on developing solid adsorbents that can efficiently capture CO2 from flue gases emitted from anthropogenic sources3. One class of materials that has attracted considerable interest in this context is metal–organic frameworks (MOFs), in which the careful combination of organic ligands with metal-ion nodes can, in principle, give rise to innumerable structurally and chemically distinct nanoporous MOFs. However, many MOFs that are optimized for the separation of CO2 from nitrogen4,5,6,7 do not perform well when using realistic flue gas that contains water, because water competes with CO2 for the same adsorption sites and thereby causes the materials to lose their selectivity. Although flue gases can be dried, this renders the capture process prohibitively expensive8,9. Here we show that data mining of a computational screening library of over 300,000 MOFs can identify different classes of strong CO2-binding sites—which we term ‘adsorbaphores’—that endow MOFs with CO2/N2 selectivity that persists in wet flue gases. We subsequently synthesized two water-stable MOFs containing the most hydrophobic adsorbaphore, and found that their carbon-capture performance is not affected by water and outperforms that of some commercial materials. Testing the performance of these MOFs in an industrial setting and consideration of the full capture process—including the targeted CO2 sink, such as geological storage or serving as a carbon source for the chemical industry—will be necessary to identify the optimal separation material.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The computed data and hypothetical materials that were used in this Article are provided free of charge on the Materials Cloud (https://doi.org/10.24435/materialscloud:2018.0016/v3). An interactive version of Fig. 1a, b can also be found on this site. Data that are not included in the paper are available upon reasonable request to the corresponding authors.
Code availability
Topology Based Crystal Constructor (ToBasCCo), the Python program used to build hypothetical MOFs, is hosted on GitHub at https://github.com/peteboyd/tobascco. The Python code that compares common chemical features between fragments is also provided on GitHub at https://github.com/peteboyd/adsorbaphore and is dependent on a C library called MCQD which performs the maximum clique detection of the chemical graphs. An interface between Python and C for this is provided here at https://github.com/peteboyd/mcqd_api. The Automatic Binding Site Locator (ABSL) program, used to identify CO2 binding sites in each MOF, is part of a broader Python-based code used to facilitate simulations of porous materials called Fully Automated Adsorption Analysis in Porous Solids (FA3PS). This is available on BitBucket at https://bitbucket.org/tdaff/automation.
References
Smit, B., Reimer, J. R., Oldenburg, C. M. & Bourg, I. C. Introduction to Carbon Capture and Sequestration (Imperial College Press, 2014).
Bui, M. et al. Carbon capture and storage (CCS): the way forward. Energy Environ. Sci. 11, 1062–1176 (2018).
D’Alessandro, D. M., Smit, B. & Long, J. R. Carbon dioxide capture: prospects for new materials. Angew. Chem. Int. Ed. 49, 6058–6082 (2010).
Sumida, K. et al. Carbon dioxide capture in metal–organic frameworks. Chem. Rev. 112, 724–781 (2012).
Furukawa, H., Cordova, K. E., O’Keeffe, M. & Yaghi, O. M. The chemistry and applications of metal–organic frameworks. Science 341, 1230444 (2013).
Huck, J. M. et al. Evaluating different classes of porous materials for carbon capture. Energy Environ. Sci. 7, 4132–4146 (2014).
Mason, J. A., Sumida, K., Herm, Z. R., Krishna, R. & Long, J. R. Evaluating metal–organic frameworks for post-combustion carbon dioxide capture via temperature swing adsorption. Energy Environ. Sci. 4, 3030–3040 (2011).
Li, G. et al. Capture of CO2 from high humidity flue gas by vacuum swing adsorption with zeolite 13X. Adsorption 14, 415–422 (2008).
Merel, J., Clausse, M. & Meunier, F. Experimental investigation on CO2 post-combustion capture by indirect thermal swing adsorption using 13X and 5A zeolites. Ind. Eng. Chem. Res. 47, 209–215 (2008).
Milner, P. J. et al. A diaminopropane-appended metal–organic framework enabling efficient CO2 capture from coal flue gas via a mixed adsorption mechanism. J. Am. Chem. Soc. 139, 13541–13553 (2017).
McDonald, T. M. et al. Cooperative insertion of CO2 in diamine-appended metal–organic frameworks. Nature 519, 303–308 (2015).
Flaig, R. W. et al. The chemistry of CO2 capture in an amine-functionalized metal–organic framework under dry and humid conditions. J. Am. Chem. Soc. 139, 12125–12128 (2017).
Couck, S. et al. An amine-functionalized MIL-53 metal–organic framework with large separation power for CO2 and CH4. J. Am. Chem. Soc. 131, 6326–6327 (2009).
Chanut, N. et al. Screening the effect of water vapour on gas adsorption performance: application to CO2 capture from flue gas in metal–organic frameworks. ChemSusChem 10, 1543–1553 (2017).
Wolber, G., Seidel, T., Bendix, F. & Langer, T. Molecule-pharmacophore superpositioning and pattern matching in computational drug design. Drug Discov. Today 13, 23–29 (2008).
Sliwoski, G., Kothiwale, S., Meiler, J. & Lowe, E. W., Jr. Computational methods in drug discovery. Pharmacol. Rev. 66, 334–395 (2013).
Ho, M. T., Allinson, G. W. & Wiley, D. E. Reducing the cost of CO2 capture from flue gases using pressure swing adsorption. Ind. Eng. Chem. Res. 47, 4883–4890 (2008).
Stylianou, K. C. et al. A guest-responsive fluorescent 3D microporous metal–organic framework derived from a long-lifetime pyrene core. J. Am. Chem. Soc. 132, 4119–4130 (2010).
Fateeva, A. et al. A water-stable porphyrin-based metal–organic framework active for visible-light photocatalysis. Angew. Chem. Int. Ed. 51, 7440–7444 (2012).
Loiseau, T. et al. A rationale for the large breathing of the porous aluminum terephthalate (MIL-53) upon hydration. Chem. Eur. J. 10, 1373–1382 (2004).
Reinsch, H. & Stock, N. High-throughput studies of highly porous Al-based MOFs. Microporous Mesoporous Mater. 171, 156–165 (2013).
Boyd, P. G. & Woo, T. K. A generalized method for constructing hypothetical nanoporous materials of any net topology from graph theory. CrystEngComm 18, 3777–3792 (2016).
Carrington, E. J., Vitórica-Yrezábal, I. J. & Brammer, L. Crystallographic studies of gas sorption in metal–organic frameworks. Acta Crystallogr. B 70, 404–422 (2014).
García, S. et al. Breakthrough adsorption study of a commercial activated carbon for pre-combustion CO2 capture. Chem. Eng. J. 171, 549–556 (2011).
García, S., Gil, M. V., Pis, J. J., Rubiera, F. & Pevida, C. Cyclic operation of a fixed-bed pressure and temperature swing process for CO2 capture: experimental and statistical analysis. Int. J. Greenhouse Gas Control 12, 35–43 (2013).
Lin, L.-C. et al. In silico screening of carbon-capture materials. Nat. Mater. 11, 633–641 (2012).
Wilmer, C. E. et al. Large-scale screening of hypothetical metal–organic frameworks. Nat. Chem. 4, 83–89 (2012).
Boyd, P. G., Lee, Y. & Smit, B. Computational development of the nanoporous materials genome. Nat. Mater. Rev. 2, 17037 (2017).
Yazaydin, A. O. et al. Screening of metal–organic frameworks for carbon dioxide capture from flue gas using a combined experimental and modeling approach. J. Am. Chem. Soc. 131, 18198–18199 (2009).
Acknowledgements
K.C.S. was supported by the Swiss National Science Foundation (SNF) under the Ambizione Energy Grant n.PZENP2_166888, P.G.B. and B.S. by the European Research Council (ERC) Advanced Grant (grant agreement no. 666983, MaGic) and the National Center of Competence in Research (NCCR), Materials’ Revolution: Computational Design and Discovery of Novel Materials (MARVEL). A.C., C.P.I., and S.M.M. were supported by European Union’s Horizon 2020 research and innovation programme under grant agreement no. 760899 (GENESIS). R.B. and J.A.R. were supported by the Center for Gas Separations Relevant to Clean Energy Technologies, an Energy Frontier Research Center funded by the Department of Energy (DOE), Office of Science, Office of Basic Energy Sciences under award DE-SC0001015. E.G.-D. is supported by the European Commission under the Research Fund for Coal and Steel (RFCS) Programme (project no. 709741). M.M.M.-V. and S.G. acknowledge the financial support from the Engineering and Physical Sciences Research Council (EP/N024540/1) and the Research Centre for Carbon Solutions (RCCS) at Heriot-Watt University. The authors thank the Swiss Norwegian Beamlines of ESRF for beamtime allocation at BM31 for the in-situ CO2 loading and variable temperature powder X-ray diffraction experiments. This work was supported by a grant from the Swiss National Supercomputing Center (CSCS) under project no. s761, as well as resources from the National Energy Research Scientific Computing Center, a DOE Office of Science User Facility supported by the Office of Science of the US Department of Energy under contract no. DE-AC02-05CH11231. J.A.R.N. acknowledges Spanish MINECO (CTQ2017-84692-R) and EU Feder funding. P.G.B., T.D.D. and T.K.W. would like to thank NSERC of Canada for financial support and Compute Canada for computing resources. S.G., J.A.R., and B.S. acknowledge support during the final stage of the work of the ACT-PrISMa project, which has received joint funding from BEIS, NERC and EPSRC (UK), funding from the Division of CCS R&D, US Department of Energy (USA), and funding from the Office Fédéral de l'Energie (Switzerland).
Author information
Authors and Affiliations
Contributions
P.G.B. and T.K.W. developed the adsorbaphore similarity algorithm and developed the library of hypothetical MOFs. P.G.B., T.K.W. and B.S. characterised the adsorbaphores; T.D.D. carried out the initial computational screening and developed the MOF-CO2 binding site identification algorithm. S.M.M. carried out the pore similarity analysis; A.C., C.P.I. and K.C.S. synthesized and characterized the materials. The breakthrough experiments were carried out by E.G.-D., C.P.I., A.C., M.M.M.-V., J.A.R.N. and S.G. The NMR experiments were carried out by R.B. and J.A.R; X-ray analysis was carried out by A.G. and P.S. All authors contributed to the analysis of the data and the writing of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
K.C.S., B.S., A.C., P.G.B. and T.K.W. have filed an international patent application (no. 18 168 544.7) that relates to water-stable polyaromatic MOF materials for CO2 separation from flue gas and natural gas streams.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Peer review information Nature thanks Philip Llewellyn and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Extended data figures and tables
Extended Data Fig. 1 Hypothetical material generation (1).
The organic secondary building units (SBUs) used in the generation of the hypothetical MOF database.
Extended Data Fig. 2 Hypothetical material generation (2).
a, Metal SBUs used in the generation of the hypothetical MOF database. b, Functional groups used to decorate the unfunctionalized hypothetical MOFs in the database. We denote a hypothetical material as mXoYY, where X refers to the metal SBU shown in a and YY refers to the organic SBU shown in Extended Data Fig. 1. Functional groups were decorated onto the base hypothetical materials using an internal numbering system.
Extended Data Fig. 3 Examples of adsorbaphores.
A selection of the most representative adsorbaphores obtained from visual inspection of the top 50 most frequent adsorbaphores found from the random pairing method described in the Supplementary Information. There are three major trends in the molecular fragments—labelled A1, A2 and A3—which can be observed upon descending each column. The number of chemical features of the fragments increases from left to right across each row. This is accomplished by increasing the minimum number of common atoms allowed during the substructure search, called the minimum clique threshold (MCT). Pictured in each adsorbaphore is a representative contour map of the energy produced from CO2 binding with the adsorbaphore atoms from each original CO2-binding site. a–c, A1, planar aromatic systems, in which CO2 binds in between the stacked rings; d–f, A2, CO2 binds near the bridging oxygen of a pillared vanadium SBU; g–i, A3, CO2 binds between open-metal Cr SBUs.
Extended Data Fig. 4 Frequently found adsorbaphores.
a–c, Frequently found adsorbaphores from the maximum clique detection method; A1 (a); A2 (b) and A3 (c). Atom colours: grey, carbon; red, oxygen; green, vanadium; blue, chromium. d, Representative adsorbaphore A1 found in different hypothetical MOFs from the hypothetical database. The adsorbaphore atoms are highlighted in green.
Extended Data Fig. 5 Effect of the adsorbaphore spacer.
Left, plot of the CO2 adsorption at 0.15 bar and 313 K in hypothetical MOFs with the frz topology, metal node number 8 and organic linker number 67 (m8o67). The interplanar spacing of the adsorbaphore atoms is adjusted by reassembling the structure with longer or shorter Al–O bonds. Right, representation of the spacing adjustments made to the material.
Extended Data Fig. 6 Hypothetical MOFs built with the frz topology.
These structures contain an Al one-dimensional rod (m8) and the organic ligands 60–82 from Extended Data Figs. 1 and 2. The spacing between parallel aromatic cores (seen in the centre of each hypothetical MOF) is around 6.7 Å, defining the adsorbaphore site in each material. No functional groups were used to decorate these materials. We refer to the synthesized versions of m8o66 and m8o67 as Al-PMOF (Al2(OH)2(H2TCPP)) and Al-PyrMOF (Al2(OH)2(TBAPy)), respectively.
Extended Data Fig. 7 CO2 adsorption capacity of a class of frz-based hypothetical MOFs at 0.15 bar and 313K under ‘wet’ (85% relative humidity) and ‘dry’ flue gas conditions.
Top, middle, hypothetical MOFs in which the organic ligand is connected to the Al ion via 4 benzoate moieties; bottom, hypothetical MOFs in which the organic ligand is connected to the Al metal ion via 4 acetylenic carboxylate moieties. The materials are ranked from lowest adsorbaphore density to highest, and the number on the x axis corresponds to the organic linker number (YY) in m8oYY (see Extended Data Fig. 6).
Extended Data Fig. 8 NMR spectra.
a, b, 13C cross-polarization MAS spectrum of Al-PMOF (a) and Al-PyrMOF (b) recorded at 9.39 T with sample spinning at 8 kHz; the contact time for the cross-polarization experiment was 2 ms. The letters labelling the peaks of the spectra correspond to the labels on the carbon atoms in the structures on the left.
Supplementary information
Supplementary Information
This file contains supplementary texts 1-3 which also contains supplementary figures and tables.
Rights and permissions
About this article
Cite this article
Boyd, P.G., Chidambaram, A., García-Díez, E. et al. Data-driven design of metal–organic frameworks for wet flue gas CO2 capture. Nature 576, 253–256 (2019). https://doi.org/10.1038/s41586-019-1798-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-019-1798-7
This article is cited by
-
A comprehensive transformer-based approach for high-accuracy gas adsorption predictions in metal-organic frameworks
Nature Communications (2024)
-
Double-walled Al-based MOF with large microporous specific surface area for trace benzene adsorption
Nature Communications (2024)
-
Progress toward the computational discovery of new metal–organic framework adsorbents for energy applications
Nature Energy (2024)
-
Gas adsorption meets deep learning: voxelizing the potential energy surface of metal-organic frameworks
Scientific Reports (2024)
-
Unconventional mechanical and thermal behaviours of MOF CALF-20
Nature Communications (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.