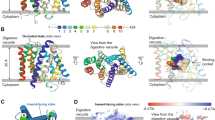
Abstract
The emergence and spread of drug-resistant Plasmodium falciparum impedes global efforts to control and eliminate malaria. For decades, treatment of malaria has relied on chloroquine (CQ), a safe and affordable 4-aminoquinoline that was highly effective against intra-erythrocytic asexual blood-stage parasites, until resistance arose in Southeast Asia and South America and spread worldwide1. Clinical resistance to the chemically related current first-line combination drug piperaquine (PPQ) has now emerged regionally, reducing its efficacy2. Resistance to CQ and PPQ has been associated with distinct sets of point mutations in the P. falciparum CQ-resistance transporter PfCRT, a 49-kDa member of the drug/metabolite transporter superfamily that traverses the membrane of the acidic digestive vacuole of the parasite3,4,5,6,7,8,9. Here we present the structure, at 3.2 Å resolution, of the PfCRT isoform of CQ-resistant, PPQ-sensitive South American 7G8 parasites, using single-particle cryo-electron microscopy and antigen-binding fragment technology. Mutations that contribute to CQ and PPQ resistance localize primarily to moderately conserved sites on distinct helices that line a central negatively charged cavity, indicating that this cavity is the principal site of interaction with the positively charged CQ and PPQ. Binding and transport studies reveal that the 7G8 isoform binds both drugs with comparable affinities, and that these drugs are mutually competitive. The 7G8 isoform transports CQ in a membrane potential- and pH-dependent manner, consistent with an active efflux mechanism that drives CQ resistance5, but does not transport PPQ. Functional studies on the newly emerging PfCRT F145I and C350R mutations, associated with decreased PPQ susceptibility in Asia and South America, respectively6,9, reveal their ability to mediate PPQ transport in 7G8 variant proteins and to confer resistance in gene-edited parasites. Structural, functional and in silico analyses suggest that distinct mechanistic features mediate the resistance to CQ and PPQ in PfCRT variants. These data provide atomic-level insights into the molecular mechanism of this key mediator of antimalarial treatment failures.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
All raw video frames, micrographs, the particle stack and relevant metadata files have been deposited into EMPIAR, with accession code EMPIAR-10330. The electron density map has been deposited into EMDB, with accession code EMD-20806. The model has been deposited in the PDB, with accession code 6UKJ. All data are available in the paper or Supplementary Information.
References
Su, X. Z., Lane, K. D., Xia, L., Sá, J. M. & Wellems, T. E. Plasmodium genomics and genetics: new insights into malaria pathogenesis, drug resistance, epidemiology, and evolution. Clin. Microbiol. Rev. 32, e00019 (2019).
van der Pluijm, R. W. et al. Determinants of dihydroartemisinin–piperaquine treatment failure in Plasmodium falciparum malaria in Cambodia, Thailand, and Vietnam: a prospective clinical, pharmacological, and genetic study. Lancet Infect. Dis. 19, 952–961 (2019).
Fidock, D. A. et al. Mutations in the P. falciparum digestive vacuole transmembrane protein PfCRT and evidence for their role in chloroquine resistance. Mol. Cell 6, 861–871 (2000).
Martin, R. E. et al. Chloroquine transport via the malaria parasite’s chloroquine resistance transporter. Science 325, 1680–1682 (2009).
Blasco, B., Leroy, D. & Fidock, D. A. Antimalarial drug resistance: linking Plasmodium falciparum parasite biology to the clinic. Nat. Med. 23, 917–928 (2017).
Ross, L. S. et al. Emerging Southeast Asian PfCRT mutations confer Plasmodium falciparum resistance to the first-line antimalarial piperaquine. Nat. Commun. 9, 3314 (2018).
Hamilton, W. L. et al. Evolution and expansion of multidrug-resistant malaria in Southeast Asia: a genomic epidemiology study. Lancet Infect. Dis. 19, 943–951 (2019).
Dhingra, S. K., Small-Saunders, J. L., Ménard, D. & Fidock, D. A. Plasmodium falciparum resistance to piperaquine driven by PfCRT. Lancet Infect. Dis. 19, 1168–1169 (2019).
Pelleau, S. et al. Adaptive evolution of malaria parasites in French Guiana: reversal of chloroquine resistance by acquisition of a mutation in pfcrt. Proc. Natl Acad. Sci. USA 112, 11672–11677 (2015).
World Health Organization. World Malaria Report 2018. https://www.who.int/malaria/publications/world-malaria-report-2018/en (2018).
Sullivan, D. J. Jr. Quinolines block every step of malaria heme crystal growth. Proc. Natl Acad. Sci. USA 114, 7483–7485 (2017).
Dhingra, S. K. et al. A variant PfCRT isoform can contribute to Plasmodium falciparum resistance to the first-line partner drug piperaquine. mBio 8, e00303-17 (2017).
Lakshmanan, V. et al. A critical role for PfCRT K76T in Plasmodium falciparum verapamil-reversible chloroquine resistance. EMBO J. 24, 2294–2305 (2005).
Sanchez, C. P. et al. Differences in trans-stimulated chloroquine efflux kinetics are linked to PfCRT in Plasmodium falciparum. Mol. Microbiol. 64, 407–420 (2007).
Paguio, M. F., Cabrera, M. & Roepe, P. D. Chloroquine transport in Plasmodium falciparum. 2. Analysis of PfCRT-mediated drug transport using proteoliposomes and a fluorescent chloroquine probe. Biochemistry 48, 9482–9491 (2009).
Sanchez, C. P. et al. Phosphomimetic substitution at Ser-33 of the chloroquine resistance transporter PfCRT reconstitutes drug responses in Plasmodium falciparum. J. Biol. Chem. 294, 12766–12778 (2019).
Renaud, J. P. et al. Cryo-EM in drug discovery: achievements, limitations and prospects. Nat. Rev. Drug Discov. 17, 471–492 (2018).
Dominik, P. K. et al. Conformational chaperones for structural studies of membrane proteins using antibody phage display with nanodiscs. Structure 24, 300–309 (2016).
Callaghan, P. S., Hassett, M. R. & Roepe, P. D. Functional comparison of 45 naturally occurring isoforms of the Plasmodium falciparum chloroquine resistance transporter (PfCRT). Biochemistry 54, 5083–5094 (2015).
Quick, M. & Javitch, J. A. Monitoring the function of membrane transport proteins in detergent-solubilized form. Proc. Natl Acad. Sci. USA 104, 3603–3608 (2007).
Lekostaj, J. K., Natarajan, J. K., Paguio, M. F., Wolf, C. & Roepe, P. D. Photoaffinity labeling of the Plasmodium falciparum chloroquine resistance transporter with a novel perfluorophenylazido chloroquine. Biochemistry 47, 10394–10406 (2008).
Bellanca, S. et al. Multiple drugs compete for transport via the Plasmodium falciparum chloroquine resistance transporter at distinct but interdependent sites. J. Biol. Chem. 289, 36336–36351 (2014).
Sá, J. M. et al. Geographic patterns of Plasmodium falciparum drug resistance distinguished by differential responses to amodiaquine and chloroquine. Proc. Natl Acad. Sci. USA 106, 18883–18889 (2009).
Juge, N. et al. Plasmodium falciparum chloroquine resistance transporter is a H+-coupled polyspecific nutrient and drug exporter. Proc. Natl Acad. Sci. USA 112, 3356–3361 (2015).
Kuhn, Y., Rohrbach, P. & Lanzer, M. Quantitative pH measurements in Plasmodium falciparum-infected erythrocytes using pHluorin. Cell. Microbiol. 9, 1004–1013 (2007).
Ashcroft, F., Gadsby, D. & Miller, C. Introduction. The blurred boundary between channels and transporters. Phil. Trans. R. Soc. Lond. B 364, 145–147 (2009).
Agrawal, S. et al. Association of a novel mutation in the Plasmodium falciparum chloroquine resistance transporter with decreased piperaquine sensitivity. J. Infect. Dis. 216, 468–476 (2017).
Bopp, S. et al. Plasmepsin II–III copy number accounts for bimodal piperaquine resistance among Cambodian Plasmodium falciparum. Nat. Commun. 9, 1769 (2018).
Duru, V. et al. Plasmodium falciparum dihydroartemisinin–piperaquine failures in Cambodia are associated with mutant K13 parasites presenting high survival rates in novel piperaquine in vitro assays: retrospective and prospective investigations. BMC Med. 13, 305 (2015).
Shi, L., Quick, M., Zhao, Y., Weinstein, H. & Javitch, J. A. The mechanism of a neurotransmitter:sodium symporter—inward release of Na+ and substrate is triggered by substrate in a second binding site. Mol. Cell 30, 667–677 (2008).
Assur, Z., Hendrickson, W. A. & Mancia, F. Tools for coproducing multiple proteins in mammalian cells. Methods Mol. Biol. 801, 173–187 (2012).
Kawate, T. & Gouaux, E. Fluorescence-detection size-exclusion chromatography for precrystallization screening of integral membrane proteins. Structure 14, 673–681 (2006).
Goehring, A. et al. Screening and large-scale expression of membrane proteins in mammalian cells for structural studies. Nat. Protoc. 9, 2574–2585 (2014).
Gibson, D. G. et al. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat. Methods 6, 343–345 (2009).
Wright, D. J., O’Reilly, M. & Tisi, D. Engineering and purification of a thermostable, high-yield, variant of PfCRT, the Plasmodium falciparum chloroquine resistance transporter. Protein Expr. Purif. 141, 7–18 (2018).
Paduch, M. et al. Generating conformation-specific synthetic antibodies to trap proteins in selected functional states. Methods 60, 3–14 (2013).
Dominik, P. K. & Kossiakoff, A. A. Phage display selections for affinity reagents to membrane proteins in nanodiscs. Methods Enzymol. 557, 219–245 (2015).
Fellouse, F. A., Wiesmann, C. & Sidhu, S. S. Synthetic antibodies from a four-amino-acid code: a dominant role for tyrosine in antigen recognition. Proc. Natl Acad. Sci. USA 101, 12467–12472 (2004).
Fellouse, F. A. et al. High-throughput generation of synthetic antibodies from highly functional minimalist phage-displayed libraries. J. Mol. Biol. 373, 924–940 (2007).
Suloway, C. et al. Automated molecular microscopy: the new Leginon system. J. Struct. Biol. 151, 41–60 (2005).
Lander, G. C. et al. Appion: an integrated, database-driven pipeline to facilitate EM image processing. J. Struct. Biol. 166, 95–102 (2009).
Scheres, S. H. RELION: implementation of a Bayesian approach to cryo-EM structure determination. J. Struct. Biol. 180, 519–530 (2012).
Kimanius, D., Forsberg, B. O., Scheres, S. H. & Lindahl, E. Accelerated cryo-EM structure determination with parallelisation using GPUs in RELION-2. eLife 5, e18722 (2016).
Cheng, A. et al. High resolution single particle cryo-electron microscopy using beam-image shift. J. Struct. Biol. 204, 270–275 (2018).
Rice, W. J. et al. Routine determination of ice thickness for cryo-EM grids. J. Struct. Biol. 204, 38–44 (2018).
Zheng, S. Q. et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331–332 (2017).
Rohou, A. & Grigorieff, N. CTFFIND4: Fast and accurate defocus estimation from electron micrographs. J. Struct. Biol. 192, 216–221 (2015).
Zhang, K. Gctf: real-time CTF determination and correction. J. Struct. Biol. 193, 1–12 (2016).
Voss, N. R., Yoshioka, C. K., Radermacher, M., Potter, C. S. & Carragher, B. DoG Picker and TiltPicker: software tools to facilitate particle selection in single particle electron microscopy. J. Struct. Biol. 166, 205–213 (2009).
Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296 (2017).
Grant, T. & Grigorieff, N. Automatic estimation and correction of anisotropic magnification distortion in electron microscopes. J. Struct. Biol. 192, 204–208 (2015).
Grant, T., Rohou, A. & Grigorieff, N. cisTEM, user-friendly software for single-particle image processing. eLife 7, e35383 (2018).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D 66, 213–221 (2010).
Pettersen, E. F. et al. UCSF Chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Wang, R. Y.-R. et al. Automated structure refinement of macromolecular assemblies from cryo-EM maps using Rosetta. eLife 5, e17219 (2016).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D 60, 2126–2132 (2004).
Stuwe, T. et al. Architecture of the nuclear pore complex coat. Science 347, 1148–1152 (2015).
Chen, V. B. et al. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. D 66, 12–21 (2010).
Barad, B. A. et al. EMRinger: side chain-directed model and map validation for 3D cryo-electron microscopy. Nat. Methods 12, 943–946 (2015).
Kucukelbir, A., Sigworth, F. J. & Tagare, H. D. Quantifying the local resolution of cryo-EM density maps. Nat. Methods 11, 63–65 (2014).
Tan, Y. Z. et al. Addressing preferred specimen orientation in single-particle cryo-EM through tilting. Nat. Methods 14, 793–796 (2017).
Rosenthal, P. B. & Henderson, R. Optimal determination of particle orientation, absolute hand, and contrast loss in single-particle electron cryomicroscopy. J. Mol. Biol. 333, 721–745 (2003).
Ludtke, S. J., Baldwin, P. R. & Chiu, W. EMAN: semiautomated software for high-resolution single-particle reconstructions. J. Struct. Biol. 128, 82–97 (1999).
Voss, N. R. & Gerstein, M. 3V: cavity, channel and cleft volume calculator and extractor. Nucleic Acids Res. 38, W555–W562 (2010).
Holm, L. & Laakso, L. M. Dali server update. Nucleic Acids Res. 44, W351–W355 (2016).
Parker, J. L. & Newstead, S. Structural basis of nucleotide sugar transport across the Golgi membrane. Nature 551, 521–524 (2017).
Tsuchiya, H. et al. Structural basis for amino acid export by DMT superfamily transporter YddG. Nature 534, 417–420 (2016).
Lee, Y. et al. Structure of the triose-phosphate/phosphate translocator reveals the basis of substrate specificity. Nat. Plants 3, 825–832 (2017).
Nji, E., Gulati, A., Qureshi, A. A., Coincon, M. & Drew, D. Structural basis for the delivery of activated sialic acid into Golgi for sialyation. Nat. Struct. Mol. Biol. 26, 415–423 (2019).
Ahuja, S. & Whorton, M. R. Structural basis for mammalian nucleotide sugar transport. eLife 8, e45221 (2019).
Chen, F., Mackey, A. J., Stoeckert, C. J. Jr & Roos, D. S. OrthoMCL-DB: querying a comprehensive multi-species collection of ortholog groups. Nucleic Acids Res. 34, D363–D368 (2006).
Edgar, R. C. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32, 1792–1797 (2004).
Robert, X. & Gouet, P. Deciphering key features in protein structures with the new ENDscript server. Nucleic Acids Res. 42, W320–W324 (2014).
Sastry, G. M., Adzhigirey, M., Day, T., Annabhimoju, R. & Sherman, W. Protein and ligand preparation: parameters, protocols, and influence on virtual screening enrichments. J. Comput. Aided Mol. Des. 27, 221–234 (2013).
Jacobson, M. P. et al. A hierarchical approach to all-atom protein loop prediction. Proteins 55, 351–367 (2004).
Lomize, M. A., Pogozheva, I. D., Joo, H., Mosberg, H. I. & Lomize, A. L. OPM database and PPM web server: resources for positioning of proteins in membranes. Nucleic Acids Res. 40, D370–D376 (2012).
Bowers, K. J. et al. Scalable algorithms for molecular dynamics simulations on commodity clusters. In Proc. the ACM/IEEE Conference on Supercomputing (SC06) (2006).
Liao, J. et al. Structural insight into the ion-exchange mechanism of the sodium/calcium exchanger. Science 335, 686–690 (2012).
Mancusso, R., Gregorio, G. G., Liu, Q. & Wang, D. N. Structure and mechanism of a bacterial sodium-dependent dicarboxylate transporter. Nature 491, 622–626 (2012).
Forrest, L. R. (Pseudo-)symmetrical transport. Science 339, 399–401 (2013).
Straimer, J. et al. Site-specific genome editing in Plasmodium falciparum using engineered zinc-finger nucleases. Nat. Methods 9, 993–998 (2012).
Sanchez, C. P., Stein, W. & Lanzer, M. Trans stimulation provides evidence for a drug efflux carrier as the mechanism of chloroquine resistance in Plasmodium falciparum. Biochemistry 42, 9383–9394 (2003).
Saliba, K. J., Horner, H. A. & Kirk, K. Transport and metabolism of the essential vitamin pantothenic acid in human erythrocytes infected with the malaria parasite Plasmodium falciparum. J. Biol. Chem. 273, 10190–10195 (1998).
Dhingra, S. K. et al. Global spread of mutant PfCRT and its pleiotropic impact on Plasmodium falciparum multidrug resistance and fitness. mBio 10, e02731-18 (2019).
Quick, M., Shi, L., Zehnpfennig, B., Weinstein, H. & Javitch, J. A. Experimental conditions can obscure the second high-affinity site in LeuT. Nat. Struct. Mol. Biol. 19, 207–211 (2012).
Miller, K. R. et al. T cell receptor-like recognition of tumor in vivo by synthetic antibody fragment. PLoS ONE 7, e43746 (2012).
Wu, T. T. & Kabat, E. A. An analysis of the sequences of the variable regions of Bence Jones proteins and myeloma light chains and their implications for antibody complementarity. J. Exp. Med. 132, 211–250 (1970).
Hohn, M. et al. SPARX, a new environment for cryo-EM image processing. J. Struct. Biol. 157, 47–55 (2007).
Wallace, A. C., Laskowski, R. A. & Thornton, J. M. LIGPLOT: a program to generate schematic diagrams of protein–ligand interactions. Protein Eng. 8, 127–134 (1995).
Francis, S. E., Sullivan, D. J. Jr & Goldberg, D. E. Hemoglobin metabolism in the malaria parasite Plasmodium falciparum. Annu. Rev. Microbiol. 51, 97–123 (1997).
Acknowledgements
We thank B. Rice, E. Eng, L. Kim, K. Jordan, M. Kopylov, V. Dandey, H. Wei, S. Dallakyan, C. Negro, S. Gabryszewski, S. Mukherjee, B. Riegel, O. Clarke and Y. Chen for their helpful contributions. This work was supported by NIH grants (R01 AI147628 to F.M., D.A.F. and M.Q.; R35 GM132120 and R01 GM111980 to F.M.; R37 AI50234 and R01 AI124678 to D.A.F.; R01 GM119396 to M.Q.; R01 AI506312 and AI111962 to P.D.R.; R01 GM117372 to A.A.K.; T32 HL120826 to J.K.; P41 GM103310 to C.S.P. and B.C.; the Agency for Science, Technology and Research Singapore (to Y.Z.T.); the Simons Foundation (SF349247 to C.S.P. and B.C.); and NYSTAR (to C.S.P. and B.C.). Some of the work was performed at the Center for Membrane Protein Production and Analysis (COMPPÅ; P41 GM116799 to Wayne Hendrickson) and at the National Resource for Automated Molecular Microscopy at the Simons Electron Microscopy Center (P41 GM103310), both located at the New York Structural Biology Center.
Author information
Authors and Affiliations
Contributions
J.K., with help from L.M.H. and S.I.G., performed protein expression and purification. S.K.E., K.N., and A.A.K. identified the Fabs. Y.Z.T. and J.K. produced and analysed the cryo-EM data and built the model with help from B.C. and C.S.P. M.Q. and A.L.W. performed the biochemical assays. K.J.W., S.K.D., J.O., P.D.R. and D.A.F. conducted mutational analyses. K.J.W. and J.V. performed computational studies, and S.K.D. and J.O. performed parasite gene editing and characterization. M.Q., D.A.F. and F.M. designed experiments and wrote the paper with J.K., Y.Z.T., K.J.W. and P.D.R. K.J.W. and S.K.E. contributed equally as second authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Peer review information Nature thanks Shangyu Dang, Leann Tilley and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Extended data figures and tables
Extended Data Fig. 1 Major PfCRT haplotypes and location of residues involved in CQ or PPQ drug-resistance phenotypes.
a, The PfCRT canonical CQ-S, PPQ-S 3D7 haplotype, the CQ-R 7G8 (South America and Western Pacific) and Dd2 (Southeast Asia) haplotypes, and the 7G8 + C350R and Dd2 + F145I variants that have emerged in P. falciparum parasites in malaria-endemic areas and are associated with PPQ resistance. b, Localization of the mutated residues in PfCRT, based on the solved PfCRT structure. Most mutated residues localize within or near the boundary of one of the 10 TM helices. Extended Data Table 2a provides additional information about the mutated residues in PfCRT observed in the field or obtained in drug-pressured cultured parasites.
Extended Data Fig. 2 Identification of PfCRT-specific Fabs and preparation of purified PfCRT with or without Fabs in nanodiscs.
a, Complementarity-determining region (CDR) sequences of unique Fabs bioassayed for binding to recombinant PfCRT 7G8 incorporated into MSP1D1 nanodiscs. Fabs were selected following multiple rounds of enrichment from the phage display library E38,39,86. Ser-Tyr-Gly-Trp (SYGW) residues, which play a dominant part in antigen recognition, are highlighted by colour code (red, yellow, green and blue, respectively) and are numbered according to the Kabat system87. CTC was selected to form a stable PfCRT 7G8–Fab complex. b, Single-point ELISA quantification of the binding of phage-displayed Fab to PfCRT-incorporated biotinylated nanodiscs, empty nanodiscs or buffer (empty wells), measured at 450 nm absorbance (n = 1). c, EC50 evaluation of purified Fab binding to PfCRT incorporated into biotinylated nanodiscs, showing high-affinity binding for the Fab CTC (3.7 nM). Data are mean ± .s.d. of n = 3 independent experiments. d, High-performance liquid chromatography profile of PfCRT 7G8 with or without bound Fab CTC. e, SDS–PAGE gel of pooled and concentrated SEC fractions from PfCRT 7G8 with or without Fab CTC. The contaminant glutamate dehydrogenase (GDH; present as a left shoulder in d) was excluded from single-particle analyses. MSP1D1 is a membrane-scaffold protein used to assemble the nanodiscs. The identity of PfCRT and GDH was confirmed using mass spectrometry. f, Representative negative-stain two-dimensional class averages from Relion42 two-dimensional classification of nanodisc-incorporated PfCRT without Fab. g, Representative negative-stain two-dimensional class averages from Relion two-dimensional classification of nanodisc-incorporated PfCRT with bound Fab CTC.
Extended Data Fig. 3 Cryo-EM analysis of the PfCRT 7G8–Fab CTC complex.
a, Representative micrograph (0.5175 Å per pixel, 1.65 μm defocus). Example picked particles contributing to the final reconstruction are circled in green. b, Representative two-dimensional class averages from Relion two-dimensional classification. c, Flowchart of cryo-EM data processing and refinement of the PfCRT 7G8–Fab CTC complex. See Methods for details. d, FSC curves for PfCRT 7G8 complexed with the Fab CTC variable domain as well as for PfCRT alone. Data show the half-map (blue) and map-to-model (purple) resolutions (at 0.143 and 0.5 cut-offs, respectively), with embedded histograms of directional resolutions sampled evenly over the three-dimensional FSC61 (yellow). The corresponding sphericity values are indicated. e, Euler angle distribution plot of the final three-dimensional reconstruction from CryoSPARC v.250. f, Local resolution88 display of unsharpened reconstructions of PfCRT complexed with the variable domain of Fab CTC, in orthogonal views, sliced through the density for the first view.
Extended Data Fig. 4 Fit of cryo-EM density with model.
Cryo-EM densities (mesh) are superimposed on TM and JM helices of the PfCRT model. The model is rendered as a cartoon, coloured in rainbow.
Extended Data Fig. 5 PfCRT symmetrical arrangement, topology of TM helices and structural comparison with other DMT superfamily members.
a, View of PfCRT from opposite directions of the digestive vacuole and cytosol, with labelling of the four helices closest to the centre (thus contributing to the cavity). b, Model of the TM helices in the PfCRT 7G8 structure in the open-to-cytosol conformation. c, Structural conservation of PfCRT compared with the DMT family members Vrg4 (a GDP-mannose transporter; PDB 5OGE and 5OGK)66, YddG (an amino acid transporter; PDB 5I20)67, TPT (a triose-phosphate/phosphate translocator; PDB 5Y78 and 5Y79)68, and Z. mays CST and M. musculus CST (two CMP-sialic acid transporters; PDB 6I1R and 6OH4, respectively)69,70. Conservation profiles were generated using the Dali server65. d, The electrostatic potential surface representation of slices reveals the cavities of these six proteins in their solved structural states. Note the highly negatively charged (red) residues in the PfCRT cavity, contrasting with the positively charged (blue) or neutral residues in the other DMT transporters (shown as slices to emphasize the individual cavities). Ligands for the non-PfCRT transporters are shown as a ball and stick representation.
Extended Data Fig. 6 The structure of the PfCRT 7G8–Fab CTC complex shows the sequence conservation, localization of CHS, chemical structure of CQ and PPQ, and conservation of CQ-R and PPQ-R mutations.
a, PfCRT and the bound Fab are rendered in cartoon. PfCRT is coloured according to sequence conservation (see Extended Data Fig. 7) and the Fab is coloured in pink. Inset shows a magnified view of the interaction between PfCRT 7G8 and the Fab CTC. b, PfCRT cleft formed by JM1, TM1, TM9 and TM10 demonstrates CHS placement. c, PfCRT is rendered as a surface coloured by hydrophobicity, from orange (hydrophobic) to blue (hydrophilic). d, Two-dimensional diagram of PfCRT–CHS interactions observed in the structure, generated using LigPlot+ v.2.189. e, Chemical structures of CQ and PPQ, with the 4-aminoquinoline rings shaded. f, Location of CQ (left) and PPQ (right) resistance-associated mutations (with side chains rendered as sticks and spheres), modelled onto the PfCRT 7G8 structure. Models are colour-coded according to sequence conservation derived from 11 apicomplexan species (Extended Data Fig. 7). TM helices associated with CQ or PPQ resistance are labelled and the areas highlighted with coloured circles.
Extended Data Fig. 7 Sequence alignment and secondary structure of PfCRT.
Sequences of different PfCRT isoforms and other CRT homologues were aligned using MUSCLE72 and displayed using ESPript73. Sequences of ten orthologues of CRT in other Plasmodium or other apicomplexan parasites were obtained from OrthoMCL-DB71. The sequences used are PfCRT 7G8 (UNIPROT W7FI62) and its orthologues PfCRT Dd2 (UNIPROT F5CEB4) and wild-type PfCRT (the canonical 3D7 wild-type sequence; UNIPROT Q9N623), P. reichenowi CRT (PrCRT; UNIPROT A0A2P9D9K2), P. vivax CRT strain Sal-1 (PvCRT; UNIPROT Q9GSD3), P. knowlesi CRT strain H (PkCRT; UNIPROT Q9GSD7), P. berghei CRT strain ANKA (PbCRT; UNIPROT Q9GSD8), P. chabaudi CRT strain chabaudi (PcCRT; UNIPROT Q7Z0V9), B. microti CRT strain RI (BmCRT; UNIPROT A0A1N6LY67), T. annulata CRT strain Ankara (TaCRT, UNIPROT Q4UDS9); E. tenella CRT (EtCRT; UNIPROT U6L1M8), T. gondii CRT (TgCRT; UNIPROT S8EU26) and C. hominis CRT strain TU502 (ChCRT; UNIPROT A0A0S4THJ3). The secondary structure of PfCRT is shown as a cartoon above the alignments with residue numbering corresponding to the PfCRT 7G8 reference, along with the positioning of the variant residues indicated in Extended Data Fig. 1 (sharing the same colour scheme). The highly conserved cysteine residues that probably form disulphide bonds are C289–C312 and C301–C309. The degree of conservation was calculated by considering all sequences including P. falciparum 3D7 (wild type) but excluding the 7G8 and Dd2 variants. Residues conserved (that is, identical or similar) in at least 10 out of 13 species are indicated in red text. Residues conserved in all species are in white text with red highlighting. None of the mutations associated with CQ or PPQ resistance mapped to residues that are fully conserved across these apicomplexan species.
Extended Data Fig. 8 Binding and transport assays for PfCRT 7G8.
a, Total binding of 125 nM [3H]Arg to 100 ng of nanodisc-incorporated PfCRT 7G8 was measured in the absence (−) or presence (+) of 800 mM imidazole (which competes with the His-tagged PfCRT 7G8 isoform for binding to the copper-coated YSi SPA beads). Data are mean ± s.e.m. (n = 3 independent experiments); grey symbols show the data mean of technical replicates (n = 3) of each independent experiment. b, Isotopic dilution of 125 nM [3H]Arg with non-radiolabelled Arg revealed a log(EC50) value of −3.40 ± 0.033 (corresponding to 397 μM). We note that the primary source of Arg in parasitized red blood cells is from the proteolysis of haemoglobin, which in its native state90 as a tetramer is present at approximately 5 mM. Data are mean ± s.e.m. (n = 3 independent experiments of technical triplicates). c, Binding of 125 nM [3H]Arg in the presence or absence of 10 μM verapamil (VP), 1 μM CQ or PPQ, 0.1 μM amodiaquine (ADQ), or 1 mM Arg, Lys or Leu. Data were normalized to the signal in the absence of the respective non-radiolabelled compound and are mean ± s.e.m. (n = 3 independent experiments); grey symbols show the data mean of technical triplicates of each independent experiment. d, e, Uptake of 370 nM [3H]CQ (d) or 250 nM [3H]Arg (e), was measured for 1-min periods in PfCRT 7G8-containing proteoliposomes preloaded with 100 mM KPi, pH 7.5 diluted in buffer composed of 50 mM Tris/MES, pH 5.5 or pH 7.5 with or without the K+ ionophore valinomycin (Vlm; 5 μM). ctl, control. The valinomycin-mediated K+ efflux proceeding down its concentration gradient generated an inside-negative membrane potential. Empty liposomes lacking PfCRT 7G8 served as controls. Data are mean ± s.e.m. (n = 3 independent experiments); grey symbols show the data mean of technical triplicates of each independent experiment.
Extended Data Fig. 9 Role of conserved cysteine residues in CQ binding and transport, and parasite PPQ dose–response data.
a, Cysteine residues that probably form disulphide bonds on the loop adjacent to JM2 and connecting to TM8 are shown in yellow and rendered as sticks. Their locations are shown as an inset of the overall structure and are coloured by conservation (as per Extended Data Fig. 7). b, Effect of reducing conditions on the binding of [3H]CQ by PfCRT 7G8, 7G8 + C289A and 7G8 + C301A. SPA binding of 100 nM [3H]CQ was performed with 200 ng of each protein variant reconstituted into nanodiscs at pH 7.5 in the presence of increasing concentrations of β-mercaptoethanol. Data (shown as mean ± s.e.m. of n = 3 independent experiments with technical triplicates) were normalized with regard to the activity measured for PfCRT 7G8 in the absence of the reducing agent. c, Effect of the reducing agent β-mercaptoethanol on [3H]CQ transport by PfCRT 7G8, 7G8 + C289A or 7G8 + C301A. Uptake of 100 nM [3H]CQ was measured for 1-min periods in control liposomes and proteoliposomes containing the indicated PfCRT variants, in the presence of increasing concentrations of β-mercaptoethanol. Data are means of n = 2 independent experiments performed as n = 4 technical replicates and were subjected to nonlinear regression fitting using hyperbolic decay models with either two (for 7G8 and 7G8 + C289A) or three (including 7G8 + C301A) parameters. Kinetic constants are shown as mean ± s.e.m. of the fits. The IC50 (concentration of β-mercaptoethanol yielding half-maximal reduction of transport) for 7G8 and 7G8 + C289A were 1.24 ± 0.14 mM and 2.58 ± 0.39 mM, respectively. For 7G8 + C301A the IC50 was 4.17 ± 0.16 mM with a remaining activity of 60.2 ± 8.2% (compared to the activity in the absence of the reducing agent). d, PPQ dose–response data for pfcrt-edited and control parasite lines, generated from 72-h growth inhibition assays. Data are mean ± s.e.m. from n = 4 independent assays performed in duplicate (n = 2). Statistical significance was determined using two-tailed Mann–Whitney U-tests.
Extended Data Fig. 10 Electrostatic potential surfaces of isoform-specific PfCRT cavities and simulations of molecular dynamics.
a, Electrostatic surfaces for the solved open-to-digestive-vacuole conformation for 7G8 and modelled isoforms, predicted at pH 5. Images are presented as a vertical slice through the transporter, showing net charges in the cavity and locations of TM helices. The row below shows the electrostatic surfaces for the homology models of these PfCRT isoforms, illustrated in their open-to-cytosol configuration, predicted at pH 7.0. b, Surface representation of the electrostatic potential of the central cavity of 7G8 and the 7G8 + C350R variant, shown in two different orientations with red and blue indicating negatively and positively charged residues, respectively. c, Simulations of molecular dynamics on the 7G8 structure (without the Fab) over 250-ns trajectories, establishing the equilibrium positions of protein side chains and distances between position 145 and residues on proximal helices. d, Simulations of molecular dynamics on the 7G8 structure (without the Fab) over 250-ns trajectories, establishing the equilibrium positions of protein side chains between position 350 and residues on proximal helices, and showing marginal movement between the C350 and the 350R isoforms.
Supplementary information
Supplementary Figure 1
This file contains the uncropped blots
Rights and permissions
About this article
Cite this article
Kim, J., Tan, Y.Z., Wicht, K.J. et al. Structure and drug resistance of the Plasmodium falciparum transporter PfCRT. Nature 576, 315–320 (2019). https://doi.org/10.1038/s41586-019-1795-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-019-1795-x
This article is cited by
-
Strong positive selection biases identity-by-descent-based inferences of recent demography and population structure in Plasmodium falciparum
Nature Communications (2024)
-
Structure of human phagocyte NADPH oxidase in the activated state
Nature (2024)
-
Antimalarial drug discovery: progress and approaches
Nature Reviews Drug Discovery (2023)
-
A minority of final stacks yields superior amplitude in single-particle cryo-EM
Nature Communications (2023)
-
pH-dependence of the Plasmodium falciparum chloroquine resistance transporter is linked to the transport cycle
Nature Communications (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.