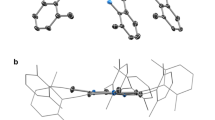
Abstract
The size-dependent and shape-dependent characteristics that distinguish nanoscale materials from bulk solids arise from constraining the dimensionality of an inorganic structure1,2,3. As a consequence, many studies have focused on rationally shaping these materials to influence and enhance their optical, electronic, magnetic and catalytic properties4,5,6. Although a select number of stable clusters can typically be synthesized within the nanoscale regime for a specific composition, isolating clusters of a predetermined size and shape remains a challenge, especially for those derived from two-dimensional materials. Here we realize a multidentate coordination environment in a metal–organic framework to stabilize discrete inorganic clusters within a porous crystalline support. We show confined growth of atomically defined nickel(ii) bromide, nickel(ii) chloride, cobalt(ii) chloride and iron(ii) chloride sheets through the peripheral coordination of six chelating bipyridine linkers. Notably, confinement within the framework defines the structure and composition of these sheets and facilitates their precise characterization by crystallography. Each metal(ii) halide sheet represents a fragment excised from a single layer of the bulk solid structure, and structures obtained at different precursor loadings enable observation of successive stages of sheet assembly. Finally, the isolated sheets exhibit magnetic behaviours distinct from those of the bulk metal halides, including the isolation of ferromagnetically coupled large-spin ground states through the elimination of long-range, interlayer magnetic ordering. Overall, these results demonstrate that the pore environment of a metal–organic framework can be designed to afford precise control over the size, structure and spatial arrangement of inorganic clusters.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Additional crystallographic information, powder X-ray diffraction data, scanning electron microscopy and energy-dispersive X-ray spectroscopy data, gas-sorption data, magnetic data, Mössbauer spectroscopy data, diffuse reflectance UV–Vis spectra, and elemental analyses are available in the Supplementary Information. Metrical data for the solid-state structures are available from the Cambridge Crystallographic Data Centre under reference numbers CCDC 1901128 to 1901144.
References
Bawendi, M. G., Steigerwald, M. L. & Brus, L. E. The quantum mechanics of larger semiconductor clusters (“quantum dots”). Annu. Rev. Phys. Chem. 41, 477–496 (1990).
Billas, I. M., Châtelain, A. & de Heer, W. A. Magnetism from the atom to the bulk in iron, cobalt, and nickel clusters. Science 265, 1682–1684 (1994).
Lee, S. C. & Holm, R. H. Nonmolecular metal chalcogenide/halide solids and their molecular cluster analogues. Angew. Chem. 29, 840–856 (1990).
Gatteschi, D., Caneschi, A., Pardi, L. & Sessoli, R. Large clusters of metal ions: the transition from molecular to bulk magnets. Science 265, 1054–1058 (1994).
Xia, Y., Xiong, Y., Lim, B. & Skrabalak, S. E. Shape-controlled synthesis of metal nanocrystals: Simple chemistry meets complex physics? Angew. Chem. 48, 60–103 (2009).
Papatriantafyllopoulou, C., Moushi, E. E., Christou, G. & Tasiopoulos, A. J. Filling the gap between the quantum and classical worlds of nanoscale magnetism: giant molecular aggregates based on paramagnetic 3d metal ions. Chem. Soc. Rev. 45, 1597–1628 (2016).
Kim, C. R., Uemura, T. & Kitagawa, S. Inorganic nanoparticles in porous coordination polymers. Chem. Soc. Rev. 45, 3828–3845 (2016).
Meilikhov, M. et al. Metals@MOFs – Loading MOFs with metal nanoparticles for hybrid functions. Eur. J. Inorg. Chem. 2010, 3701–3714 (2010).
Lin, H. et al. Bulk assembly of organic metal halide nanotubes. Chem. Sci. 8, 8400–8404 (2017).
Chen, K.-J., Perry, J. J., Scott, H. S., Yang, Q.-Y. & Zaworotko, M. J. Double-walled pyr topology networks from a novel fluoride-bridged heptanuclear metal cluster. Chem. Sci. 6, 4784–4789 (2015).
Gallington, L. C. et al. Regioselective atomic layer deposition in metal–organic frameworks directed by dispersion interactions. J. Am. Chem. Soc. 138, 13513–13516 (2016).
Braglia, L. et al. Tuning Pt and Cu sites population inside functionalized UiO-67 MOF by controlling activation conditions. Faraday Discuss. 201, 265–286 (2017).
An, B. et al. Confinement of ultrasmall Cu/ZnOx nanoparticles in metal-organic frameworks for selective methanol synthesis from catalytic hydrogenation of CO2. J. Am. Chem. Soc. 139, 3834–3840 (2017).
Chen, L., Chen, H., Luque, R. & Li, Y. Metal−organic framework encapsulated Pd nanoparticles: towards advanced heterogeneous catalysts. Chem. Sci. 5, 3708–3714 (2014).
Wang, X.-N. et al. Crystallographic visualization of postsynthetic nickel clusters into metal–organic framework. J. Am. Chem. Soc. 141, 13654–13663 (2019).
Gonzalez, M. I., Bloch, E. D., Mason, J. A., Teat, S. J. & Long, J. R. Single-crystal-to-single-crystal metalation of a metal-organic framework: a route toward structurally well-defined catalysts. Inorg. Chem. 54, 2995–3005 (2015).
Gonzalez, M. I., Oktawiec, J. & Long, J. R. Ethylene oligomerization in metal-organic frameworks bearing nickel(ii) 2,2′-bipyridine complexes. Faraday Discuss. 201, 351–367 (2017).
Ketelaar, J. A. A. Die Kristallstruktur des Nickelbromids und -jodids. Z. Kristallogr. Cryst. Mater. 88, 26–34 (1934).
Ferrari, A., Braibanti, A. & Bigliardi, G. Refinement of the crystal structure of NiCl2 and of unit-cell parameters of some anhydrous chlorides of divalent metals. Acta Crystallogr. 16, 846–847 (1963).
George, P. & McClure, D. S. The effect of inner orbital splitting on the thermodynamic properties of transition metal compounds and coordination complexes. In Progress in Inorganic Chemistry Vol. 1 (ed. Cotton, F. A.) 381–463 (Interscience, 1959).
Wilkinson, M. K., Cable, J. W., Wollan, E. O. & Koehler, W. C. Neutron diffraction investigations of the magnetic ordering in FeBr2, CoBr2, FeCl2, and CoCl2. Phys. Rev. 113, 497–507 (1959).
Tsubokawa, I. The magnetic properties of NiBr2. J. Phys. Soc. Jpn. 15, 2109 (1960).
Mydosh, J. A. Spin Glasses (Taylor & Francis, 1993).
Milios, C. J. & Winpenny, R. E. P. in Molecular Nanomagnets and Related Phenomena (ed. Gao, S.) 1–109 (Springer, 2014).
Huang, B. et al. Layer-dependent ferromagnetism in a van der Waals crystal down to the monolayer limit. Nature 546, 270–273 (2017).
Gong, C. et al. Discovery of intrinsic ferromagnetism in two-dimensional van der Waals crystals. Nature 546, 265–269 (2017).
Wang, Y. et al. Cryo-mediated exfoliation and fracturing of layered materials into 2D quantum dots. Sci. Adv. 3, e1701500 (2017).
Lin, L. et al. Fabrication of luminescent monolayered tungsten dichalcogenides quantum dots with giant spin-valley coupling. ACS Nano 7, 8214–8223 (2013).
Ritter, K. A. & Lyding, J. W. The influence of edge structure on the electronic properties of graphene quantum dots and nanoribbons. Nat. Mater. 8, 235–242 (2009).
Wilcoxon, J. P. & Samara, G. A. Strong quantum-size effects in a layered semiconductor: MoS2 nanoclusters. Phys. Rev. B 51, 7299–7302 (1995).
Rama, G. et al. Stereoselective formation of chiral metallopeptides. Chemistry 18, 7030–7035 (2012).
Trickett, C. A. et al. Definitive molecular level characterization of defects in UiO-66 crystals. Angew. Chem. 54, 11162–11167 (2015).
Bruker Analytical X-ray Systems. SAINT and APEX 2 Software for CCD Diffractometers (2000).
Sheldrick, G. M. SADABS (Bruker Analytical X-ray Systems, 2014).
Sheldrick, G. M. SHELXS (Univ. Göttingen, 2014).
Sheldrick, G. M. A short history of SHELX. Acta Crystallogr. A 64, 112–122 (2008).
Sheldrick, G. M. SHELXL (Univ. Göttingen, 2014).
Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K. & Puschmann, H. OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Cryst. 42, 339–341 (2009).
Shearer, G. C. et al. Tuned to perfection: ironing out the defects in metal–organic framework UiO-66. Chem. Mater. 26, 4068–4071 (2014).
Spek, A. L. PLATON SQUEEZE: a tool for the calculation of the disordered solvent contribution to the calculated structure factors. Acta Crystallogr. C 71, 9–18 (2015).
Spek, A. L. Single-crystal structure validation with the program PLATON. J. Appl. Cryst. 36, 7–13 (2003).
Coelho, A. A. Indexing of powder diffraction patterns by iterative use of singular value decomposition. J. Appl. Cryst. 36, 86–95 (2003).
Coelho, A. A. TOPAS-Academic v.4.1 (Coelho Software, 2007).
Acknowledgements
This research was supported through a Multidisciplinary University Research Initiatives Program funded by the US Department of Defense, Office of Naval Research under award N00014-15-1-2681. Single-crystal X-ray diffraction experiments were performed at beamline 11.3.1 at the Advanced Light Source at Lawrence Berkeley National Laboratory. The Advanced Light Source is supported by the Director, Office of Science, Office of Basic Energy Sciences, of the US Department of Energy under contract no. DE-AC02-05CH11231. Powder X-ray diffraction data were collected at beamline 17-BM-B at the Advanced Photon Source, a US Department of Energy, Office of Science User Facility operated by the DOE Office of Science by Argonne National Laboratory under contract no. DE-AC02-06CH11357. Work at the Molecular Foundry was supported by the Office of Science, Office of Basic Energy Sciences, of the US Department of Energy under contract no. DE-AC02-05CH11231. We thank the US National Science Foundation for providing graduate fellowship support for A.B.T, L.E.D. and J.O. In addition, we thank S. J. Teat, K. Chakarawet, M. Jackson and N. Masciocchi for experimental assistance and helpful discussions. We also thank K. R. Meihaus for editorial assistance.
Author information
Authors and Affiliations
Contributions
M.I.G., A.B.T. and J.R.L. formulated the project. M.I.G. and A.B.T. synthesized the compounds. M.I.G. collected and analysed the single-crystal X-ray diffraction data, with the assistance of A.B.T. J.O. collected and analysed the powder X-ray diffraction data. A.B.T. and L.E.D. collected and analysed the magnetic susceptibility data. A.B.T. and K.B. collected and analysed the electron microscopy data. A.B.T. collected the Mössbauer spectra, and F.G. and G.J.L. analysed the spectra. M.I.G. collected and analysed the gas adsorption data. M.I.G., A.B.T. and J.R.L. wrote the paper, and all authors contributed to revising it.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature thanks Felipe Gándara, Mohamedally Kurmoo and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Extended data figures and tables
Extended Data Fig. 1 High-angle annular dark field images and energy-dispersive spectroscopy data for 1(NiBr2)15.
a, High-angle annular dark field (HAADF) image (a, top left) and energy-dispersive X-ray spectroscopy (EDS) Zr (a, top right; yellow), Ni (a, bottom left; green) and Br (a, bottom right; red) mapping of a microcrystalline powder sample of 1(NiBr2)15. b, STEM-EDS line scan analysis for Ni (red) and Zr (yellow) across the length of the crystallite plotted as normalized atom per cent. The average amount for the two elements was determined to be 72 ± 12% for Ni and 28 ± 12% for Zr, corresponding to a Ni:Zr ratio of 2.6. c, EDS spectrum for the crystallite of 1(NiBr2)15. Signals for Cu and Au originate from the space-filling washer and sample grid, respectively.
Extended Data Fig. 2 High-angle annular dark field images and energy-dispersive spectroscopy data for 1(NiCl2)15.
a, High-angle annular dark field (HAADF) image (a, top left) and energy-dispersive X-ray spectroscopy (EDS) Zr (a, top right; yellow), Ni (a, bottom left; green) and Cl (a, bottom right; red) mapping of a microcrystalline powder sample of 1(NiCl2)15. b, STEM-EDS line scan analysis for Ni (green) and Zr (yellow) across the length of the crystallite plotted as normalized atom per cent. The average amount for the two elements was determined to be 70.7 ± 11% for Ni and 29 ± 11% for Zr, corresponding to a Ni:Zr ratio of 2.4. c, EDS spectrum for the crystallite of 1(NiCl2)15. Signals for Cu and Au originate from the space-filling washer and sample grid, respectively. cps, counts per second.
Extended Data Fig. 3 High-angle annular dark field images and energy-dispersive spectroscopy data for 1(CoCl2)18.
a, High-angle annular dark field (HAADF) image (a, top left) and energy-dispersive X-ray spectroscopy (EDS) Zr (a, top right; yellow), Co (a, bottom left; violet) and Cl (a, bottom right; green) mapping of a microcrystalline powder sample of 1(CoCl2)18. b, STEM-EDS line scan analysis for Co (violet) and Zr (yellow) across the length of the crystallite plotted as normalized atom per cent. The average amount for the two elements was determined to be 75 ± 13% for Co and 25 ± 13% for Zr, corresponding to a Co:Zr ratio of 3.0. c, EDS spectrum for the crystallite of 1(CoCl2)18. Signals for Cu and Au originate from the space-filling washer and sample grid, respectively.
Extended Data Fig. 4 High-angle annular dark field images and energy-dispersive spectroscopy data for 1(FeCl2)19.
a, High-angle annular dark field (HAADF) image (a, top left) and energy-dispersive X-ray spectroscopy (EDS) mapping Zr (a, top right; yellow), Fe (a, bottom left; orange) and Cl (a, bottom right; green of a microcrystalline powder sample of 1(FeCl2)19. b, STEM-EDS line scan analysis for Fe (orange) and Zr (yellow) across the length of the crystallite plotted as normalized atom per cent. The average amount for the two elements was determined to be 77 ± 9% for Fe and 23 ± 9% for Zr, corresponding to a Fe:Zr ratio of 3.3. c, EDS spectrum for the crystallite of 1(FeCl2)19. Signals for Cu and Au originate from the space-filling washer and sample grid, respectively.
Extended Data Fig. 5 Comparison of Ni(ii) site occupancies.
a, b, Nickel site occupancies for single-crystal structures of NiBr2-loaded (a, light to dark red) and NiCl2-loaded (b, light to dark green) 1 obtained by reaction of 1 with 1.00 equiv to excess (>50 equiv) NiX2 (X = Br, Cl) in diglyme. Error bars represent the crystallographic standard uncertainties of the Ni(ii) site occupancies. Lines are included to guide the eye.
Extended Data Fig. 6 a.c. magnetic susceptibility data.
a, b, In-phase (a) and out-of-phase (b) variable-temperature a.c. magnetic susceptibility data from 2 to 10 K for 1(FeCl2)19 under zero d.c. magnetic field and a 0.4 mT a.c. magnetic field oscillating at frequencies of 1, 5, 7.5, 10, 50, 75, 100, 500, 750 and 1,000 Hz (blue to red). Coloured lines are guides for the eye.
Extended Data Fig. 7 Mössbauer spectra.
a, b, 57Fe Mössbauer spectra for 1(FeCl2)19 at 100 K (a) and 5 K (b). The data were fit with four high-spin octahedral Fe(ii) components (green), two high-spin four- and five-coordinate Fe(ii) components (red), and a magnetic hyperfine component (grey). Spontaneous oxidation leads to a high-spin Fe(iii) impurity (blue) visible at 5 K. Overall fits are depicted in black.
Supplementary information
Supplementary Information
This file includes Supplementary Text, Supplementary Figures 1–29, Supplementary Tables 1–11, and associated references.
Rights and permissions
About this article
Cite this article
Gonzalez, M.I., Turkiewicz, A.B., Darago, L.E. et al. Confinement of atomically defined metal halide sheets in a metal–organic framework. Nature 577, 64–68 (2020). https://doi.org/10.1038/s41586-019-1776-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-019-1776-0
This article is cited by
-
Enantiomeric analysis of chiral phenyl aromatic compounds by coated capillary electrochromatography based on a MOF-on-MOF stationary phase
Microchimica Acta (2024)
-
Silver nanoparticle enhanced metal-organic matrix with interface-engineering for efficient photocatalytic hydrogen evolution
Nature Communications (2023)
-
Ultrafine Fe-modulated Ni nanoparticles embedded within nitrogen-doped carbon from Zr-MOFs-confined conversion for efficient oxygen evolution reaction
Frontiers of Chemical Science and Engineering (2022)
-
Phase-enabled metal-organic framework homojunction for highly selective CO2 photoreduction
Nature Communications (2021)
-
Recent advances in the synthesis of monolithic metal-organic frameworks
Science China Materials (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.