Abstract
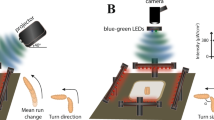
Light discrimination according to colour can confer survival advantages by guiding animals towards food and shelter and away from potentially harmful situations1,2. Such colour-dependent behaviour can be learned or innate. Data on innate colour preference in mammals remain controversial3 and there are limited data for simpler organisms4,5,6,7. Here we show that, when given a choice among blue, green and dim light, fruit flies exhibit an unexpectedly complex pattern of colour preference that changes according to the time of day. Flies show a strong preference for green in the early morning and late afternoon, a reduced green preference at midday and a robust avoidance of blue throughout the day. Genetic manipulations reveal that the peaks in green preference require rhodopsin-based visual photoreceptors and are controlled by the circadian clock. The midday reduction in green preference in favour of dim light depends on the transient receptor potential (TRP) channels dTRPA1 and Pyrexia, and is also timed by the clock. By contrast, avoidance of blue light is primarily mediated by multidendritic neurons, requires rhodopsin 7 and the TRP channel Painless, and is independent of the clock. Our findings show that several TRP channels are involved in colour-driven behaviour in Drosophila, and reveal distinct pathways of innate colour preference that coordinate the behavioural dynamics of flies in ambient light.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data sets generated during this study are available from the corresponding author upon reasonable request.
Code availability
The custom computer code used in this study is available in GitHub repository (https://github.com/StasLaz/Tracking-flies) and from the corresponding author upon reasonable request.
References
Dominy, N. J. & Lucas, P. W. Ecological importance of trichromatic vision to primates. Nature 410, 363–366 (2001).
Osorio, D. & Vorobyev, M. A review of the evolution of animal colour vision and visual communication signals. Vision Res. 48, 2042–2051 (2008).
McManus, I. C., Jones, A. L. & Cottrell, J. The aesthetics of colour. Perception 10, 651–666 (1982).
Fischbach, K. F. Simultaneous and successive colour contrast expressed in ‘slow’ phototactic behaviour of walking Drosophila melanogaster. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 130, 161–171 (1979).
Yamaguchi, S., Desplan, C. & Heisenberg, M. Contribution of photoreceptor subtypes to spectral wavelength preference in Drosophila. Proc. Natl Acad. Sci. USA 107, 5634–5639 (2010).
Gao, S. et al. The neural substrate of spectral preference in Drosophila. Neuron 60, 328–342 (2008).
Schumperli, R. A. Evidence for colour vision in Drosophila melanogaster through spontaneous phototactic choice behaviour. J. Comp. Physiol. 86, 77–94 (1973).
Otsuna, H., Shinomiya, K. & Ito, K. Parallel neural pathways in higher visual centers of the Drosophila brain that mediate wavelength-specific behavior. Front. Neural Circuits 8, 8 (2014).
Yoshii, T., Rieger, D. & Helfrich-Förster, C. Two clocks in the brain: an update of the morning and evening oscillator model in Drosophila. Prog. Brain Res. 199, 59–82 (2012).
Hori, M., Shibuya, K., Sato, M. & Saito, Y. Lethal effects of short-wavelength visible light on insects. Sci. Rep. 4, 7383 (2014).
Hall, H., Ma, J., Shekhar, S., Leon-Salas, W. D. & Weake, V. M. Blue light induces a neuroprotective gene expression program in Drosophila photoreceptors. BMC Neurosci. 19, 43 (2018).
Kistenpfennig, C. et al. A new rhodopsin influences light-dependent daily activity patterns of fruit flies. J. Biol. Rhythms 32, 406–422 (2017).
Ni, J. D., Baik, L. S., Holmes, T. C. & Montell, C. A rhodopsin in the brain functions in circadian photoentrainment in Drosophila. Nature 545, 340–344 (2017).
Sakai, K. et al. Drosophila melanogaster rhodopsin Rh7 is a UV-to-visible light sensor with an extraordinarily broad absorption spectrum. Sci. Rep. 7, 7349 (2017).
Senthilan, P. R. & Helfrich-Förster, C. Rhodopsin 7—the unusual rhodopsin in Drosophila. PeerJ 4, e2427 (2016).
Xiang, Y. et al. Light-avoidance-mediating photoreceptors tile the Drosophila larval body wall. Nature 468, 921–926 (2010).
Zanini, D. et al. Proprioceptive opsin functions in Drosophila larval locomotion. Neuron 98, 67–74.e4 (2018).
Im, S. H. & Galko, M. J. Pokes, sunburn, and hot sauce: Drosophila as an emerging model for the biology of nociception. Dev. Dyn. 241, 16–26 (2012).
Fowler, M. A. & Montell, C. Drosophila TRP channels and animal behavior. Life Sci. 92, 394–403 (2013).
Heisenberg, M. & Buchner, E. The role of retinula cell types in visual behavior of Drosophila melanogaster. J. Comp. Physiol. A 117, 127–162 (1977).
Schnaitmann, C., Garbers, C., Wachtler, T. & Tanimoto, H. Color discrimination with broadband photoreceptors. Curr. Biol. 23, 2375–2382 (2013).
Green, E. W. et al. Drosophila circadian rhythms in seminatural environments: Summer afternoon component is not an artifact and requires TrpA1 channels. Proc. Natl Acad. Sci. USA 112, 8702–8707 (2015).
Roessingh, S., Wolfgang, W. & Stanewsky, R. Loss of Drosophila melanogaster TRPA1 function affects “siesta” behavior but not synchronization to temperature cycles. J. Biol. Rhythms 30, 492–505 (2015).
Wolfgang, W., Simoni, A., Gentile, C. & Stanewsky, R. The Pyrexia transient receptor potential channel mediates circadian clock synchronization to low temperature cycles in Drosophila melanogaster. Proc. R. Soc. Lond. B 280, 20130959 (2013).
Sokabe, T., Chen, H.-C., Luo, J. & Montell, C. A switch in thermal preference in Drosophila larvae depends on multiple rhodopsins. Cell Rep. 17, 336–344 (2016).
Shen, W. L. et al. Function of rhodopsin in temperature discrimination in Drosophila. Science 331, 1333–1336 (2011).
Senthilan, P. R. et al. Drosophila auditory organ genes and genetic hearing defects. Cell 150, 1042–1054 (2012).
Sun, Y. et al. TRPA channels distinguish gravity sensing from hearing in Johnston’s organ. Proc. Natl Acad. Sci. USA 106, 13606–13611 (2009).
Schloss, K. B., Nelson, R., Parker, L., Heck, I. A. & Palmer, S. E. Seasonal variations in color preference. Cogn. Sci. 41, 1589–1612 (2017).
Yamaguchi, S., Wolf, R., Desplan, C. & Heisenberg, M. Motion vision is independent of color in Drosophila. Proc. Natl Acad. Sci. USA 105, 4910–4915 (2008).
Konopka, R. J. & Benzer, S. Clock mutants of Drosophila melanogaster. Proc. Natl Acad. Sci. USA 68, 2112–2116 (1971).
Schlichting, M. et al. A neural network underlying circadian entrainment and photoperiodic adjustment of sleep and activity in Drosophila. J. Neurosci. 36, 9084–9096 (2016).
Guo, F., Cerullo, I., Chen, X. & Rosbash, M. PDF neuron firing phase-shifts key circadian activity neurons in Drosophila. eLife 3, e02780 (2014).
Dolezelova, E., Dolezel, D. & Hall, J. C. Rhythm defects caused by newly engineered null mutations in Drosophila’s cryptochrome gene. Genetics 177, 329–345 (2007).
Beaver, L. M. & Giebultowicz, J. M. Regulation of copulation duration by period and timeless in Drosophila melanogaster. Curr. Biol. 14, 1492–1497 (2004).
Tang, X., Platt, M. D., Lagnese, C. M., Leslie, J. R. & Hamada, F. N. Temperature integration at the AC thermosensory neurons in Drosophila. J. Neurosci. 33, 894–901 (2013).
Sweeney, S. T., Broadie, K., Keane, J., Niemann, H. & O’Kane, C. J. Targeted expression of tetanus toxin light chain in Drosophila specifically eliminates synaptic transmission and causes behavioral defects. Neuron 14, 341–351 (1995).
Seidner, G. et al. Identification of neurons with a privileged role in sleep homeostasis in Drosophila melanogaster. Curr. Biol. 25, 2928–2938 (2015).
Szular, J. et al. Rhodopsin 5- and Rhodopsin 6-mediated clock synchronization in Drosophila melanogaster is independent of retinal phospholipase C-β signaling. J. Biol. Rhythms 27, 25–36 (2012).
Gorczyca, D. A. et al. Identification of Ppk26, a DEG/ENaC channel functioning with Ppk1 in a mutually dependent manner to guide locomotion behavior in Drosophila. Cell Rep. 9, 1446–1458 (2014).
Shimono, K. et al. Multidendritic sensory neurons in the adult Drosophila abdomen: origins, dendritic morphology, and segment- and age-dependent programmed cell death. Neural Dev. 4, 37 (2009).
Lamaze, A. et al. Regulation of sleep plasticity by a thermo-sensitive circuit in Drosophila. Sci. Rep. 7, 40304 (2017).
Bernardo-Garcia, F. J., Humberg, T.-H., Fritsch, C. & Sprecher, S. G. Successive requirement of Glass and Hazy for photoreceptor specification and maintenance in Drosophila. Fly (Austin) 11, 112–120 (2017).
Pearn, M. T., Randall, L. L., Shortridge, R. D., Burg, M. G. & Pak, W. L. Molecular, biochemical, and electrophysiological characterization of Drosophila norpA mutants. J. Biol. Chem. 271, 4937–4945 (1996).
Döring, F., Wischmeyer, E., Kühnlein, R. P., Jäckle, H. & Karschin, A. Inwardly rectifying K+ (Kir) channels in Drosophila. A crucial role of cellular milieu factors for Kir channel function. J. Biol. Chem. 277, 25554–25561 (2002).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012).
Lazopulo, S., Lopez, J. A., Levy, P. & Syed, S. A stochastic burst follows the periodic morning peak in individual Drosophila locomotion. PLoS ONE 10, e0140481 (2015).
Hernándes de Salomon, C. & Spatz, H.-C. Colour vision in Dropsophila melanogaster: wavelength discrimination. J. Comp. Physiol. A 150, 31–37 (1983).
Salcedo, E. et al. Blue- and green-absorbing visual pigments of Drosophila: ectopic expression and physiological characterization of the R8 photoreceptor cell-specific Rh5 and Rh6 rhodopsins. J. Neurosci. 19, 10716–10726 (1999).
Acknowledgements
We thank C. Desplan, J. Giebultowicz, F. Hamada, C. Helfrich-Förster, L. and Y.-N. Jan, W. Joiner, M. Kim, M. Klein, C. Montell, R. Stanewsky, D. Top, Y. Xiang, M. Young, the Kyoto Stock Centre, and the Bloomington Drosophila Stock Centre for fly stocks; J. Dallman, M. Dallman, B. de Bivort, M. Klein and J. Truman for comments on the manuscript; M. Klein and W. Li for discussions; and J. A. Lopez and M. Collazo for technical support. This work was supported in part by the National Science Foundation under grant IOS-1656603 to S.S.
Author information
Authors and Affiliations
Contributions
S.L. and S.S. conceived and designed the study, S.L and A.L performed the behavioural experiments and analysed the data, J.D.B. and S.L. performed imaging experiments, S.L. and S.S. wrote the paper with feedback from all authors, and S.S. and J.D.B. supervised the study.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Peer review information Nature thanks Charlotte Förster, Craig Montell, Ralf Stanewsky and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Extended data figures and tables
Extended Data Fig. 1 Properties of unfiltered and filtered light, and temperature inside the tubes.
a, Measured values for the broadband ‘white’ LED light of about 8,800 lx used in the experiments and the corresponding total irradiance of the light before and after passing through the filters. b, Spectral irradiance of the LED light and light transmitted through the green, blue and red filters. Peaks for the filtered light are at 450 nm (blue), 528 nm (green), and 620 nm (red). c, Normalized spectral sensitivity of Drosophila rhodopsins (solid lines) plotted together with transmission data through green and blue filters from b (dashed lines). Rhodopsin sensitivity data were adapted from previous work13,49. d–f, Internal tube temperature in the green (d), blue (e) and red (f) zones during one day. Temperature was recorded every 10 s and averaged from 2 consecutive days. Deviation between the 2 days was smaller than 0.1 °C (shaded bands). Temperature was constant (with fluctuations of less than approximately ±0.05 °C) during the day and night with average values of about 25.5 °C and about 25.3 °C, respectively. Transitions between lights on and lights off caused a brief temperature change of approximately ±0.5 °C; the temperature stabilized in <30 min, before any change in colour preference started. g, Average daily preference between red zones (fraction of flies shown in red) and dark zones (black) for wild-type flies. The dark zone was obtained by overlaying three colour filters (Rosco no. 27, no. 382 and no. 389). Shaded bands show s.d. between four consecutive days. Twenty flies were used. h, Distribution of flies across colour zones in 12 h:12 h light–dark conditions, followed by constant darkness (n = 24 flies). Green, blue and red lines show the fractions of flies in green, blue and red zones, respectively. g, h, Black and white horizontal bars indicate dark and light conditions, respectively.
Extended Data Fig. 2 Experimental setup with tubes in filters.
a, Typical spatial arrangement of tubes (each approximately 6-cm long and 5-mm wide) in an experiment with three colour filters (green, blue and red) and two colour filters (green and red). n = 132 flies. b, Example frames of automatic tracking of flies in tubes with filters (right) and without filters (left). c, Background subtraction was applied after stabilizing video. d, Flies (green objects) were detected following digital filtering and noise elimination. e, Example of 12 randomly picked single fly trajectories, moving between green (G), blue (B) and red (R) zones over 24 h. Fly positions were determined every minute but data shown are 5 min apart for clarity. f, Population-averaged colour preference of the 12 flies shown in e. As with the individual data, the time interval here is 5 min. g, Same colour preference data as in f averaged over 1-h intervals. h, Sketch of a typical order of filters around tubes, with a total of six possible combinations. i, j, An example of the randomized order of filters (i) and the resulting colour preference data (j). Patterns in colour preference with randomized filter order were identical (within error bars) to that for a typical arrangement of filters. This indicates that the positions of colour filters on neighbouring tubes did not affect fly behaviour. j, Green, blue and red lines show fractions of flies in the corresponding coloured zones; shaded areas show s.d. between five consecutive days (n = 18 flies). Black and white horizontal bars indicate dark and light parts of light–dark cycle.
Extended Data Fig. 3 Colour preference in additional wild-type genotypes.
a, Typical fly locations during green peaks and midday change in green/red (dim) preference. The coloured rectangles of corresponding filters overlay the video frames. The single-day colour preference is the average of a six-day experiment with s.d. between days shown in bands (n = 24 flies). i–iii show single greyscale frames with indicated colour filters during the first green preference peak, the midday green/red switch and the second green peak. b, Preference among three zones for each genotype during one day. Solid lines green, blue and red lines show average fractions of flies in corresponding coloured zones; shaded areas show s.d. a, b, Black and white bars indicate dark and light parts of light–dark cycle. c, Comparison of blue avoidance between different genotypes. The avoidance index was calculated (Methods) for each hour from ZT2 to ZT10, then averaged. d, Preference between green and dim light near the time of the first green peak (ZT2) and midday (ZT6). Preference for green is shown as positive and preference for dim light as negative. c, d, Data are means ± s.d. between independent experiments; n is number of independent experiments (n in d is the same as in c). *P < 0.05, **P < 0.01, ***P < 0.001; two-sided Fisher’s exact test. For the number of flies and statistical details, see Supplementary Tables 1, 2.
Extended Data Fig. 4 Colour preference behaviour is driven by colour and not light intensity.
a, Colour preference with different blue light intensities. Top, average daily preference between green, red (dim) and blue light with 100%, 50%, 25% or 10% of maximum (572 μW cm−2) intensity through a blue filter. Bottom, average blue avoidance at different intensities of blue light. b, Colour preference with reduced intensity of white light. Top, average daily preference between green, red (dim) and blue light with different intensities of white light incident on the filters. Incident intensities are indicated above each plot. Bottom, average blue avoidance with different intensities of white light. The average intensity of green, red and blue light transmitted through the filters is shown on the top x-axis. a, b, Coloured lines show the average fraction of flies in corresponding coloured zones; s.d. between five consecutive days shown as shading (top) and error bars (bottom; n = 24 flies for each experiment). Black and white bars indicate dark and light parts of light–dark cycle. The avoidance index was calculated (Methods) for each hour from ZT2 to ZT10, then averaged. c–f, Preference in a two-choice assay with green or white light of different intensities. c, Average daily preference between 2 intensities of green light with the second option being 1, 0.5, 0.25, 0.1 or 0.04 of the intensity of the first. For comparison, the last column shows typical response in a similar assay, but with green and dim lights as the two choices. d, Average daily preference between bright (about 662 μW cm−2) and dim (about 138 μW cm−2) white light. The intensity of the bright option is similar to that of light transmitted through the green filter in the colour choice assays. e, Average preference for green light of the higher intensity in the first and last 3 h of the day. Red line with shaded zone shows preference and s.d. for green in green/red assay. The abscissa shows the ratio of the two intensities in logarithmic scale. f, Change in preference for green of higher intensity during first and last 3 h of the day (M and E) and at ZT6 (midday). c–f, Data are means ± s.d. between three consecutive days (n = 18 flies for each experiment). All experiments were repeated twice with similar results.
Extended Data Fig. 5 Flies tend to stay near food in the middle of the day.
a–f, Examples of fly positions during ZT3.5 to ZT8.5, with food at the bottom of each tube. Flies tend to stay near food when a green or red filter is nearest to the food (a, c, e, f). When a blue filter is nearest to the food (b, d), flies enter the blue zone only briefly to eat. Ordinates represent the coordinates along the tube length. Horizontal orange lines represent the edges of the colour zones.
Extended Data Fig. 6 Locomotor activity of wild-type flies and flies with various genetic defects.
a, Average activity of flies in tubes with colour filters (black line) and without filters (orange line) under 12 h:12 h light-dark. Light source was set at 2,000 lx for tubes without filters. This was comparable to the irradiance of light transmitted through the filters in the colour preference experiments (Methods). The morning (M) and evening (E) bursts of activity are similar in the two experiments. However, the level of activity during the middle of the day is substantially lower in tubes with filters (n = 18 flies for each experiment). b–i, Average activities of tested genotypes with abnormal colour preference (n shows number of flies). All flies except gl60j (c) and circadian-clock mutants (d) show well-defined morning and evening bursts. The activity peaks seen in per0 and tim0 data (d) are not anticipatory morning and evening peaks, but instead are startle responses to lights turning on or off. Average activity was calculated as the distance moved by individual flies between consecutive video frames (captured at 1 frame per s) and binned over 3-min intervals. Black and white bars indicate dark and light portions of light–dark cycle.
Extended Data Fig. 7 Colour preference in additional genotypes.
a–o, Preference of each genotype for three colours during one day. Average fraction of flies (solid lines) with associated s.d. (shaded bands) in green, blue and red zones shown in the corresponding colour, calculated from n independent experiments. Twelve, 18 or 24 flies were used for each experiment. Black and white bars indicate dark and light parts of light–dark cycle. A white-eyed strain (w1118) showed a modified green preference pattern (h). Flies with eliminated gustatory receptor 28b (gr28b, i) or TRPL channel (trplMB10553, j) are on this white-eyed background and showed colour preference patterns closely resembling that of their control (h). For the number of flies and statistical details, see Supplementary Table 2.
Extended Data Fig. 8 Colour preference in additional genotypes and two-choice assays.
a, b, Preference of each genotype for three colours during one day. Average fraction of flies (solid lines) with associated s.d. (shaded bands) in green, blue and red zones shown in the corresponding colour. Black and white bars indicate the dark and light parts of the light–dark cycle. c, Silencing of small ventral lateral neurons (s-LNvs) leads to loss of switch in green/dim preference. Comparison of colour preference of flies with silenced large ventral lateral neurons (l-LNvs) (C929-Gal4), both large and small ventral lateral neurons (pdf-Gal4), and posterior dorsal neurons 1 (DN1ps) (Clk4.1-Gal4) (right) and corresponding control flies (left). Targeted neurons are pictured on the right. Each experiment was repeated twice with similar results. d, Average daily preference between green and dim lights for each genotype. Average fraction of flies (solid lines) with associated s.d. (shaded bands) in green, blue and red zones shown in the corresponding colours (number of experiments as in f). e, Average daily preference between blue and dim light for each genotype (number of experiments as in g). Apart from the difference in colour choices, all experimental details are the same as in d. f, Preference between green and dim light at around ZT2 and ZT6 (midday). Preference for green shown as positive and preference for dim light as negative. g, Comparison of blue avoidance between different genotypes. The avoidance index was calculated (Methods) for each hour from ZT2 to ZT10, then averaged. a, b, e, g, Data are means ± s.d. between independent experiments; n number of independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001; two-sided Fisher’s exact test. For the number of flies and statistical details, see Supplementary Tables 1–3.
Extended Data Fig. 9 Rh7 expression in adult multidendritic neurons.
a–d, Hemisegment of an adult abdomen with multidendritic neurons labelled with ppk–Gal4;UAS-CD4::GFP (a) and stained with an antibody against Elav (c). b, Merge of a and c. White arrows show multidendritic neurons (dendritic arborization D neuron (ddaD) and ddaC) and dorsal bipolar dendrite (dbd) neuron. Orange arrow shows a dorsal bipolar dendrite neuron dendrite. e–h, Rh7 expression in multidendritic neurons labelled with ppk–Gal4;UAS-CD4::GFP (e) and stained with an antibody against Rh7 (g). f, Merge of e and g. Scale bars, 10 µm. Each experiment was repeated three times independently with similar results.
Extended Data Fig. 10 Correlation between colour preference and activity for wild-type and per mutants.
a, b, Colour preferences and activities of CantonS, perS, perL and per0 flies under 9.5 h:9.5 h (19 h), 12 h:12 h (24 h) and 14.75 h:14.75 h (29.5 h) light–dark cycles. The fraction of flies in the green, blue and red zones is shown in the corresponding colour. Two independent experiments were carried out with similar results, each with 18 or 24 flies of each genotype. Average activity of flies is shown with a black line (b). Activity was calculated from video recordings as the distance moved by a fly between two consecutive frames, binned over 3-min intervals, then normalized. M and E, morning and evening bursts in locomotor activity. c, Timing of the morning (squares) and evening (circles) activity bursts in relation to peaks in green colour preference (green diamonds), under three separate light–dark cycles. The green peaks always appear near the end of the morning activity and the start of the evening activity when the morning and evening bursts occur during the lights-on portion of the cycle. a–c, Grey shaded regions represent lights-off portions of light–dark cycles.
Supplementary information
Supplementary Information
The Supplementary Information file contains additional discussion regarding data in the manuscript. It also contains three tables (Supplementary Tables 1-3) and description of three videos (Supplementary Video ZT4-12, Supplementary Video ZT12-20, Supplementary Video ZT20-4).
Rights and permissions
About this article
Cite this article
Lazopulo, S., Lazopulo, A., Baker, J.D. et al. Daytime colour preference in Drosophila depends on the circadian clock and TRP channels. Nature 574, 108–111 (2019). https://doi.org/10.1038/s41586-019-1571-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-019-1571-y
This article is cited by
-
Optimized design and in vivo application of optogenetically functionalized Drosophila dopamine receptors
Nature Communications (2023)
-
A simple high-throughput method for automated detection of Drosophila melanogaster light-dependent behaviours
BMC Biology (2022)
-
Monochromatic visible lights modulate the timing of pre-adult developmental traits in Drosophila melanogaster
Photochemical & Photobiological Sciences (2022)
-
Timing requires the right amount and type of light
Nature Ecology & Evolution (2021)
-
Color vision in insects: insights from Drosophila
Journal of Comparative Physiology A (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.