Abstract
New antibiotics are needed to combat rising levels of resistance, with new Mycobacterium tuberculosis (Mtb) drugs having the highest priority. However, conventional whole-cell and biochemical antibiotic screens have failed. Here we develop a strategy termed PROSPECT (primary screening of strains to prioritize expanded chemistry and targets), in which we screen compounds against pools of strains depleted of essential bacterial targets. We engineered strains that target 474 essential Mtb genes and screened pools of 100–150 strains against activity-enriched and unbiased compound libraries, probing more than 8.5 million chemical–genetic interactions. Primary screens identified over tenfold more hits than screening wild-type Mtb alone, with chemical–genetic interactions providing immediate, direct target insights. We identified over 40 compounds that target DNA gyrase, the cell wall, tryptophan, folate biosynthesis and RNA polymerase, as well as inhibitors that target EfpA. Chemical optimization yielded EfpA inhibitors with potent wild-type activity, thus demonstrating the ability of PROSPECT to yield inhibitors against targets that would have eluded conventional drug discovery.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Source Data for Figs. 1a, b, 2, 3a, c, e, 4a, 5b and Extended Data Figs. 1b, 2a, c, d, 4a, 5, 7e, 8, 9a, b, c, f, i, l are provided with the online version of the paper. The raw primary screen data, calculated fold changes and P values, and compound annotations, are available online at https://broad.io/cgtb.
Code availability
ConcensusGLM is available at https://doi.org/10.5281/zenodo.3235787 and on GitHub at http://github.com/broadinstitute/concensusGLM. Other computer code is available from the corresponding author upon request.
References
World Health Organization. Antimicrobial Resistance: Global Report on Surveillance 2014. (WHO, 2014).
World Health Organization. Global Tuberculosis Report 2018. (WHO, 2018).
Andries, K. et al. A diarylquinoline drug active on the ATP synthase of Mycobacterium tuberculosis. Science 307, 223–227 (2005).
Matsumoto, M. et al. OPC-67683, a nitro-dihydro-imidazooxazole derivative with promising action against tuberculosis in vitro and in mice. PLoS Med. 3, e466 (2006).
Dheda, K. et al. The epidemiology, pathogenesis, transmission, diagnosis, and management of multidrug-resistant, extensively drug-resistant, and incurable tuberculosis. Lancet Respir. Med. 5, 291–360 (2017).
Christophe, T. et al. High content screening identifies decaprenyl-phosphoribose 2′ epimerase as a target for intracellular antimycobacterial inhibitors. PLoS Pathog. 5, e1000645 (2009).
Grzegorzewicz, A. E. et al. Inhibition of mycolic acid transport across the Mycobacterium tuberculosis plasma membrane. Nat. Chem. Biol. 8, 334–341 (2012).
Stanley, S. A. et al. Diarylcoumarins inhibit mycolic acid biosynthesis and kill Mycobacterium tuberculosis by targeting FadD32. Proc. Natl Acad. Sci. USA 110, 11565–11570 (2013).
Giaever, G. et al. Genomic profiling of drug sensitivities via induced haploinsufficiency. Nat. Genet. 21, 278–283 (1999).
Nelson, J. et al. MOSAIC: a chemical-genetic interaction data repository and web resource for exploring chemical modes of action. Bioinformatics 34, 1251–1252 (2018).
Xu, D. et al. Genome-wide fitness test and mechanism-of-action studies of inhibitory compounds in Candida albicans. PLoS Pathog. 3, e92 (2007).
Wang, J. et al. Platensimycin is a selective FabF inhibitor with potent antibiotic properties. Nature 441, 358–361 (2006).
Evans, J. C. et al. Validation of CoaBC as a bactericidal target in the coenzyme a pathway of Mycobacterium tuberculosis. ACS Infect. Dis. 2, 958–968 (2016).
Abrahams, G. L. et al. Pathway-selective sensitization of Mycobacterium tuberculosis for target-based whole-cell screening. Chem. Biol. 19, 844–854 (2012).
Donald, R. G. et al. A Staphylococcus aureus fitness test platform for mechanism-based profiling of antibacterial compounds. Chem. Biol. 16, 826–836 (2009).
Huber, J. et al. Chemical genetic identification of peptidoglycan inhibitors potentiating carbapenem activity against methicillin-resistant Staphylococcus aureus. Chem. Biol. 16, 837–848 (2009).
Typas, A. et al. High-throughput, quantitative analyses of genetic interactions in E. coli. Nat. Methods 5, 781–787 (2008).
Kim, J. H. et al. Protein inactivation in mycobacteria by controlled proteolysis and its application to deplete the beta subunit of RNA polymerase. Nucleic Acids Res. 39, 2210–2220 (2011).
Kim, J. H. et al. A genetic strategy to identify targets for the development of drugs that prevent bacterial persistence. Proc. Natl Acad. Sci. USA 110, 19095–19100 (2013).
DeJesus, M. A. et al. Comprehensive essentiality analysis of the Mycobacterium tuberculosis genome via saturating transposon mutagenesis. m Bio 8, e02133-16 (2017).
Yu, C. et al. High-throughput identification of genotype-specific cancer vulnerabilities in mixtures of barcoded tumor cell lines. Nat. Biotechnol. 34, 419–423 (2016).
Han, T. X., Xu, X.-Y., Zhang, M.-J., Peng, X. & Du, L.-L. Global fitness profiling of fission yeast deletion strains by barcode sequencing. Genome Biol. 11, R60 (2010).
Smith, A. M. et al. Highly-multiplexed barcode sequencing: an efficient method for parallel analysis of pooled samples. Nucleic Acids Res. 38, e142 (2010).
Smith, A. M. et al. Quantitative phenotyping via deep barcode sequencing. Genome Res. 19, 1836–1842 (2009).
Vilchèze, C. et al. Coresistance to isoniazid and ethionamide maps to mycothiol biosynthetic genes in Mycobacterium bovis. Antimicrob. Agents Chemother. 55, 4422–4423 (2011).
Wellington, S. et al. A small-molecule allosteric inhibitor of Mycobacterium tuberculosis tryptophan synthase. Nat. Chem. Biol. 13, 943–950 (2017).
Moradigaravand, D. et al. dfrA thyA double deletion in para-aminosalicylic acid-resistant Mycobacterium tuberculosis Beijing strains. Antimicrob. Agents Chemother. 60, 3864–3867 (2016).
Tibshirani, R. Regression shrinkage and selection via the lasso. J. Roy. Stat. Soc. B 58, 267 (1994).
Scovill, J., Blank, E., Konnick, M., Nenortas, E. & Shapiro, T. Antitrypanosomal activities of tryptanthrins. Antimicrob. Agents Chemother. 46, 882–883 (2002).
Hwang, J. M. et al. Design, synthesis, and structure–activity relationship studies of tryptanthrins as antitubercular agents. J. Nat. Prod. 76, 354–367 (2013).
Medapi, B., Meda, N., Kulkarni, P., Yogeeswari, P. & Sriram, D. Development of acridine derivatives as selective Mycobacterium tuberculosis DNA gyrase inhibitors. Bioorg. Med. Chem. 24, 877–885 (2016).
Banerjee, A. et al. inhA, a gene encoding a target for isoniazid and ethionamide in Mycobacterium tuberculosis. Science 263, 227–230 (1994).
Yu, S., Girotto, S., Lee, C. & Magliozzo, R. S. Reduced affinity for isoniazid in the S315T mutant of Mycobacterium tuberculosis KatG is a key factor in antibiotic resistance. J. Biol. Chem. 278, 14769–14775 (2003).
Negatu, D. A. et al. Whole-cell screen of fragment library identifies gut microbiota metabolite indole propionic acid as antitubercular. Antimicrob. Agents Chemother. 62, e01571-17 (2018).
Negatu, D. A. et al. Gut microbiota metabolite indole propionic acid targets tryptophan biosynthesis in Mycobacterium tuberculosis. mBio 10, e02781-18 (2019).
Tibshirani, R., Walther, G. & Hastie, T. Estimating the number of clusters in a data set via the gap statistic. J. Roy. Stat. Soc. B 63, 411–423 (2001).
Kool, E. T. Circular DNA vectors for synthesis of RNA and DNA. US patent WO1998038300A1 (2000).
Daubendiek, S. L. & Kool, E. T. Generation of catalytic RNAs by rolling transcription of synthetic DNA nanocircles. Nat. Biotechnol. 15, 273–277 (1997).
Silver, L. L. Challenges of antibacterial discovery. Clin. Microbiol. Rev. 24, 71–109 (2011).
Grant, S. S. et al. Identification of novel inhibitors of nonreplicating Mycobacterium tuberculosis using a carbon starvation model. ACS Chem. Biol. 8, 2224–2234 (2013).
Li, X. Z., Zhang, L. & Nikaido, H. Efflux pump-mediated intrinsic drug resistance in Mycobacterium smegmatis. Antimicrob. Agents Chemother. 48, 2415–2423 (2004).
Li, G. et al. Efflux pump gene expression in multidrug-resistant Mycobacterium tuberculosis clinical isolates. PLoS ONE 10, e0119013 (2015).
Rodrigues, L., Machado, D., Couto, I., Amaral, L. & Viveiros, M. Contribution of efflux activity to isoniazid resistance in the Mycobacterium tuberculosis complex. Infect. Genet. Evol. 12, 695–700 (2012).
Silver, L. L. in Antibacterials Vol. 1 (eds Fisher, J. F. et al.) Ch. 24, 31–67 (Springer, 2017).
Singh, V. et al. The complex mechanism of antimycobacterial action of 5-fluorouracil. Chem. Biol. 22, 63–75 (2015).
Snapper, S. B., Melton, R. E., Mustafa, S., Kieser, T. & Jacobs, W. R. Jr. Isolation and characterization of efficient plasmid transformation mutants of Mycobacterium smegmatis. Mol. Microbiol. 4, 1911–1919 (1990).
Murphy, K. C., Papavinasasundaram, K. & Sassetti, C. M. Mycobacterial recombineering. Methods Mol. Biol. 1285, 177–199 (2015).
Murphy, K. C. et al. ORBIT: a new paradigm for genetic engineering of mycobacterial chromosomes. mBio 9, e01467-18 (2018).
Ehrt, S. et al. Controlling gene expression in mycobacteria with anhydrotetracycline and Tet repressor. Nucleic Acids Res. 33, e21 (2005).
Carey, M. F., Peterson, C. L. & Smale, S. T. PCR-mediated site-directed mutagenesis. Cold Spring Harb. Protoc. 2013, 738–742 (2013).
Wards, B. J. & Collins, D. M. Electroporation at elevated temperatures substantially improves transformation efficiency of slow-growing mycobacteria. FEMS Microbiol. Lett. 145, 101–105 (1996).
Stanley, S. A. et al. Identification of novel inhibitors of M. tuberculosis growth using whole cell based high-throughput screening. ACS Chem. Biol. 7, 1377–1384 (2012).
Reynolds, R. C. et al. High throughput screening of a library based on kinase inhibitor scaffolds against Mycobacterium tuberculosis H37Rv. Tuberculosis (Edinb.) 92, 72–83 (2012).
Ananthan, S. et al. High-throughput screening for inhibitors of Mycobacterium tuberculosis H37Rv. Tuberculosis (Edinb.) 89, 334–353 (2009).
Maddry, J. A. et al. Antituberculosis activity of the molecular libraries screening center network library. Tuberculosis (Edinb.) 89, 354–363 (2009).
Ballell, L. et al. Fueling open-source drug discovery: 177 small-molecule leads against tuberculosis. ChemMedChem 8, 313–321 (2013).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Venables, W. N. & Ripley, B. D. Modern Applied Statistics with S 4th edn (Springer, 2002).
van der Maaten, L. & Hinton, G. Visualizing data using t-SNE. J. Mach. Learn. Res. 9, 2579–2605 (2008).
Murtagh, F. & Legendre, P. Ward’s hierarchical agglomerative clustering method: which algorithms implement Ward’s criterion? J. Classif. 31, 274–295 (2014).
Kanehisa, M., Furumichi, M., Tanabe, M., Sato, Y. & Morishima, K. KEGG: new perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 45, D353–D361 (2017).
Friedman, J., Hastie, T. & Tibshirani, R. Regularization paths for generalized linear models via coordinate descent. J. Stat. Softw. 33, 1–22 (2010).
Vilcheze, C. & Jacobs, W. R. Isolation and analysis of Mycobacterium tuberculosis mycolic acids. Curr. Protoc. Microbiol. 5, 10A.3.1–10A.2.11 (2007).
Cole, S. T. et al. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393, 537–544 (1998).
Gerstung, M. et al. Reliable detection of subclonal single-nucleotide variants in tumour cell populations. Nat. Commun. 3, 811 (2012).
Paixão, L. et al. Fluorometric determination of ethidium bromide efflux kinetics in Escherichia coli. J. Biol. Eng. 3, 18 (2009).
Michaelis, L., Menten, M. L., Johnson, K. A. & Goody, R. S. The original Michaelis constant: translation of the 1913 Michaelis–Menten paper. Biochemistry 50, 8264–8269 (2011).
Nelder, J. A. & Mead, R. A simplex method for function minimization. Comput. J. 7, 308–313 (1965).
Fick, A. Ueber diffusion. Ann. Phys. 170, 59–86 (1855).
Vergalli, J. et al. Spectrofluorimetric quantification of antibiotic drug concentration in bacterial cells for the characterization of translocation across bacterial membranes. Nat. Protocols 13, 1348–1361 (2018).
Acknowledgements
Funding was provided by the Bill and Melinda Gates Foundation, Broad Institute TB Gift Donors and Pershing Square Foundation.
Reviewer information
Nature thanks Terry Roemer and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
The manuscript was written by E.O.J., E.S.L. and D.T.H. Statistical analysis was carried out by E.O.J. Computational pipelines were written by E.O.J. and N. Bandyopadhyay. Experiments were designed as follows. Strain construction: K.M.G., K.C.M., T.R.I., S.E., E.J.R., C.M.S., D.S. Assay development and screening: E.O.J., J.E.G., M.F., D.T.H. Medicinal chemistry: T.K., B.K.H., M.H.S.-W., D.T.H. Mechanism of action follow-up: E.O.J., J.E.G., D.T.H. Experiments were carried out as follows. Strain construction: R.E.A., N. Betancourt, I.D.S., M.G., J.A.M., C.E.M., K.G.P., J.T.P., P.A.P., M.K.P., N.R., N.S., C.T., S.W., J.B.W. Assay development: E.O.J., M.T. Compound screening: E.O.J., E.L., M.S., K.D., J.D., C.G., A.J.G., R.K., R.M.N., M.T., C.W. Mechanism of action follow-up: E.O.J., J.E.G., E.O., E.M., S.K.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Mtb hypomorph strain creation.
a, Hypomorph strains were constructed by introducing a DAS tag at the 3ʹ end of the gene of interest, with concomitant introduction of a 20-nucleotide barcode and an episomally encoded, regulated SspB gene to control the level of protein depletion. b, Degradation of a DAS-tagged target gene product was mediated by SspB, the expression of which was driven by an ATC-inducible TetON promoter. To allow individualized degrees of knockdown for each gene product, a series of TetON promoters with varying strengths was generated. Regulated promoter strength was quantified by fusion to a luciferase gene and measuring luminescence in the presence and absence of the ATC inducer. In the screen, strains containing the TetON-1, -2, -6, -10 and -18 promoters were used for subsequent strain construction. Independent biological replicates (n = 8) are shown as open circles; means are shown as horizontal bars; error bars denote 95% confidence intervals. c, A range of up to five different knockdown levels was attempted using five different promoters for each target gene, allowing the generation of 2,014 hypomorphs. d, Barcoded hypomorph strains were pooled and distributed into 384-well plates containing the compound library and incubated for 14 days. e, Chromosomal strain barcodes were inserted into each engineered hypomorph, thus allowing PCR amplification by an array of primers containing 5′ overhangs encoding screen location (well and plate) barcodes. For census enumeration of pooled strains, PCR products were combined and subjected to Illumina NGS. f, Dose response of trimethoprim, a DHFR inhibitor, against wild-type Mtb and the DHFR hypomorph, showing the hypersensitivity of the hypomorph. Growth was calculated as OD600 normalized to untreated controls. Independent biological replicates (n = 4) are shown as open circles; means are shown as filled circles; error bars denote 95% confidence intervals.
Extended Data Fig. 2 Strain and compound collection characterization.
a, Histogram of growth of the individual hypomorphs in the screening pool over the 14-day duration of the screen. Most hypomorphs were within a tenfold window of growth rates. b, Histogram of hypomorph Zʹ factors between the untreated wells and rifampin control wells of the screening assay for the hypomorphs. All Zʹ factors were greater than 0.5, indicating an excellent screening assay. c, The composition of the bioactive compound library. The right bar shows the broad classes within the segment of known drugs. d, The bioactive compound library was tested up to 50 µM against a GFP-expressing Mtb strain. Most compounds had detectable activity for at least one concentration tested. e, Volcano plot (maximum likelihood mean fold change from n = 2 biologically independent samples against the unadjusted two-sided Wald test P value) of chemical–genetic interactions from the bioactive library. Each point represents a single strain–compound interaction at a single concentration. Some interactions of interest are highlighted: compounds are designated by colour, with wild-type Mtb interactions shown as open circles and hypomorphs interactions of interest shown as solid circles. Most interactions were inhibitory, because the compound library was confirmed to be enriched for antitubercular activity.
Extended Data Fig. 3 Feature weights of Lasso classifiers.
a, Feature weights of the hypomorphs used by the Lasso binary classifier to predict DNA gyrase inhibitors. We trained on the fluoroquinolones, with the GyrA hypomorph being the most predictive strain. Features are denoted as the target gene of the hypomorph and compound concentration separated by an underscore. b, As in a for the Lasso binary classifier to predict inhibitors of mycolic acid biosynthesis. We trained on known InhA inhibitors, with the MshC hypomorph being a prominent discriminator. c, As in a for the Lasso binary classifier to predict inhibitors of folate biosynthesis. We trained on the sulfonamides, with the TrpG hypomorph being a prominent discriminator. d, As in a but trained on chemical–genetic interaction profiles from the sulfonamides and confirmed new folate biosynthesis inhibitors. e, As in a but trained on chemical–genetic interaction profiles of the newly identified inhibitors of tryptophan biosynthesis.
Extended Data Fig. 4 Validation of predicted DNA gyrase inhibitors.
a, Actual compound performance of predicted DNA gyrase inhibitors in an agarose gel-based in vitro assay of DNA gyrase supercoiling inhibition. The ratio of imaged pixel intensities (Supplementary Fig. 1a–l) for supercoiled and relaxed bands was indicative of inhibition, shown by the ciprofloxacin control. Eight of the twenty-seven compounds with the greatest effect sizes that showed statistically significant (P < 0.05, two-sided Wald test) inhibition are shown. Open circles show the independent samples (n = 23 for ciprofloxacin and novobiocin, n = 2 for all other conditions); filled circles indicate the mean pixel intensity ratio; error bars denote the 95% confidence interval of the mean. b, Agarose gel showing increasing inhibition of Mtb DNA gyrase supercoiling activity with increasing tryptanthrin concentration. DNA gyrase catalyses supercoiling of pBR322; inhibitors prevent the accumulation of supercoiled gel bands. This experiment was repeated independently once with similar results. The uncropped version is shown in Supplementary Fig. 1m.
Extended Data Fig. 5 Validation of predicted inhibitors of mycolic acid biosynthesis.
Actual performance of predicted inhibitors of mycolic acid biosynthesis in an in vitro assay of inhibition of incorporation of14C-acetic acid into mycolic acid. The ratio of imaged pixel intensities for fatty acid methyl ester (FAME) and mycolic acid methyl ester (MAME) bands was indicative of inhibition activity, as shown by the isoniazid and ethionamide controls. Open circles show the independent biological replicates (n = 5 for vehicle control, n = 4 for BRD-1728 and BRD-7564, n = 3 for BRD-4384 and BRD-9942, n = 1 for ethionamide control, and n = 2 for all other conditions); filled circles indicate the mean pixel intensity ratio; error bars denote the 95% confidence interval of the mean (statistical significance determined by P < 0.05, two-sided Wald test).
Extended Data Fig. 6 New classes of inhibitors of folate and tryptophan biosynthesis.
a, Schematic of the folate and tryptophan biosynthesis pathways. TrpG is an amphibolic enzyme, upstream of both PABA and tryptophan. Biosynthetic enzymes mentioned in the text are indicated in their metabolic context. 3-IGP, 3-indoleglycerol phosphate; ADC, 4-amino-4-deoxychorismate; DHF, dihydrofolate; DHFR, dihydrofolate reductase; DHFS, dihydrofolate synthase; DHP, dihydropteroate; DHP-PAS: adduct of DHP and PAS; DHPS, dihydropteroate synthase; HMDP-P2, 6-hydroxymethyl-7,8-dihydropterin diphosphate; MTX: methotrexate; PABA, para-amino-benzoic acid; PAS, para-aminosalicylic acid; Sulfa drugs, sulfonamide antibiotics. b, Dose–response curves of known inhibitors of folate biosynthesis and the validated tryptophan biosynthesis inhibitor scaffold, 3-indole propionic acid, supplemented with PABA, folic acid or tryptophan. Chemical structures of the known inhibitors are shown. Independent biological replicates (n = 4) are shown as open circles; means are shown as filled circles; error bars show 95% confidence intervals. c, Actual performance of predicted inhibitors of folate biosynthesis in a metabolite rescue assay. Mtb was treated with predicted inhibitors in the presence or absence of tryptophan, folate or PABA. The effect of BRD-7721, a 3-indole propionic acid ester, is abolished by supplementation with tryptophan, indicating it is an inhibitor of tryptophan biosynthesis. By contrast, the effect of the nitrothiophene BRD-2550 is abolished by folate and PABA, and that of BRD-8884 is abolished by folate alone, showing that they are inhibitors of folate biosynthesis with distinct mechanisms. Independent biological replicates (n = 4) are shown as open circles; means are shown as filled circles; error bars denote the 95% confidence intervals. d, Chemical structures of predicted and subsequently validated inhibitors of folate biosynthesis, including the nitrothiophenes and para-aminosalicylic acid derivative BRD-9819.
Extended Data Fig. 7 Performance of a large, unbiased compound library.
a, ROC curve showing that primary data were predictive of activity in a confirmatory secondary growth assay (n = 4 biologically independent experiments). We retested 454 compound–strain interactions using a resazurin, growth-based colorimetric assay. Taking 75% inhibition in the secondary assay as the ground truth, we demonstrated the primary assay as predictive of real activity that could be detected by conventional methods. Using 50% and 90% inhibition as the ground-truth assumption, the ROC AUC values were 0.61 and 0.69. b, Volcano plot (maximum likelihood mean fold change from n = 2 biologically independent samples against the unadjusted two-sided Wald test P value) of chemical–genetic interactions from the larger unbiased chemical library. Each point represents a single strain–compound interaction at a single concentration. c, Compounds in the library of bioactive compounds generally hit more strains than compounds in the unbiased library. Empirical cumulative distribution functions of number of hypomorphs hit by compounds in the two screens is plotted. Shown by the dotted lines, 36% of compounds in the bioactive library and 75% of compounds in the larger library hit 10 strains or fewer, suggesting that activity detected in the larger screen was generally more hypomorph-specific. d, Clustering of chemical–genetic interaction profiles. The number of chemical–genetic interaction profile clusters in the two libraries was determined by finding the minimum Gap statistic, a measure of within-cluster similarity compared to clustering at random. The minimum, denoted by the dotted lines, shows the estimate of the true number of clusters in the unbiased and bioactive libraries, with the unbiased library containing many more unique chemical-genetic clusters. e, Chemical–genetic clusters (n = 1,864) are enriched for chemically similar compounds in the unbiased library. The y axis shows the frequency of chemical–genetic clusters with a particular Tanimoto WSS Z score (x axis), which is an indicator of in-cluster chemical similarity. A total of 221 (12%) of the clusters have meaningful structure–activity relationships—that is, compounds within a cluster have significantly greater chemical similarities than by chance—as indicated by a one-sided permutation test (unadjusted P < 0.05, 10,000 permutations). f, Actual compound performance of predicted inhibitors of folate biosynthesis in a metabolite rescue assay. Mtb was treated with predicted inhibitors in the presence or absence of folate or PABA. The effect of BRD-1242 is abolished by PABA alone, and the effects of BRD-4308 and BRD-9309 are abolished by folate alone, suggesting that they are inhibitors of folate biosynthesis with distinct mechanisms. Independent biological replicates (n = 3) are shown as open circles; means are shown as filled circles; error bars denote 95% confidence intervals.
Extended Data Fig. 8 Validation of predicted RNAP inhibitors.
Actual compound performance of predicted RNAP inhibitors in an in vitro assay for inhibition of RNA synthesis by E. coli RNAP. Three compounds that showed statistically significant inhibition are shown with a rifampin control (P < 0.05, two-tailed Wald test). Open circles show the independent replicates (n = 36 for vehicle control, n = 4 for BRD-6652, n = 8 for BRD-8565 and rifampin, n = 12 for actinomycin D), filled circles indicate the mean ratio of treatment-to-vehicle fluorescence, and error bars show the 95% confidence interval of the mean. Act. D, actinomycin D.
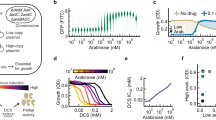
Extended Data Fig. 9 Inhibitors of EfpA, in M. tuberculosis.
a, Dose–response analysis of BRD-8000 on the growth of wild-type Mtb, the EfpA hypomorph, and a mutant overexpressing EfpA (pUV15::efpA), demonstrating hypersensitivity of the hypomorph. Independent biological replicates (n = 4) are shown as open circles; means are shown as filled circles; error bars denote 95% confidence intervals. b, BRD-8000.2 is bactericidal as demonstrated by reducing colony-forming units over time. Independent biological replicates (n = 8) are shown as open circles; means are shown as filled circles; error bars denote 95% confidence intervals. c, Toxicity and bioavailability measurements of BRD-8000.2 and BRD-8000.3. hERG, human ether a-go-go related gene. d, Cytochrome P450 (CYP) inhibition measurements of BRD-8000.2 and BRD-8000.3. e, Schematic of the EtBr efflux assay. Bacteria were loaded with EtBr and its efflux was monitored by change in fluorescence. f, Example kinetic time courses of EtBr fluorescence decay for Msm with BRD-8000.3. The concentration of EtBr used for pre-incubation is indicated in colour, with two different inhibitor concentrations shown. Numerically integrated Michaelis–Menten best-fit time courses are shown in red. Experiments were repeated independently once with similar results. g, Kinetic time course of EtBr fluorescence decay for Mtb. Numerically integrated Michaelis–Menten best-fit time courses are shown in red. The table shows best-fit Michaelis–Menten parameters and Fick’s diffusion constant for wild-type Mtb, an EfpA-overexpressor (pUV15::efpA), and the BRD-8000 resistant mutant (efpAV319F). Although the in vivo apparent maximal efflux rate (Vmax) of the EfpA overexpressor is higher than the wild-type Mtb, that of the BRD-8000 resistant mutant is not, indicating that the resistant mutant is not hyperactive for efflux. Experiments were repeated independently once with similar results. h, Dose–response analysis of isoniazid against wild-type Mtb and the BRD-8000 resistant mutant (efpAV319F). Because isoniazid is a substrate of EfpA, no shift in the MIC90 value for isoniazid with the BRD-8000 resistant mutant indicates that EfpA(V319F) is not hyperactive for efflux. Mean growth (n = 4 biologically independent replicates) is shown, with error bars indicating 95% confidence intervals. i, Results of an assay for intracellular accumulation of BRD-8000.3 in MsmΔefpA complemented with either Mtb wild-type efpA or efpAV319F. Fluorescence of BRD-8000.3 in bacterial lysates was measured, and lysate background fluorescence was subtracted. Although verapamil, a general efflux pump inhibitor, caused statistically significant intracellular accumulation of BRD-8000.3, there was no significant difference in accumulation between the different strains in the absence of verapamil, indicating that EfpA(V319F) is not hyperactive for efflux, and that BRD-8000.3 is not a substrate of EfpA. Independent biological replicates (n = 9 for wild-type control without verapamil; n = 3 for other conditions) are shown as open circles; means are shown as filled circles; error bars denote s.e.m. j, Additional compounds predicted and validated to be efflux inhibitors. k, Dose–response analysis of kinetic time courses of EtBr fluorescence decay for compounds in h. Increasing inhibitor concentration is denoted by colour. Experiments were repeated independently once with similar results. l, Example kinetic time courses of EtBr fluorescence decay for Msm at one concentration (6 µM) of BRD-9327. Numerically integrated Michaelis–Menten best-fit time courses are shown in red. The table shows global best-fit kinetic inhibition parameters across ten concentrations for this inhibitor. Experiments were repeated independently once with similar results.
Supplementary information
41586_2019_1315_MOESM1_ESM.pdf
Supplementary Information Supplementary Notes 1-8: Additional detail on statistical methods and chemical characterization.
Supplementary Figure 1 Agarose gel electrophoresis for
in vitro DNA gyrase assay. a-l, Gel images used to validate predicted DNA gyrase inhibitors using an in vitro assay of DNA gyrase supercoiling and decatenation activity. Analysis of supercoiling band intensity is presented in Extended Data Fig. 4a. i, was rotated to be square. Detailed lane annotations are in Supplementary Table 3. m, Uncropped agarose gel image for the dose response of tryptanthrin inhibition of DNA gyrase supercoiling and decatenation activity. The white box indicates the cropped region used in Extended Data Fig. 4b.
Supplementary Data 1
Strains and genotypes used in chemical genetic screens.
Supplementary Data 2
Ground truth reference set of compounds used for training machine learning models for mechanism of action prediction.
Supplementary Data 3
Annotation of Supplementary Figure 1.
Source data
Rights and permissions
About this article
Cite this article
Johnson, E.O., LaVerriere, E., Office, E. et al. Large-scale chemical–genetics yields new M. tuberculosis inhibitor classes. Nature 571, 72–78 (2019). https://doi.org/10.1038/s41586-019-1315-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-019-1315-z
This article is cited by
-
Antimicrobial resistance crisis: could artificial intelligence be the solution?
Military Medical Research (2024)
-
ABT-MPNN: an atom-bond transformer-based message-passing neural network for molecular property prediction
Journal of Cheminformatics (2023)
-
Multivariate chemogenomic screening prioritizes new macrofilaricidal leads
Communications Biology (2023)
-
Deep clustering of small molecules at large-scale via variational autoencoder embedding and K-means
BMC Bioinformatics (2022)
-
Deep learning-driven prediction of drug mechanism of action from large-scale chemical-genetic interaction profiles
Journal of Cheminformatics (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.