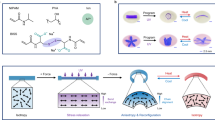
Abstract
Modern robots lack the multifunctional interconnected systems found in living organisms and are consequently unable to reproduce their efficiency and autonomy. Energy-storage systems are among the most crucial limitations to robot autonomy, but their size, weight, material and design constraints can be re-examined in the context of multifunctional, bio-inspired applications. Here we present a synthetic energy-dense circulatory system embedded in an untethered, aquatic soft robot. Modelled after redox flow batteries, this synthetic vascular system combines the functions of hydraulic force transmission, actuation and energy storage into a single integrated design that geometrically increases the energy density of the robot to enable operation for long durations (up to 36 hours). The fabrication techniques and flexible materials used in its construction enable the vascular system to be created with complex form factors that continuously deform with the robot’s movement. This use of electrochemical energy storage in hydraulic fluids could facilitate increased energy density, autonomy, efficiency and multifunctionality in future robot designs.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Code availability
The Arduino code used to control the pumps within the robot is available from the corresponding author upon reasonable request.
Data availability
The datasets generated and analysed during this study are available from the corresponding author upon reasonable request.
References
Silverthorn, D. U. Human Physiology: An Integrated Approach 7th edn (Pearson, 2015).
Kim, T. H., Lee, S. J. & Choi, W. Design and control of the phase shift full bridge converter for the on-board battery charger of electric forklifts. J. Power Electron. 12, 113–119 (2012).
Holness, A. E., Perez-Rosado, A., Bruck, H. A., Peckerar, M. & Gupta, S. K. in Challenges in Mechanics of Time Dependent Materials (eds Antoun, B. et al.) Vol. 2, 155–162 (Springer, 2017).
Liu, P., Sherman, E. & Jacobsen, A. Design and fabrication of multifunctional structural batteries. J. Power Sources 189, 646–650 (2009).
Snyder, J. F., Carter, R. H. & Wetzel, E. D. Electrochemical and mechanical behavior in mechanically robust solid polymer electrolytes for use in multifunctional structural batteries. Chem. Mater. 19, 3793–3801 (2007).
Aglietti, G. S., Schwingshackl, C. W. & Roberts, S. C. Multifunctional structure technologies for satellite applications. Shock Vib. Dig. 39, 381–391 (2007).
Thomas, J. P. & Qidwai, M. A. The design and application of multifunctional structure–battery materials systems. JOM 57, 18–24 (2005).
Soloveichik, G. L. Flow batteries: current status and trends. Chem. Rev. 115, 11533–11558 (2015).
Weber, A. Z. et al. Redox flow batteries: a review. J. Appl. Electrochem. 41, 1137–1164 (2011).
Weng, G.-M., Li, Z., Cong, G., Zhou, Y. & Lu, Y.-C. Unlocking the capacity of iodide for high-energy-density zinc/polyiodide and lithium/polyiodide redox flow batteries. Energy Environ. Sci. 10, 735–741 (2017).
Li, B. et al. Ambipolar zinc–polyiodide electrolyte for a high-energy density aqueous redox flow battery. Nat. Commun. 6, 6303 (2015).
Janoschka, T. et al. An aqueous, polymer-based redox-flow battery using non-corrosive, safe, and low-cost materials. Nature 527, 78–81 (2015).
Duduta, M. et al. Semi-solid lithium rechargeable flow battery. Adv. Energy Mater. 1, 511–516 (2011).
Ponce de León, C., Frías-Ferrer, A., González-García, J., Szánto, D. A. & Walsh, F. C. Redox flow cells for energy conversion. J. Power Sources 160, 716–732 (2006).
Dunn, B., Kamath, H. & Tarascon, J. Electrical energy storage for the grid: a battery of choices. Science 334, 928–935 (2011).
Skyllas-Kazacos, M., Chakrabarti, M. H., Hajimolana, S. A., Mjalli, F. S. & Saleem, M. Progress in flow battery research and development. J. Electrochem. Soc. 158, R55–R59 (2011).
Doughty, D. H., Butler, P. C., Akhil, A. A., Clark, N. H. & Boyes, J. D. Stationary electrical energy storage. Electrochem. Soc. Interface 19, 49–53 (2010).
Lönnstedt, O. M., Ferrari, M. C. O. & Chivers, D. P. Lionfish predators use flared fin displays to initiate cooperative hunting. Biol. Lett. 10 20140281 (2014).
Mohamed, M. R., Sharkh, S. M. & Walsh, F. C. Redox flow batteries for hybrid electric vehicles: progress and challenges. In 2009 IEEE Vehicle Power and Propulsion Conf. 551–557 (IEEE, 2010).
Huskinson, B. et al. A metal-free organic–inorganic aqueous flow battery. Nature 505, 195–198 (2014).
Yang, Z. et al. Electrochemical energy storage for green grid. Chem. Rev. 111, 3577–3613 (2011).
Xia, Y. & Whitesides, G. M. Soft lithography. Angew. Chem. Int. Ed. 37, 550–575 (1998).
Shepherd, R. F. et al. Multigait soft robot. Proc. Natl Acad. Sci. USA 108, 20400–20403 (2011).
Sfakiotakis, M., Lane, D. M. & Davies, J. B. C. Review of fish swimming modes for aquatic locomotion. J. Oceanic Eng. 24, 237–252 (1999).
Lauder, G. V. & Tytell, E. D. Hydrodynamics of undulatory propulsion. Fish Physiol. 23, 425–468 (2005).
Summers, A. P. & Long, J. H. Skin and bones, sinew and gristle: the mechanical behavior of fish skeletal tissues. Fish Physiol. 23, 141–177 (2005).
Long, J. H., Hale, M. E., McHenry, M. J. & Westneat, M. W. Functions of fish skin: flexural stiffness and steady swimming of longnose gar Lepisosteus osseus. J. Exp. Biol. 199, 2139–2151 (1996).
Xie, C., Zhang, H., Xu, W., Wang, W. & Li, X. A long cycle life, self-healing zinc–iodine flow battery with high power density. Angew. Chem. Int. Ed. 57, 11171–11176 (2018).
Wehner, M. et al. An integrated design and fabrication strategy for entirely soft, autonomous robots. Nature 536, 451–455 (2016).
Shepherd, R. F. et al. Using explosions to power a soft robot. Angew. Chem. Int. Ed. 52, 2892–2896 (2013).
Katzschmann, R. K., DelPreto, J., MacCurdy, R. & Rus, D. Exploration of underwater life with an acoustically controlled soft robotic fish. Sci. Robot. 3, eaar3449 (2018).
Christianson, C., Goldberg, N. N., Deheyn, D. D., Cai, S. & Tolley, M. T. Translucent soft robots driven by frameless fluid electrode dielectric elastomer actuators. Sci. Robot. 3, eaat1893 (2018).
Li, T. et al. Fast-moving soft electronic fish. Sci. Adv. 3, e1602045 (2017).
Katzschmann, R. K., Marchese, A. D. & Rus, D. in Experimental Robotics Vol. 109 (eds Hsieg, M. A. et al.) 405–420 (Springer, 2016).
Marchese, A. D., Onal, C. D. & Rus, D. Autonomous soft robotic fish capable of escape maneuvers using fluidic elastomer actuators. Soft Robot. 1, 75–87 (2014).
Jusufi, A., Vogt, D. M., Wood, R. J. & Lauder, G. V. Undulatory swimming performance and body stiffness modulation in a soft robotic fish-inspired physical model. Soft Robot. 4, 202–210 (2017).
Yu, J., Wang, K., Tan, M. & Zhang, J. Design and control of an embedded vision guided robotic fish with multiple control surfaces. Sci. World J. 2014, 631296 (2014).
Donatelli, C. M. et al. Prototype of a fish inspired swimming silk robot. In 2018 IEEE Int. Conf. on Soft Robotics (RoboSoft) 60–65 (IEEE, 2018).
Suzumori, K., Endo, S., Kanda, T., Kato, N. & Suzuki, H. A bending pneumatic rubber actuator realizing soft-bodied manta swimming robot. In Proc. 2007 IEEE Int. Conf. on Robotics and Automation 4975–4980 (IEEE, 2007).
Valdivia y Alvarado, P. & Youcef-Toumi, K. in Robot Fish: Bio-inspired Fishlike Underwater Robots (eds Du, R. et al.) 161–191 (Springer, 2015).
Winsberg, J. et al. Poly(TEMPO)/zinc hybrid-flow battery: a novel, “green” high voltage, and safe energy storage system. Adv. Mater. 28, 2238–2243 (2016).
Perry, M. L., Darling, R. M. & Zaffou, R. High power density redox flow battery cells. ECS Trans. 53, 7–16 (2013).
Davies, T. & Tummino, J. High-performance vanadium redox flow batteries with graphite felt electrodes. J. Carbon Res. 4, 8 (2018).
Aaron, D. S. et al. Dramatic performance gains in vanadium redox flow batteries through modified cell architecture. J. Power Sources 206, 450–453 (2012).
Liu, Q. H. et al. High performance vanadium redox flow batteries with optimized electrode configuration and membrane selection. J. Electrochem. Soc. 159, A1246–A1252 (2012).
Acknowledgements
This work was supported by the US Office of Naval Research, grant number N00014-17-1-2837.
Reviewer information
Nature thanks Herbert Shea and Wei Wang for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
R.F.S. and J.H.P. conceived the idea of the study. C.A.A., S.C., J.H.P. and R.J. planned and carried out experiments. L.A.A., J.H.P. and R.F.S. supervised the project. All authors discussed the results and contributed to the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Replica moulding of robot parts.
Left, one half of the robot’s silicone is cast in a 3D-printed mould. Right, the finished part. A two-part mould is used in the fabrication process to generate the pleats and fluidic channels of the final robot.
Extended Data Fig. 2 The effects of design alternatives on the energy density of the synthetic vascular system.
a, Figure 4f is reproduced here for comparative purposes, showing the impact of the volume fraction and energy density of the hydraulic fluid on the overall increase in system energy density. b, The effects of ZnI2 catholyte concentration on the overall increase in system energy density for our robot. The estimated energy density values at certain concentrations (solid red circles) are shown11.
Extended Data Fig. 3 Cell capacity and cycling efficiency data for the pelvic fin cell.
A pelvic fin cell, filled with 5 ml of ZnI2 catholyte, was subjected to n = 19 charging and discharging cycles to examine how cell capacity, coulombic efficiency and battery longevity are affected by reuse and recharging of the battery cells.
Extended Data Fig. 4 Buckling test for fluid-filled battery actuator cell.
Water, serving as a substitute for the ZnI2 catholyte solution, was injected into the battery cell composite depicted in Fig. 3 to examine how inclusion of incompressible fluids affected the bending stiffness of the flow battery cells during buckling testing (n = 8). The maximum buckling force and the bending stiffness increased by approximately 10% compared to the cell tested without fluid. Error bars show 1σ uncertainties.
Supplementary information
Video 1
Tail fin actuation. Tail fin actuation is demonstrated while the robot is submerged in a salt water tank. This actuation is initiated by the reversible pumping of ZnI2 catholyte solution from one side of the tail to the other.
Video 2
Pectoral fin actuation. Pectoral fin actuation is demonstrated while the robot is submerged in a salt water tank. This actuation is initiated when catholyte solution contained in the left and right dorsal fins is pumped into the corresponding pectoral fin, thereby inflating them and pushing them outward from the body.
Video 3
Underwater locomotion. The robot swims in a salt water tank via tail fin actuation. The robot moves at a rate of 1.56 body lengths per minute against a current.
Rights and permissions
About this article
Cite this article
Aubin, C.A., Choudhury, S., Jerch, R. et al. Electrolytic vascular systems for energy-dense robots. Nature 571, 51–57 (2019). https://doi.org/10.1038/s41586-019-1313-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-019-1313-1
This article is cited by
-
Robot, repair thyself: laying the foundations for self-healing machines
Nature (2024)
-
Inherently integrated microfiber-based flexible proprioceptive sensor for feedback-controlled soft actuators
npj Flexible Electronics (2024)
-
Self-sufficient metal–air batteries for autonomous systems
Nature Chemical Engineering (2024)
-
Bioinspired soft robots for deep-sea exploration
Nature Communications (2023)
-
Pleobot: a modular robotic solution for metachronal swimming
Scientific Reports (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.