Abstract
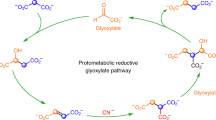
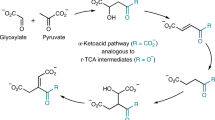
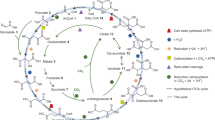
Life builds its molecules from carbon dioxide (CO2) and breaks them back down again through the intermediacy of just five metabolites, which are the universal hubs of biochemistry1. However, it is unclear how core biological metabolism began and why it uses the intermediates, reactions and pathways that it does. Here we describe a purely chemical reaction network promoted by ferrous iron, in which aqueous pyruvate and glyoxylate—two products of abiotic CO2 reduction2,3,4—build up 9 of the 11 intermediates of the biological Krebs (or tricarboxylic acid) cycle, including all 5 universal metabolic precursors. The intermediates simultaneously break down to CO2 in a life-like regime that resembles biological anabolism and catabolism5. Adding hydroxylamine6,7,8 and metallic iron into the system produces four biological amino acids in a manner that parallels biosynthesis. The observed network overlaps substantially with the Krebs and glyoxylate cycles9,10, and may represent a prebiotic precursor to these core metabolic pathways.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Change history
17 April 2021
A Correction to this paper has been published: https://doi.org/10.1038/s41586-021-03383-9
References
Braakman, R. & Smith, E. The compositional and evolutionary logic of metabolism. Phys. Biol. 10, 011001 (2013).
Varma, S. J., Muchowska, K. B., Chatelain, P. & Moran, J. Native iron reduces CO2 to intermediates and end-products of the acetyl-CoA pathway. Nat. Ecol. Evol. 2, 1019–1024 (2018).
Eggins, B. R., Brown, E. M., McNeill, E. A. & Grimshaw, J. Carbon dioxide fixation by electrochemical reduction in water to oxalate and glyoxylate. Tetrahedr. Lett. 29, 945–948 (1988).
Marín-Yaseli, M. R., González-Toril, E., Mompeán, C. & Ruiz-Bermejo, M. The role of aqueous aerosols in the “glyoxylate scenario”: an experimental approach. Chemistry 22, 12785–12799 (2016).
Goldford, J. E. & Segrè, D. Modern views of ancient metabolic networks. Curr. Opin. Syst. Biol. 8, 117–124 (2018).
Canfield, D. E., Glazer, A. N. & Falkowski, P. G. The evolution and future of Earth’s nitrogen cycle. Science 330, 192–196 (2010).
Kalson, N.-H., Furman, D. & Zeiri, Y. Cavitation-induced synthesis of biogenic molecules on primordial Earth. ACS Cent. Sci. 3, 1041–1049 (2017).
Sakurai, M. & Yanagawa, H. Prebiotic synthesis of amino acids from formaldehyde and hydroxylamine in a modified sea medium. Orig. Life 14, 171–176 (1984).
Muchowska, K. B. et al. Metals promote sequences of the reverse Krebs cycle. Nat. Ecol. Evol. 1, 1716–1721 (2017).
Zubay, G. The glyoxylate cycle, a possible evolutionary precursor of the TCA cycle. Chemtracts 16, 783–788 (2003).
Ruiz-Mirazo, K., Briones, C. & de la Escosura, A. Prebiotic systems chemistry: new perspectives for the origins of life. Chem. Rev. 114, 285–366 (2014).
Sutherland, J. D. Studies on the origin of life—the end of the beginning. Nat. Rev. Chem. 1, 0012 (2017).
Harrison, S. & Lane, N. Life as a guide to prebiotic nucleotide synthesis. Nat. Commun. 9, 5176 (2018).
Wächtershäuser, G. Evolution of the first metabolic cycles. Proc. Natl Acad. Sci. USA 87, 200–204 (1990).
Smith, E. & Morowitz, H. J. Universality in intermediary metabolism. Proc. Natl Acad. Sci. USA 101, 13168–13173 (2004).
Martin, W. F. & Russell, M. J. On the origin of biochemistry at an alkaline hydrothermal vent. Phil. Trans. R. Soc. Lond. B 362, 1887–1925 (2007).
Hartman, H. Speculations on the origin and evolution of metabolism. J. Mol. Evol. 4, 359–370 (1975).
Camprubi, E., Jordan, S., Vasiliadou, R. & Lane, N. Iron catalysis at the origin of life. IUBMB Life 69, 373–381 (2017).
Roldan, A. et al. Bio-inspired CO2 conversion by iron sulfide catalysts under sustainable conditions. Chem. Commun. 51, 7501–7504 (2015).
Keller, M. A., Kampjut, D., Harrison, S. A. & Ralser, M. Sulfate radicals enable a non-enzymatic Krebs cycle precursor. Nat. Ecol. Evol. 1, 0083 (2017).
Zhang, X. V. & Martin, S. T. Driving parts of Krebs cycle in reverse through mineral photochemistry. J. Am. Chem. Soc. 128, 16032–16033 (2006).
Novikov, Y. & Copley, S. D. Reactivity landscape of pyruvate under simulated hydrothermal vent conditions. Proc. Natl Acad. Sci. USA 110, 13283–13288 (2013).
Goldford, J. E., Hartman, H., Smith, T. F. & Segrè, D. Remnants of an ancient metabolism without phosphate. Cell 168, 1126–1134 (2017).
Coggins, A. & Powner, M. Prebiotic synthesis of phosphoenol pyruvate by α-phosphorylation-controlled triose glycolysis. Nat. Chem. 9, 310–317 (2017).
Springsteen, G., Yerabolu, J., Nelson, J., Rhea, C. & Krishnamurthy, R. Linked cycles of oxidative decarboxylation of glyoxylate as protometabolic analogs of the citric acid cycle. Nat. Commun. 9, 91 (2018).
Keller, M., Turchyn, A. & Ralser, M. Non-enzymatic glycolysis and pentose phosphate pathway-like reactions in a plausible Archean ocean. Mol. Syst. Biol. 10, 725 (2014).
Rouxel, O. J., Bekker, A. & Edwards, K. J. Iron isotope constraints on the Archean and Paleoproterozoic ocean redox state. Science 307, 1088–1091 (2005).
Eschenmoser, A. The search for the chemistry of life’s origin. Tetrahedron 63, 12821–12844 (2007).
Mall, A. et al. Reversibility of citrate synthase allows autotrophic growth of a thermophilic bacterium. Science 359, 563–567 (2018).
Nunoura, T. et al. A primordial and reversible TCA cycle in a facultatively chemolithoautotrophic thermophile. Science 359, 559–563 (2018).
Orgel, L. E. The implausibility of metabolic cycles on the prebiotic Earth. PLoS Biol. 6, e18 (2008).
Vasas, V., Szathmáry, E. & Santos, M. Lack of evolvability in self-sustaining autocatalytic networks constraints metabolism-first scenarios for the origin of life. Proc. Natl Acad. Sci. USA 107, 1470–1475 (2010).
De Duve, C. Blueprint For a Cell: The Nature and Origin of Life (Neil Patterson Publishers, 1991).
Acknowledgements
We thank W. F. Martin and D. Segrè for discussions. This project has received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (grant agreement 639170) and from the French Agence Nationale de la Recherche (ANR) Laboratoires d’Excellence ‘Chemistry of Complex Systems’ (ANR-10-LABX-0026 CSC).
Reviewer information
Nature thanks Eric Smith, Peter Strazewski and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
J.M. supervised the research and the other authors performed the experiments. All authors contributed intellectually throughout the study. J.M. and K.B.M wrote the paper, and K.B.M. and S.J.V. assembled the Supplementary Information.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Screen for transition metal promoters.
These GC chromatograms show the reaction networks that arise from pyruvate and glyoxylate at 70 °C, as promoted by different transition metal ions (qualitative screen).
Extended Data Fig. 2 Calibration lines for carboxylic acids.
Shown are the correlations between the concentrations of aqueous solutions of carboxylic acids (glyoxylic, glycolic, oxalic, malonic, levulinic, mesaconic, and hydroxyketoglutaric plus oxopentenedioic acids) and the measured GC peak area. Error bars correspond to the standard deviation (three independent runs). Orange lines show the 95% confidence bounds computed for second-degree polynomial fits (OriginPro). Calibration lines for glycine, aspartic acid and glutamic acid are shown in Supplementary Fig. 10. Calibration lines for the remaining compounds detected here (pyruvate, malate, fumarate, succinate, α-ketoglutarate, isocitrate, cis-aconitate, tricarballylate and alanine) are identical to those we reported previously for the same analytical set-up9 (Supplementary Table 1).
Supplementary information
Supplementary Information
This file contains Supplementary Materials and Methods, Supplementary References, Supplementary Figures 1-31 and Supplementary Tables 1-5.
Rights and permissions
About this article
Cite this article
Muchowska, K.B., Varma, S.J. & Moran, J. Synthesis and breakdown of universal metabolic precursors promoted by iron. Nature 569, 104–107 (2019). https://doi.org/10.1038/s41586-019-1151-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-019-1151-1
This article is cited by
-
Primitive purine biosynthesis connects ancient geochemistry to modern metabolism
Nature Ecology & Evolution (2024)
-
Hexose phosphorylation for a non-enzymatic glycolysis and pentose phosphate pathway on early Earth
Scientific Reports (2024)
-
Heat flows enrich prebiotic building blocks and enhance their reactivity
Nature (2024)
-
Mechanisms controlling cellular and systemic iron homeostasis
Nature Reviews Molecular Cell Biology (2024)
-
Formation, stabilization and fate of acetaldehyde and higher aldehydes in an autonomously changing prebiotic system emerging from acetylene
Communications Chemistry (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.