Abstract
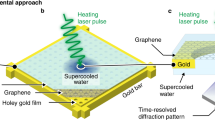
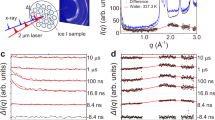
Since Bridgman’s discovery of five solid water (H2O) ice phases1 in 1912, studies on the extraordinary polymorphism of H2O have documented more than seventeen crystalline and several amorphous ice structures2,3, as well as rich metastability and kinetic effects4,5. This unique behaviour is due in part to the geometrical frustration of the weak intermolecular hydrogen bonds and the sizeable quantum motion of the light hydrogen ions (protons). Particularly intriguing is the prediction that H2O becomes superionic6,7,8,9,10,11,12—with liquid-like protons diffusing through the solid lattice of oxygen—when subjected to extreme pressures exceeding 100 gigapascals and high temperatures above 2,000 kelvin. Numerical simulations suggest that the characteristic diffusion of the protons through the empty sites of the oxygen solid lattice (1) gives rise to a surprisingly high ionic conductivity above 100 Siemens per centimetre, that is, almost as high as typical metallic (electronic) conductivity, (2) greatly increases the ice melting temperature7,8,9,10,11,12,13 to several thousand kelvin, and (3) favours new ice structures with a close-packed oxygen lattice13,14,15. Because confining such hot and dense H2O in the laboratory is extremely challenging, experimental data are scarce. Recent optical measurements along the Hugoniot curve (locus of shock states) of water ice VII showed evidence of superionic conduction and thermodynamic signatures for melting16, but did not confirm the microscopic structure of superionic ice. Here we use laser-driven shockwaves to simultaneously compress and heat liquid water samples to 100–400 gigapascals and 2,000–3,000 kelvin. In situ X-ray diffraction measurements show that under these conditions, water solidifies within a few nanoseconds into nanometre-sized ice grains that exhibit unambiguous evidence for the crystalline oxygen lattice of superionic water ice. The X-ray diffraction data also allow us to document the compressibility of ice at these extreme conditions and a temperature- and pressure-induced phase transformation from a body-centred-cubic ice phase (probably ice X) to a novel face-centred-cubic, superionic ice phase, which we name ice XVIII2,17.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Bridgman, P. W. Water, in the liquid and five solid forms, under pressure. Proc. Am. Acad. Arts Sci. 47, 441–558 (1912).
Bartels-Rausch, T. et al. Ice structures, patterns, and processes: a view across the icefields. Rev. Mod. Phys. 84, 885–944 (2012).
Malenkov, G. Liquid water and ices: understanding the structure and physical properties. J. Phys. Condens. Matter 21, 283101 (2009).
Pallares, G. et al. Anomalies in bulk supercooled water at negative pressure. Proc. Natl Acad. Sci. USA 111, 7936–7941 (2014).
Mishima, O. & Stanley, H. E. The relationship between liquid, supercooled and glassy water. Nature 396, 329–335 (1998).
Demontis, P., LeSar, R. & Klein, M. L. New high-pressure phases of ice. Phys. Rev. Lett. 60, 2284–2287 (1988).
Cavazzoni, C. et al. Superionic and metallic states of water and ammonia at giant planet conditions. Science 283, 44–46 (1999).
Mattsson, T. R. & Desjarlais, M. P. Phase diagram and electrical conductivity of high energy-density water from density functional theory. Phys. Rev. Lett. 97, 017801 (2006).
Schwegler, E., Sharma, M., Gygi, F. & Galli, G. Melting of ice under pressure. Proc. Natl Acad. Sci. USA 105, 14779–14783 (2008).
French, M., Mattsson, T., Nettelmann, N. & Redmer, R. Equation of state and phase diagram of water at ultrahigh pressures as in planetary interiors. Phys. Rev. B 79, 054107 (2009).
Redmer, R., Mattsson, T. R., Nettelmann, N. & French, M. The phase diagram of water and the magnetic fields of Uranus and Neptune. Icarus 211, 798–803 (2011).
Hernandez, J.-A. & Caracas, R. Superionic-superionic phase transitions in body-centered cubic H2O ice. Phys. Rev. Lett. 117, 135503 (2016).
Wilson, H. F., Wong, M. L. & Militzer, B. Superionic to superionic phase change in water: consequences for the interiors of Uranus and Neptune. Phys. Rev. Lett. 110, 151102 (2013).
Sun, J., Clark, B. K., Torquato, S. & Car, R. The phase diagram of high-pressure superionic ice. Nat. Commun. 6, 8156 (2015).
French, M., Desjarlais, M. P. & Redmer, R. Ab initio calculation of thermodynamic potentials and entropies for superionic water. Phys. Rev. E 93, 022140 (2016).
Millot, M. et al. Experimental evidence for superionic water ice using shock compression. Nat. Phys. 14, 297–302 (2018).
del Rosso, L., Celli, M. & Ulivi, L. A new porous water ice stable at atmospheric pressure obtained by emptying a hydrogen filled ice. Nat. Commun. 7, 1–19 (2016).
Rygg, J. R. et al. Powder diffraction from solids in the terapascal regime. Rev. Sci. Instrum. 83, 113904 (2012).
Coppari, F. et al. Experimental evidence for a phase transition in magnesium oxide at exoplanet pressures. Nat. Geosci. 6, 926–929 (2013).
Dolan, D. H. & Gupta, Y. M. Nanosecond freezing of water under multiple shock wave compression: optical transmission and imaging measurements. J. Chem. Phys. 121, 9050–9057 (2004).
Chau, R., Mitchell, A. C., Minich, R. W. & Nellis, W. J. Electrical conductivity of water compressed dynamically to pressures of 70–180 GPa (0.7–1.8 Mbar). J. Chem. Phys. 114, 1361–1365 (2001).
Dolan, D. H., Knudson, M. D., Hall, C. A. & Deeney, C. A metastable limit for compressed liquid water. Nat. Phys. 3, 339–342 (2007).
Gleason, A. E. et al. Compression freezing kinetics of water to ice VII. Phys. Rev. Lett. 119, 025701 (2017).
Gleason, A. E. et al. Ultrafast visualization of crystallization and grain growth in shock-compressed SiO2. Nat. Commun. 6, 8191 (2015).
Shibuta, Y. et al. Heterogeneity in homogeneous nucleation from billion-atom molecular dynamics simulation of solidification of pure metal. Nat. Commun. 8, 10 (2017).
Sugimura, E. et al. Compression of H2O ice to 126 GPa and implications for hydrogen-bond symmetrization: synchrotron X-ray diffraction measurements and density-functional calculations. Phys. Rev. B 77, 214103 (2008).
French, M. & Redmer, R. Construction of a thermodynamic potential for the water ices VII and X. Phys. Rev. B 91, 014308 (2015).
Benoit, M., Bernasconi, M., Focher, P. & Parrinello, M. New high-pressure phase of ice. Phys. Rev. Lett. 76, 2934–2936 (1996).
Caracas, R. Dynamical instabilities of ice X. Phys. Rev. Lett. 101, 085502 (2008).
Militzer, B. & Wilson, H. F. New phases of water ice predicted at megabar pressures. Phys. Rev. Lett. 105, 195701 (2010).
Hermann, A., Ashcroft, N. W. & Hoffmann, R. High pressure ices. Proc. Natl Acad. Sci. USA 109, 745–750 (2012).
Nettelmann, N., Helled, R., Fortney, J. J. & Redmer, R. New indication for a dichotomy in the interior structure of Uranus and Neptune from the application of modified shape and rotation data. Planet. Space Sci. 77, 143–151 (2013).
Hemley, R. J. et al. Static compression of H2O-ice to 128 GPa (1.28 Mbar). Nature 330, 737–740 (1987).
Loubeyre, P., LeToullec, R., Wolanin, E., Hanfland, M. & Hausermann, D. Modulated phases and proton centring in ice observed by X-ray diffraction up to 170 GPa. Nature 397, 503–506 (1999).
Frank, M. R. M., Fei, Y. & Hu, J. Constraining the equation of state of fluid H2O to 80 GPa using the melting curve, bulk modulus, and thermal expansivity of ice VII. Geochim. Cosmochim. Acta 68, 2781–2790 (2004).
Sugimura, E. et al. Simultaneous high-pressure and high-temperature volume measurements of ice VII and its thermal equation of state. Phys. Rev. B 82, 134103 (2010).
Sugimura, E. et al. Experimental evidence of superionic conduction in H2O ice. J. Chem. Phys. 137, 194505 (2012).
Acknowledgements
We acknowledge C. Davis, J. Emig, E. Folsom, R. Posadas Soriano, S. Uhlich, T. Uphaus and W. Unites for target preparation, the Omega Laser Facility management, staff and support crew for shot and diagnostic support and G. W. Collins, F. Datchi, R. Jeanloz and P. F. McMillan for discussions. This work was prepared by LLNL under contract DE-AC52-07NA27344. Omega shots were allocated through the LLE Laboratory Basic Science programme. Partial support was provided by LLNL LDRD programmes 12-SI-007, 14-SI-003 and 19-ERD-031 and the US Department of Energy through the joint FES/NNSA HEDLP programme.
Reviewer information
Nature thanks Stephane Mazevet and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
M.M. and F.C. contributed equally to this work, designed and fielded the laser shots, analysed the data and wrote the manuscript. M.M. initiated the project and performed hydrodynamic simulations (with input from D.C.S.). A.C.B. prepared the water targets. J.R.R. was the principal investigator of the Omega laser experimental campaign. S.H. performed molecular dynamics numerical simulations. J.H.E., J.R.R. and F.C. contributed to the development of the diffraction data analysis software. All authors discussed the data analysis and interpretation and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
The Supplementary Information document contains a description of the methods, additional velocimetry and diffraction data and an extended discussion of the results with 24 supplementary figures and 2 tables.
Rights and permissions
About this article
Cite this article
Millot, M., Coppari, F., Rygg, J.R. et al. Nanosecond X-ray diffraction of shock-compressed superionic water ice. Nature 569, 251–255 (2019). https://doi.org/10.1038/s41586-019-1114-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-019-1114-6
This article is cited by
-
Rich proton dynamics and phase behaviours of nanoconfined ices
Nature Physics (2024)
-
Tidal Dissipation in Giant Planets
Space Science Reviews (2024)
-
Fluxional Hydrogen Bonds in Small Water Clusters (H2O)n (n = 2–6)
Journal of Cluster Science (2024)
-
Thermal and Tidal Evolution of Ice Giants with Growing Frozen Cores: The Case of Neptune
Space Science Reviews (2024)
-
Diamond precipitation dynamics from hydrocarbons at icy planet interior conditions
Nature Astronomy (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.