Abstract
Loss-of-function mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) compromise epithelial HCO3− and Cl− secretion, reduce airway surface liquid pH, and impair respiratory host defences in people with cystic fibrosis1,2,3. Here we report that apical addition of amphotericin B, a small molecule that forms unselective ion channels, restored HCO3− secretion and increased airway surface liquid pH in cultured airway epithelia from people with cystic fibrosis. These effects required the basolateral Na+, K+-ATPase, indicating that apical amphotericin B channels functionally interfaced with this driver of anion secretion. Amphotericin B also restored airway surface liquid pH, viscosity, and antibacterial activity in primary cultures of airway epithelia from people with cystic fibrosis caused by different mutations, including ones that do not yield CFTR, and increased airway surface liquid pH in CFTR-null pigs in vivo. Thus, unselective small-molecule ion channels can restore host defences in cystic fibrosis airway epithelia via a mechanism that is independent of CFTR and is therefore independent of genotype.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Ratjen, F. et al. Cystic fibrosis. Nat. Rev. Dis. Primers 1, 15010 (2015).
Quinton, P. M. The neglected ion: HCO3 −. Nat. Med. 7, 292–293 (2001).
Shah, V. S. et al. Airway acidification initiates host defense abnormalities in cystic fibrosis mice. Science 351, 503–507 (2016).
Ramsey, B. W. et al. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N. Engl. J. Med. 365, 1663–1672 (2011).
Wainwright, C. E. et al. Lumacaftor–ivacaftor in patients with cystic fibrosis homozygous for Phe508del CFTR. N. Engl. J. Med. 373, 220–231 (2015).
Oliver, K. E., Han, S. T., Sorscher, E. J. & Cutting, G. R. Transformative therapies for rare CFTR missense alleles. Curr. Opin. Pharmacol. 34, 76–82 (2017).
El-Etri, M. & Cuppoletti, J. Metalloporphyrin chloride ionophores: induction of increased anion permeability in lung epithelial cells. Am. J. Physiol. 270, L386–L392 (1996).
Jiang, C. et al. Partial correction of defective Cl− secretion in cystic fibrosis epithelial cells by an analog of squalamine. Am. J. Physiol. Lung Cell. Mol. Physiol. 281, L1164–L1172 (2001).
Koulov, A. V. et al. Chloride transport across vesicle and cell membranes by steroid-based receptors. Angew. Chem. Int. Ed. 42, 4931–4933 (2003).
Shen, B., Li, X., Wang, F., Yao, X. & Yang, D. A synthetic chloride channel restores chloride conductance in human cystic fibrosis epithelial cells. PLoS ONE 7, e34694 (2012).
Poulsen, J. H., Fischer, H., Illek, B. & Machen, T. E. Bicarbonate conductance and pH regulatory capability of cystic fibrosis transmembrane conductance regulator. Proc. Natl Acad. Sci. USA 91, 5340–5344 (1994).
Chang, E. H. et al. Medical reversal of chronic sinusitis in a cystic fibrosis patient with ivacaftor. Int. Forum Allergy Rhinol. 5, 178–181 (2015).
Garland, A. L. et al. Molecular basis for pH-dependent mucosal dehydration in cystic fibrosis airways. Proc. Natl Acad. Sci. USA 110, 15973–15978 (2013).
Garnett, J. P. et al. Hyperglycaemia and Pseudomonas aeruginosa acidify cystic fibrosis airway surface liquid by elevating epithelial monocarboxylate transporter 2 dependent lactate–H+ secretion. Sci. Rep. 6, 37955 (2016).
Farha, M. A., French, S., Stokes, J. M. & Brown, E. D. Bicarbonate alters bacterial susceptibility to antibiotics by targeting the proton motive force. ACS Infect. Dis. 4, 382–390 (2018).
Peckham, D., Holland, E., Range, S. & Knox, A. J. Na+/K+ ATPase in lower airway epithelium from cystic fibrosis and non-cystic-fibrosis lung. Biochem. Biophys. Res. Commun. 232, 464–468 (1997).
Widdicombe, J. H., Welsh, M. J. & Finkbeiner, W. E. Cystic fibrosis decreases the apical membrane chloride permeability of monolayers cultured from cells of tracheal epithelium. Proc. Natl Acad. Sci. USA 82, 6167–6171 (1985).
Grillo, A. S. et al. Restored iron transport by a small molecule promotes absorption and hemoglobinization in animals. Science 356, 608–616 (2017).
Ermishkin, L. N., Kasumov, K. M. & Potseluyev, V. M. Properties of amphotericin B channels in a lipid bilayer. Biochim. Biophys. Acta 470, 357–367 (1977).
Gray, K. C. et al. Amphotericin primarily kills yeast by simply binding ergosterol. Proc. Natl Acad. Sci. USA 109, 2234–2239 (2012).
Anderson, T. M. et al. Amphotericin forms an extramembranous and fungicidal sterol sponge. Nat. Chem. Biol. 10, 400–406 (2014).
Cioffi, A. G., Hou, J., Grillo, A. S., Diaz, K. A. & Burke, M. D. Restored physiology in protein-deficient yeast by a small molecule channel. J. Am. Chem. Soc. 137, 10096–10099 (2015).
Van Goor, F. et al. Rescue of CF airway epithelial cell function in vitro by a CFTR potentiator, VX-770. Proc. Natl Acad. Sci. USA 106, 18825–18830 (2009).
Abou Alaiwa, M. H. et al. Repurposing tromethamine as inhaled therapy to treat CF airway disease. JCI Insight 1, e87535 (2016).
Wallace, D. P. et al. A synthetic channel-forming peptide induces Cl− secretion: modulation by Ca2+-dependent K+ channels. Biochim. Biophys. Acta 1464, 69–82 (2000).
Derichs, N., Jin, B.-J., Song, Y., Finkbeiner, W. E. & Verkman, A. S. Hyperviscous airway periciliary and mucous liquid layers in cystic fibrosis measured by confocal fluorescence photobleaching. FASEB J. 25, 2325–2332 (2011).
Monforte, V. et al. Nebulized liposomal amphotericin B prophylaxis for Aspergillus infection in lung transplantation: pharmacokinetics and safety. J. Heart Lung Transplant. 28, 170–175 (2009).
Saint-Criq, V. & Gray, M. A. Role of CFTR in epithelial physiology. Cell. Mol. Life Sci. 74, 93–115 (2017).
Ringden, O. et al. Efficacy of amphotericin B encapsulated in liposomes (AmBisome) in the treatment of invasive fungal infections in immunocompromised patients. J. Antimicrob. Chemother. 28, 73–82 (1991).
Palmer, S. M. et al. Safety of aerosolized amphotericin B lipid complex in lung transplant recipients. Transplantation 72, 545–548 (2001).
Drew, R. et al. Comparative safety of amphotericin B lipid complex and amphotericin B deoxycholate as aerosolized antifungal prophylaxis in lung-transplant recipients. Transplantation 77, 232–237 (2004).
Perfect, J. R., Dodds Ashley, E. & Drew, R. Design of aerosolized amphotericin B formulations for prophylaxis trials among lung transplant recipients. Clin. Infect. Dis. 39, S207–S210 (2004).
Lowry, C. M. et al. Safety of aerosolized liposomal versus deoxycholate amphotericin B formulations for prevention of invasive fungal infections following lung transplantation: a retrospective study. Transpl. Infect. Dis. 9, 121–125 (2007).
Castagnola, E. et al. Nebulized liposomal amphotericin B and combined systemic antifungal therapy for the treatment of severe pulmonary aspergillosis after allogeneic hematopoietic stem cell transplant for a fatal mitochondrial disorder. J. Chemother. 19, 339–342 (2013).
Slobbe, L., Boersma, E. & Rijnders, B. J. A. Tolerability of prophylactic aerosolized liposomal amphotericin-B and impact on pulmonary function: data from a randomized placebo-controlled trial. Pulm. Pharmacol. Ther. 21, 855–859 (2008).
Rijnders, B. J. A. et al. Aerosolized liposomal amphotericin B for the prevention of invasive pulmonary aspergillosis during prolonged neutropenia: a randomized, placebo-controlled trial. Clin. Infect. Dis. 46, 1401–1408 (2008).
Monforte, V. et al. Feasibility, tolerability, and outcomes of nebulized liposomal amphotericin B for Aspergillus infection prevention in lung transplantation. J. Heart Lung Transplant. 29, 523–530 (2010).
Hullard-Pulstinger, A., Holler, E., Hahn, J., Andreesen, R. & Krause, S. W. Prophylactic application of nebulized liposomal amphotericin B in hematologic patients with neutropenia. Onkologie 34, 254–258 (2011).
Monforte, V. et al. Prophylaxis with nebulized liposomal amphotericin B for Aspergillus infection in lung transplant patients does not cause changes in the lipid content of pulmonary surfactant. J. Heart Lung Transplant. 32, 313–319 (2013).
Stone, N. R. H., Bicanic, T., Salim, R. & Hope, W. Liposomal amphotericin B (AmBisome®): a review of the pharmacokinetics, pharmacodynamics, clinical experience and future directions. Drugs 76, 485–500 (2016).
Zabner, J. et al. Development of cystic fibrosis and noncystic fibrosis airway cell lines. Am. J. Physiol. Lung Cell. Mol. Physiol. 284, L844–L854 (2003).
Karp, P. H. et al. An in vitro model of differentiated human airway epithelia. Methods for establishing primary cultures. Methods Mol. Biol. 188, 115–137 (2002).
Chen, P. S., Toribara, T. Y. & Warner, H. Microdetermination of phosphorus. Anal. Chem. 28, 1756–1758 (1956).
Andrews, N. J. et al. Structurally simple lipid bilayer transport agents for chloride and bicarbonate. Chem. Sci. 2, 256–260 (2011).
Busschaert, N. et al. Tripodal transmembrane transporters for bicarbonate. Chem. Commun. 46, 6252–6254 (2010).
Davis, J. T. et al. Using small molecules to facilitate exchange of bicarbonate and chloride anions across liposomal membranes. Nat. Chem. 1, 138–144 (2009).
Busschaert, N. et al. Synthetic transporters for sulfate: a new method for the direct detection of lipid bilayer sulfate transport. Chem. Sci. 5, 1118–1127 (2014).
Denmark, S. E., Williams, B. J., Eklov, B. M., Pham, S. M. & Beutner, G. L. Design, validation, and implementation of a rapid-injection NMR system. J. Org. Chem. 75, 5558–5572 (2010).
Thomas, A. A. & Denmark, S. E. Pre-transmetalation intermediates in the Suzuki–Miyaura reaction revealed: the missing link. Science 352, 329–332 (2016).
Davis, S. A. et al. C3-OH of amphotericin B plays an important role in ion conductance. J. Am. Chem. Soc. 137, 15102–15104 (2015).
Pezzulo, A. A. et al. Reduced airway surface pH impairs bacterial killing in the porcine cystic fibrosis lung. Nature 487, 109–113 (2012).
Cho, D. Y., Hajighasemi, M., Hwang, P. H., Illek, B. & Fischer, H. Proton secretion in freshly excised sinonasal mucosa from asthma and sinusitis patients. Am. J. Rhinol. Allergy 23, e10–e13 (2009).
Cho, D. Y., Hwang, P. H., Illek, B. & Fischer, H. Acid and base secretion in freshly excised nasal tissue from cystic fibrosis patients with ΔF508 mutation. Int. Forum Allergy Rhinol. 1, 123–127 (2011).
Fischer, H. & Widdicombe, J. H. Mechanisms of acid and base secretion by the airway epithelium. J. Membr. Biol. 211, 139–150 (2006).
Fischer, H. Function of proton channels in lung epithelia. Wiley Interdiscip. Rev. Membr. Transp. Signal. 1, 247–258 (2012).
Devor, D. C. et al. Bicarbonate and chloride secretion in Calu-3 human airway epithelial cells. J. Gen. Physiol. 113, 743–760 (1999).
Myerburg, M. M. et al. AMPK agonists ameliorate sodium and fluid transport and inflammation in cystic fibrosis airway epithelial cells. Am. J. Respir. Cell Mol. Biol. 42, 676–684 (2010).
Worthington, E. N. & Tarran, R. Methods for ASL measurements and mucus transport rates in cell cultures. Methods Mol. Biol. 742, 77–92 (2011).
Schneider, C. A., Rasband, W. S. & Eliceiri, K. W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675 (2012).
Tang, X. X. et al. Acidic pH increases airway surface liquid viscosity in cystic fibrosis. J. Clin. Invest. 126, 879–891 (2016).
Acknowledgements
We acknowledge M. Sivaguru at the Carl R. Woese Institute for Genomic Biology for assistance with confocal microscopy, S. E. Denmark and C. Delaney for assistance with rapid injection NMR and D. J. Blair for helpful discussions. Support was provided by NIH (5R35GM118185 to M.D.B. and HL091842 to M.J.W.), and by Emily’s Entourage. M.J.W. is an HHMI Investigator. K.A.M. is a Medical Scholars Fellow.
Author information
Authors and Affiliations
Contributions
K.A.M., R.S.C., B.R.K., M.J.W. and M.D.B. designed experiments and interpreted data. K.A.M., R.S.C., M.J.W. and M.D.B. wrote the manuscript. K.A.M., R.S.C. and P.N.D. cultured epithelia and performed Ussing chamber experiments. P.H.K. cultured primary epithelia. R.S.C. measured ion efflux, ASL ion concentrations and H14CO3− transport. K.A.M. measured ASL pH, Rt and LDH. K.A.M. and R.S.C. performed pH-stat titration. K.A.M. and A.G.C. measured ASL height. K.A.M. and X.X.T. measured ASL viscosity. K.A.M. and V.S.S. measured ASL antibacterial activity. K.A.M., R.S.C. and B.R.K. measured ASL pH in CFTR−/− pigs. A.S.G. synthesized compounds. L.Z. assisted NMR studies.
Corresponding author
Ethics declarations
Competing interests
K.A.M., A.G.C., A.S.G., M.J.W. and M.D.B. are inventors on patent applications PCT/US15/58806, PCT/US18/55435 and/or PCT/US2017/26806, submitted by UIUC, which cover use of AmB and AmB–cholesterol to treat CF.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
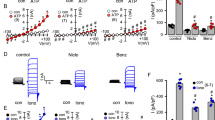
Extended Data Fig. 1 AmB can transport K+, Na+, Cl−, H+ and HCO3− across a lipid membrane.
Traces indicate the percentage of maximum ion efflux after Triton X-100 addition. a, Schematic for the 13C NMR HCO3− efflux experiment. b, 13C NMR spectra of H13CO3−-loaded POPC/10% cholesterol liposomes treated with AmB, C35deOAmB, or DMSO vehicle. NaH13CO3 was loaded inside the liposomes and the intravesicular solution was buffered to pH 7.5, whereas the extravesicular solution was buffered to pH 7.3. Owing to this pH difference, intravesicular HCO3− displays a more downfield chemical shift relative to extravesicular HCO3−. Addition of AmB (1:4,000 AmB:POPC) produces an upfield 13C signal corresponding to extravesicular HCO3−, while the addition C35deOAmB or DMSO vehicle does not, demonstrating that AmB is able to facilitate HCO3− efflux. c, The percentage of efflux of HCO3− mediated by DMSO, AmB or C35deOAmB, quantified 10 min after addition to POPC liposomes (n = 3 biologically independent samples). Data from each run were normalized to the percentage of total ion release from 0 to 100%. After lysis of the liposome suspension, the integration of the signal corresponding to extravesicular HCO3− relative to the integration of a 13C-glucose standard was scaled to correspond to 100% efflux. d, To confirm that the upfield signal corresponds to extravesicular HCO3−, Mn2+—which binds to HCO3− and quenches the observed 13C signal via paramagnetic relaxation enhancement—was added to the extravesicular solution. Because Mn2+ is impermeable to the POPC bilayer, Mn2+ can only affect the signal corresponding to HCO3− outside the liposomes. Addition of Mn2+ quenched the upfield signal produced with the addition of AmB but not the signal corresponding to intravesicular HCO3−, confirming that AmB causes efflux of HCO3−. e, To effect complete ion release, the POPC liposomes were lysed with Triton X-100 at the conclusion of the experiment. f, K+ efflux from POPC/10% cholesterol liposomes after addition of AmB equivalent to 1:1,000 AmB:lipid, or DMSO vehicle. g, Na+ efflux from POPC/10% cholesterol liposomes after addition of AmB equivalent to 1:1,000 AmB:lipid, or DMSO vehicle. h, Cl− efflux from POPC/10% cholesterol liposomes after addition of AmB equivalent to 1:1,000 AmB:lipid, or DMSO vehicle. i, HCO3− efflux from POPC/10% cholesterol liposomes after addition of AmB equivalent to 1:1,000 AmB:lipid, or DMSO vehicle. Kinetics of efflux were measured using rapid-injection NMR to add AmB to liposomes. j, H+ efflux from POPC/10% cholesterol liposomes after addition of AmB equivalent to 1:1,000 AmB:lipid, or DMSO vehicle. b, d–j, Panels show a representative spectrum or graph from at least three independent experiments. In all panels, measurements were taken from distinct samples. In c, the graph depicts mean ± s.e.m.
Extended Data Fig. 2 AmB-mediated pH changes are HCO3−-dependent and do not alter major cation concentrations in the ASL.
a, Base secretion and acid absorption rates in NuLi (HCO3− +: n = 8 biologically independent samples; HCO3− −: n = 4 biologically independent samples) or CuFi-1 (ΔF508/ΔF508) epithelia (HCO3− +: n = 23 biologically independent samples, P < 0.0001; HCO3− −: n = 18 biologically independent samples) over 20 min after acute addition of increasing AmB concentrations, as measured by pH-stat titration. All n are biologically independent samples. HCO3− +: 0.5, 1 μM, n = 6; 5 μM, n = 7. 0.5 μM, P = 0.9663; 1 μM, P = 0.7328; 5 μM, P = 0.1459. HCO3− −: 0.5, 1, 5 μM, n = 6. The apical pH was titrated to a target pH of 6.0. b–e, The effect of AmB (2 μM) compared to perfluorocarbon (FC-72) vehicle after 48 h on Na+ (b; P = 0.7855), K+ (c; P = 0.2892), 24Mg2+ (d; P = 0.8339) and Ca2+ (e; P = 0.2708 with Welch’s correction) concentrations in the ASL in CuFi-1 (ΔF508/ΔF508), as measured by ICP-MS (n = 16 biologically independent samples). ANOVA (a), two-sided unpaired Student’s t-test (b–d) or two-sided unpaired Student’s t-test with Welch’s correction (e) were used to assess statistical significance. Data are mean ± s.e.m.; NS, not significant; ****P ≤ 0.0001. In all panels, measurements were taken from biologically independent samples.
Extended Data Fig. 3 AmB treatment is sustained, is ineffective on wild type, is not due to increased CFTR activity, does not disturb membrane integrity, and is non-toxic.
a–c, The effect of AmB (2 μM) compared to FC-72 vehicle left on the surface of CuFi-1 (ΔF508/ΔF508) epithelia for 7 (a; n = 6 biologically independent samples, P = 0.0004), 14 (b; n = 9 biologically independent samples, P = 0.5138) or 28 (c; n = 6 biologically independent samples, P = 0.3421) days on H14CO3− movement from the basolateral buffer to the ASL over 10 min after radiolabel addition, normalized to FC-72 vehicle addition. d–i, Changes in transepithelial current (It) after treatment with 10 μM forskolin/100 μM IBMX (FI) to activate CFTR and 1 μM CFTRinh-172 to inhibit CFTR in NuLi (CFTR+/+) epithelia (d, g), CuFi-1 (ΔF508/ΔF508) epithelia (e, h), and CuFi-1 epithelia treated with AmB (2 μM, 48 h) (f, i) (n = 6 biologically independent samples). g–i show a representative graph from six independent experiments repeated with similar results. j, Transepithelial electrical resistance (Rt) in CuFi-1 epithelia did not differ between treatment with vehicle or increasing doses of AmB over increasing time periods after a single treatment (n = 9 biologically independent samples). k, Cytotoxicity, as measured by detection of lactase dehydrogenase in CuFi-1 epithelia over increasing time periods after a single AmB or vehicle treatment, is represented as percentage of total cellular lysis by Triton X-100. AmB treatment did not cause increased cytotoxicity compared to vehicle (n = 12 biologically independent samples). In a–c, two-sided unpaired Student’s t-test was used to assess statistical significance. In a–f, j, k, graphs show mean ± s.e.m.; NS, not significant; ***P ≤ 0.001. In all panels, measurements were taken from biologically independent samples.
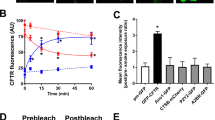
Extended Data Fig. 4 AmB increases ASL height.
ASL height, as imaged by confocal microscopy, in NuLi (CFTR+/+) epithelia (a), CuFi-1 epithelia (b), CuFi-1 epithelia with apical addition of AmB (c), CuFi-1 epithelia with apical addition of C35deOAmB (d), CuFi-1 epithelia with basolateral addition of AmB (e), and NuLi (f) and AmB-treated CuFi-1 epithelia with basolateral addition of bumetanide (500 μM) (g). Representative images from at least six independent experiments are shown. In all panels, measurements were taken from biologically independent samples. Scale bars, 10 μm.
Extended Data Fig. 5 AmB restores ASL pH and antibacterial activity in primary cultures of human airway epithelia from donors with CF.
a, Genotypes and ΔpH measurements from patient donors in ASL pH assay. b, The effects of AmB (2 μM, 48 h; n = 9 biologically independent samples, P = 0.446), C35deOAmB (2 μM, 48 h; n = 5 biologically independent samples, P = 0.9994) and basolateral addition of AmB (2 μM, 48 h; n = 3 biologically independent samples, P = 0.6359) compared to vehicle on the average ASL pH of primary cultured airway epithelia derived from CF humans with different CFTR mutations. c, The effect of AmB (2 μM, 48 h; n = 7 biologically independent samples, P = 0.4866) compared to vehicle on ASL pH in non-CF epithelia. d, Genotypes of patient donors in ASL viscosity assay. e, Genotypes of patient donors in ASL antibacterial activity assay. f, The effect of AmB (2 μM, 48 h; n = 8 biologically independent samples, P = 0.0042) and C35deOAmB (2 μM, 48 h; n = 5 biologically independent samples, P = 0.9626) compared to vehicle on the average ASL antibacterial activity of primary cultured airway epithelia derived from humans with CF harbouring different CFTR mutations. Antibacterial activity is measured by the percentage of S. aureus killed after exposure to ASL. g, The ability of AmB (2 μM) alone to kill S. aureus compared to saline (n = 36 biologically independent samples, P = 0.1569). h–j, Representative FRAP traces for measuring ASL viscosity from six independent experiments repeated with similar results for non-CF (h), CF (i), and AmB-treated CF (j) epithelia. In b and f, ANOVA was used to assess statistical significance. Two-sided unpaired Student’s t-test with Welch’s correction (c) or two-sided unpaired Student’s t-test (g) was used to assess statistical significance. Graphs show mean ± s.e.m.; NS, not significant; *P ≤ 0.05; **P ≤ 0.01. In all panels, measurements were taken from biologically independent samples.
Extended Data Fig. 6 AmBisome increases transepithelial H14CO3− secretion and ASL pH in a time- and dose-dependent manner.
a, The effect of AmBisome (1:1,000 AmB:lipid ratio), AmB:cholesterol (1:1,000 AmB:lipid in DMSO), and sterile water or DMSO vehicle on H13CO3− transport across a POPC/10% cholesterol lipid membrane. b, The effect of AmBisome (1 mg ml−1, 48 h; n = 16 biologically independent samples, P = 0.0477) compared to FC-72 vehicle on H14CO3− movement from the basolateral buffer to the ASL over 10 min after radiolabel addition in CuFi-1 (ΔF508/ΔF508), normalized to FC-72 vehicle addition. c, The effect of increasing AmBisome concentration (1 mg ml−1, 48 h) compared to vehicle on ASL pH in CuFi-1 epithelia. All concentrations, n = 9 biologically independent samples; 0.25 μM, P = 0.0106; 2 μM, P < 0.0001; 25 μM, P = 0.0002; 50 μM, P < 0.0001; 100 μM, P < 0.0001. In a, a representative graph from at least three independent experiments is shown. In b, two-sided unpaired Student’s t-test was used to assess statistical significance. In c, ANOVA was used to assess statistical significance. Graphs depict means ± s.e.m.; NS, not significant; *P ≤ 0.05; ****P ≤ 0.0001. In a, the same sample for each replicate was measured repeatedly over time. In b and c, measurements were taken from biologically independent samples.
Supplementary information
Rights and permissions
About this article
Cite this article
Muraglia, K.A., Chorghade, R.S., Kim, B.R. et al. Small-molecule ion channels increase host defences in cystic fibrosis airway epithelia. Nature 567, 405–408 (2019). https://doi.org/10.1038/s41586-019-1018-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-019-1018-5
This article is cited by
-
Mesenchymal stem cells in fibrotic diseases—the two sides of the same coin
Acta Pharmacologica Sinica (2023)
-
Aromatic pentaamide macrocycles bind anions with high affinity for transport across biomembranes
Nature Chemistry (2023)
-
Self-assembled Supramolecular Artificial Transmembrane Ion Channels: Recent Progress and Application
Chemical Research in Chinese Universities (2023)
-
Measuring anion binding at biomembrane interfaces
Nature Communications (2022)
-
Ion transporters: emerging agents for anticancer therapy
Science China Chemistry (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.