Abstract
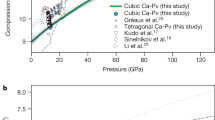
Laboratory measurements of sound velocities of high-pressure minerals provide crucial information on the composition and constitution of the deep mantle via comparisons with observed seismic velocities. Calcium silicate (CaSiO3) perovskite (CaPv) is a high-pressure phase that occurs at depths greater than about 560 kilometres in the mantle1 and in the subducting oceanic crust2. However, measurements of the sound velocity of CaPv under the pressure and temperature conditions that are present at such depths have not previously been performed, because this phase is unquenchable (that is, it cannot be physically recovered to room conditions) at atmospheric pressure and adequate samples for such measurements are unavailable. Here we report in situ X-ray diffraction and ultrasonic-interferometry sound-velocity measurements at pressures of up to 23 gigapascals and temperatures of up to 1,700 kelvin (similar to the conditions at the bottom of the mantle transition region) using sintered polycrystalline samples of cubic CaPv converted from bulk glass and a multianvil apparatus. We find that cubic CaPv has a shear modulus of 126 ± 1 gigapascals (uncertainty of one standard deviation), which is about 26 per cent lower than theoretical predictions3,4 (about 171 gigapascals). This value leads to substantially lower sound velocities of basaltic compositions than those predicted for the pressure and temperature conditions at depths between 660 and 770 kilometres. This suggests accumulation of basaltic crust in the uppermost lower mantle, which is consistent with the observation of low-seismic-velocity signatures below 660 kilometres5,6 and the discovery of CaPv in natural diamond of super-deep origin7. These results could contribute to our understanding of the existence and behaviour of subducted crust materials in the deep mantle.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Source data for Fig. 3 are provided in Supplementary Information.
References
Irifune, T. Absence of an aluminous phase in the upper part of the Earth’s lower mantle. Nature 370, 131–133 (1994).
Hirose, K. & Fei, Y. Subsolidus and melting phase relations of basaltic composition in the uppermostlower mantle. Geochim. Cosmochim. Acta 66, 2099–2108 (2002).
Stixrude, L., Lithgow-Bertelloni, C., Kiefer, B. & Fumagalli, P. Phase stability and shear softening in CaSiO3 perovskite at high pressure. Phys. Rev. B 75, 024108 (2007).
Tsuchiya, T. Elasticity of subducted basaltic crust at the lower mantle pressures: Insights on the nature of deep mantle heterogeneity. Phys. Earth Planet. Inter. 188, 142–149 (2011).
Schmandt, B., Jacobsen, S. D., Becker, T. W. & Liu, Z. Dehydration melting at the top of the lower mantle. Science 344, 1265–1268 (2014).
Liu, Z., Park, J. & Karato, S.-i. Seismological detection of low-velocity anomalies surrounding the mantle transition zone in Japan subduction zone. Geophys. Res. Lett. 43, 2480–2487 (2016).
Nestola, F. et al. CaSiO3 perovskite in diamond indicates the recycling of oceanic crust into the lower mantle. Nature 555, 237–241 (2018).
Irifune, T. et al. Sound velocities of majorite garnet and the composition of the mantle transition region. Nature 451, 814–817 (2008).
Kurnosov, A., Marquardt, H., Frost, D. J., Ballaran, T. B. & Ziberna, L. Evidence for a Fe3+-rich pyrolitic lower mantle from (Al,Fe)-bearing bridgmanite elasticity data. Nature 37, 1–16 (2017).
Irifune, T. & Ringwood, A. E. Phase transformations in a harzburgite composition to 26 GPa: implications for dynamical behaviour of the subducting slab. Earth Planet. Sci. Lett. 86, 365–376 (1987).
Xu, W., Lithgow-Bertelloni, C., Stixrude, L. & Ritsema, J. The effect of bulk composition and temperature on mantle seismic structure. Earth Planet. Sci. Lett. 275, 70–79 (2008).
Ballmer, M. D., Schmerr, N. C., Nakagawa, T. & Ritsema, J. Compositional mantle layering revealed by slab stagnation at ~1000-km depth. Sci. Adv. 1, e1500815 (2015).
Saikia, A., Frost, D. J. & Rubie, D. C. Splitting of the 520-kilometer seismic discontinuity and chemical heterogeneity in the mantle. Science 319, 1515–1518 (2008).
Irifune, T. & Ringwood, A. E. Phase transformation in subducted oceanic crust and buoyancy relationships at depths of 600–800 km in the mantle. Earth Planet. Sci. Lett. 117, 101–110 (1993).
Kesson, S. E., Fitz Gerald, J. D. & Shelley, J. M. G. Mineral chemistry and density of subducted basaltic crust at lower mantle pressures. Nature 372, 767–769 (1994).
Li, B., Kung, J. & Liebermann, R. C. Modern techniques in measuring elasticity of Earth materials at high pressure and high temperature using ultrasonic interferometry in conjunction with synchrotron X-radiation in multi-anvil apparatus. Phys. Earth Planet. Inter. 143–144, 559–574 (2004).
Kudo, Y. et al. Sound velocity measurements of CaSiO3 perovskite to 133 GPa and implications for lowermost mantle seismic anomalies. Earth Planet. Sci. Lett. 349–350, 1–7 (2012).
Shim, S.-H. Tetragonal structure of CaSiO3 perovskite above 20 GPa. Geophys. Res. Lett. 29, 2166 (2002).
Komabayashi, T., Hirose, K., Sata, N., Ohishi, Y. & Dubrovinsky, L. S. Phase transition in CaSiO3 perovskite. Earth Planet. Sci. Lett. 260, 564–569 (2007).
Carpenter, M. A., Li, B. & Liebermann, R. C. Elastic anomalies accompanying phase transitions in (Ca,Sr)TiO3 perovskites: part III. Experimental investigation of polycrystalline samples. Am. Mineral. 92, 344–355 (2007).
Sun, T., Zhang, D.-B. & Wentzcovitch, R. M. Dynamic stabilization of cubic CaSiO3 perovskite at high temperatures and pressures from ab initio molecular dynamics. Phys. Rev. B 89, 094109 (2014).
Davies, G. F. & Dziewonski, A. M. Homogeneity and constitution of the earth’s lower mantle and outer core. Phys. Earth Planet. Inter. 10, 336–343 (1975).
Sinelnikov, Y. D., Chen, G. & Liebermann, R. C. Elasticity of CaTiO3-CaSiO3 perovskites. Phys. Chem. Miner. 25, 515–521 (1998).
Kawai, K. & Tsuchiya, T. Small shear modulus of cubic CaSiO3 perovskite. Geophys. Res. Lett. 42, 2718–2726 (2015).
Tauzin, B., Kim, S. & Kennett, B. L. N. Pervasive seismic low-velocity zones within stagnant plates in the mantle transition zone: thermal or compositional origin? Earth Planet. Sci. Lett. 477, 1–13 (2017).
Kono, Y., Irifune, T., Ohfuji, H., Higo, Y. & Funakoshi, K.-I. Sound velocities of MORB and absence of a basaltic layer in the mantle transition region. Geophys. Res. Lett. 39, L24306 (2012).
Murakami, M., Hirose, K., Yurimoto, H., Nakashima, S. & Takafuji, N. Water in Earth’s lower mantle. Science 295, 1885–1887 (2002).
Litasov, K. et al. Water solubility in Mg-perovskites and water storage capacity in the lower mantle. Earth Planet. Sci. Lett. 211, 189–203 (2003).
Brodholt, J. P. Pressure-induced changes in the compression mechanism of aluminous perovskite in the Earth’s mantle. Nature 407, 620–622 (2000).
Bolfan-Casanova, N., Keppler, H. & Rubie, D. C. Water partitioning at 660 km depth and evidence for very low water solubility in magnesium silicate perovskite. Geophys. Res. Lett. 30, 1905 (2003).
Walter, M. J. et al. Deep mantle cycling of oceanic crust: evidence from diamonds and their mineral inclusions. Science 334, 54–57 (2011).
Kaneshima, S. Seismic scatterers at the shallowest lower mantle beneath subducted slabs. Earth Planet. Sci. Lett. 286, 304–315 (2009).
Wang, Y., Weidner, D. J. & Guyot, F. Thermal equation of state of CaSiO3 perovskite. J. Geophys. Res. B Solid Earth 101, 661–672 (1996).
Matsui, M., Higo, Y., Okamoto, Y., Irifune, T. & Funakoshi, K. I. Simultaneous sound velocity and density measurements of NaCl at high temperatures and pressures: application as a primary pressure standard. Am. Mineral. 97, 1670–1675 (2012).
Li, B. & Liebermann, R. C. Study of the Earth’s interior using measurements of sound velocities in minerals by ultrasonic interferometry. Phys. Earth Planet. Inter. 233, 135–153 (2014).
Kono, Y. et al. Simultaneous structure and elastic wave velocity measurement of SiO2 glass at high pressures and high temperatures in a Paris–Edinburgh cell. Rev. Sci. Instrum. 83, 033905–033909 (2012).
Irifune, T., Shinmei, T., McCammon, C. A. & Miyajima, N. Iron partitioning and density changes of pyrolite in Earth’s lower mantle. Science 327, 193–195 (2010).
Ricolleau, A. et al. Phase relations and equation of state of a natural MORB: implications for the density profile of subducted oceanic crust in the Earth’s lower mantle. J. Geophys. Res. 115, B08202 (2010).
Sueda, Y. et al. The phase boundary between CaSiO3 perovskite and Ca2SiO4+CaSi2O5 determined by in situ X-ray observations. Geophys. Res. Lett. 33, L10307 (2006).
Higo, Y., Inoue, T., Irifune, T., Funakoshi, K.-I. & Li, B. Elastic wave velocities of (Mg0.91Fe0.09)2SiO4 ringwoodite under P–T conditions of the mantle transition region. Phys. Earth Planet. Inter. 166, 167–174 (2008).
Sinogeikin, S. V. & Bass, J. D. Elasticity of majorite and a majorite-pyrope solid solution to high pressure: implications for the transition zone. Geophys. Res. Lett. 29, 1017 (2002).
Kono, Y., Higo, Y., Ohfuji, H., Inoue, T. & Irifune, T. Elastic wave velocities of garnetite with a MORB composition up to 14 GPa. Geophys. Res. Lett. 34, L14308 (2007).
Nishihara, Y., Aoki, I., Takahashi, E., Matsukage, K. N. & Funakoshi, K.-I. Thermal equation of state of majorite with MORB composition. Phys. Earth Planet. Inter. 148, 73–84 (2005).
Morishima, H. et al. The high-pressure and temperature equation of state of a majorite solid solution in the system of Mg4Si4O12-Mg3Al2Si3O12. Phys. Chem. Miner. 27, 3–10 (1999).
Chantel, J., Frost, D. J., McCammon, C. A., Jing, Z. & Wang, Y. Acoustic velocities of pure and iron-bearing magnesium silicate perovskite measured to 25 GPa and 1200 K. Geophys. Res. Lett. 39, L19307 (2012).
Wentzcovitch, R. M., Karki, B. B., Cococcioni, M. & De Gironcoli, S. Thermoelastic properties of MgSiO3-perovskite: insights on the nature of the Earth’s lower mantle. Phys. Rev. Lett. 92, 18501 (2004).
Marquardt, H., Speziale, S., Reichmann, H. J., Frost, D. J. & Schilling, F. R. Single-crystal elasticity of (Mg0.9Fe0.1)O to 81 GPa. Earth Planet. Sci. Lett. 287, 345–352 (2009).
Kono, Y. et al. P–V–T relation of MgO derived by simultaneous elastic wave velocity and in situ X-ray measurements: a new pressure scale for the mantle transition region. Phys. Earth Planet. Inter. 183, 196–211 (2010).
Gréaux, S. et al. Sound velocities of aluminum-bearing stishovite in the mantle transition zone. Geophys. Res. Lett. 43, 4239–4246 (2016).
Shinmei, T. et al. High-temperature and high-pressure equation of state for the hexagonal phase in the system NaAlSiO4 – MgAl2O4. Phys. Chem. Miner. 32, 594–602 (2005).
Wu, Y. et al. Elasticity of single-crystal NAL phase at high pressure: A potential source of the seismic anisotropy in the lower mantle. J. Geophys. Res. B 121, 1–12 (2016).
Acknowledgements
We thank T. Kunimoto for technical assistance in the experiments at beamline BL04B1 in SPring-8. We acknowledge Y. Kono for providing data analysis software; H. Dekura, Y. Nishihara and Y. Kudo for discussions; and G. Helffrich for comments on the manuscript. This work was supported by the JSPS Kakenhi programmes (to T.I.; number 25220712 and 15H05829).
Reviewer information
Nature thanks J. Buchen, L. Stixrude and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
S.G. and T.I. designed the research project. A.Y. and S.G. prepared the glass starting material. Y.H., Y.T. and S.G. prepared the ultrasonic experiments in SPring-8. S.G., T.A. and Z.L. carried out the ultrasonic experiments at SPring-8 and S.G. and Y.T. analysed the data. T.I. and S.G. wrote the manuscript. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Pressure and temperature conditions of sound-velocity and density measurements conducted in the stability field of CaPv.
The pressure is calibrated against the NaCl scale34. The red line indicates the decomposition boundary of CaPv to Ca2SiO4 larnite and CaSi2O5 titanite39. Dashed lines represent the pressure–temperature paths followed during the travel-time measurements in the two cooling cycles at 15 GPa (blue) and 17 GPa (red) shown in Fig. 2b.
Extended Data Fig. 2 Schematic diagram of the high-pressure cell used in the present ultrasonic experiments.
Buffer rod, Al2O3; sample, CaSiO3 glass (Fig. 1a); pressure marker, NaCl and BN; soft medium, MgO; pressure medium, (Mg,Co)O; insulator, LaCrO3. ⊗ denotes the thermocouple hot junction.
Extended Data Fig. 3 Cross-section of the experimental cell recovered after the ultrasonic experiment.
The elemental energy-dispersive spectroscopy mappings of Ca, Si, Al, Mg, Na, Cl and Au, superimposed on the backscattered-electron image of the cross-section, showed no chemical zoning in the CaSiO3 sample or at the interface with the sample and the other cell components.
Extended Data Fig. 4 Experimental longitudinal and shear moduli.
a–f, Results are shown for tetragonal CaPv at 300 K (a, b) and cubic CaPv at 900 K (c, d) and 1,500 K (e, f) as a function of unit-cell volume of CaPv. The solid lines represent fits of the experimental data with the finite-strain EOS (equations (1)–(12)) and the shaded areas represent the 95% confidence intervals, calculated from the trade-off between KS0 and K′ (Extended Data Fig. 4a–e) and G0 and G′ (Extended Data Fig. 4b–f). Most of our experimental data agree within the 95% confidence intervals, demonstrating the small correlation coefficients between the fitted variables.
Supplementary information
Supplementary Table 1
Pressure and temperature conditions of the P- and S-wave velocity and 567 density measurements.
Rights and permissions
About this article
Cite this article
Gréaux, S., Irifune, T., Higo, Y. et al. Sound velocity of CaSiO3 perovskite suggests the presence of basaltic crust in the Earth’s lower mantle. Nature 565, 218–221 (2019). https://doi.org/10.1038/s41586-018-0816-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-018-0816-5
This article is cited by
-
Remnant of the late Permian superplume that generated the Siberian Traps inferred from geomagnetic data
Nature Communications (2023)
-
Primitive noble gases sampled from ocean island basalts cannot be from the Earth’s core
Nature Communications (2022)
-
Weak cubic CaSiO3 perovskite in the Earth’s mantle
Nature (2022)
-
Formation of large low shear velocity provinces through the decomposition of oxidized mantle
Nature Communications (2021)
-
Pressure calibration and sound velocity measurement to 12 GPa in multi-anvil apparatus
Acta Geochimica (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.