Abstract
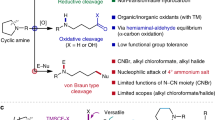
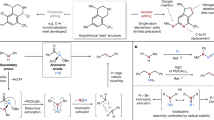
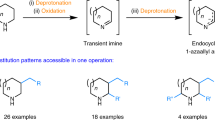
Deconstructive functionalization involves carbon–carbon (C–C) bond cleavage followed by bond construction on one or more of the constituent carbons. For example, ozonolysis1 and olefin metathesis2,3 have allowed each carbon in C=C double bonds to be viewed as a functional group. Despite the substantial advances in deconstructive functionalization involving the scission of C=C double bonds, there are very few methods that achieve C(sp3)–C(sp3) single-bond cleavage and functionalization, especially in relatively unstrained cyclic systems. Here we report a deconstructive strategy to transform saturated nitrogen heterocycles such as piperidines and pyrrolidines, which are important moieties in bioactive molecules, into halogen-containing acyclic amine derivatives through sequential C(sp3)–N and C(sp3)–C(sp3) single-bond cleavage followed by C(sp3)–halogen bond formation. The resulting acyclic haloamines are versatile intermediates that can be transformed into various structural motifs through substitution reactions. In this way we achieve the skeletal remodelling of cyclic amines, an example of scaffold hopping. We demonstrate this deconstructive strategy by the late-stage diversification of proline-containing peptides.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data supporting the findings of this study are available within the paper and its Supplementary Information, or from the corresponding author upon reasonable request.
Change history
01 April 2020
An amendment to this paper has been published and can be accessed via a link at the top of the paper.
References
Bailey, P. S. The reactions of ozone with organic compounds. Chem. Rev. 58, 925–1010 (1958).
Hoveyda, A. H. & Zhugralin, A. R. The remarkable metal-catalysed olefin metathesis reaction. Nature 450, 243–251 (2007).
Vougioukalakis, G. C. & Grubbs, R. H. Ruthenium-based heterocyclic carbene-coordinated olefin metathesis catalysts. Chem. Rev. 110, 1746–1787 (2010).
Galloway, W. R. J. D., Isidro-Llobet, A. & Spring, D. R. Diversity-oriented synthesis as a tool for the discovery of novel biologically active small molecules. Nat. Commun. 1, 80 (2010).
Chen, W., Ma, L., Paul, A. & Seidel, D. Direct α-C–H bond functionalization of unprotected cyclic amines. Nat. Chem. 10, 165–169 (2018).
Cernak, T., Dykstra, K. D., Tyagarajan, S., Vachal, P. & Krska, S. W. The medicinal chemist’s toolbox for late stage functionalization of drug-like molecules. Chem. Soc. Rev. 45, 546–576 (2016).
Sun, H., Tawa, G. & Wallqvist, A. Classification of scaffold-hopping approaches. Drug Discov. Today 17, 310–324 (2012).
Hu, Y., Stumpfe, D. & Bajorath, J. Recent advances in scaffold hopping. J. Med. Chem. 60, 1238–1246 (2017).
Shawcross, A. P. & Stanforth, S. P. Reaction of N-nitroaryl-1,2,3,4-tetrahydroisoquinoline derivatives with oxygen. J. Heterocycl. Chem. 27, 367–369 (1990).
Han, G., McIntosh, M. C. & Weinreb, S. M. A convenient synthetic method for amide oxidation. Tetrahedr. Lett. 35, 5813–5816 (1994).
Ito, R., Umezawa, N. & Higuchi, T. Unique oxidation reaction of amides with pyridine-N-oxide catalyzed by ruthenium porphyrin: direct oxidative conversion of N-acyl-l-proline to N-acyl-l-glutamate. J. Am. Chem. Soc. 127, 834–835 (2005).
Kaname, M., Yoshifuji, S. & Sashida, H. Ruthenium tetroxide oxidation of cyclic N-acylamines by a single layer method: formation of ω-amino acids. Tetrahedr. Lett. 49, 2786–2788 (2008).
Osberger, T. J., Rogness, D. C., Kohrt, J. T., Stepan, A. F. & White, M. C. Oxidative diversification of amino acids and peptides by small-molecule iron catalysis. Nature 537, 214–219 (2016).
Henninot, A., Collins, J. C. & Nuss, J. M. The current state of peptide drug discovery: Back to the future? J. Med. Chem. 61, 1382–1414 (2018).
Yu, C. et al. Selective ring-opening of N-alkyl pyrrolidines with chloroformates to 4-chlorobutyl carbamates. J. Org. Chem. 82, 6615–6620 (2017).
Roque, J. B., Kuroda, Y., Göttemann, L. T. & Sarpong, R. Deconstructive fluorination of cyclic amines by carbon–carbon cleavage. Science 361, 171–174 (2018).
Wang, Z. et al. Silver-catalyzed decarboxylative chlorination of aliphatic carboxylic acids. J. Am. Chem. Soc. 134, 4258–4263 (2012).
Anderson, J. M. & Kochi, J. K. Silver(i)-catalyzed oxidative decarboxylation of acids by peroxydisulfate. Role of silver(ii). J. Am. Chem. Soc. 92, 1651–1659 (1970).
Dai, C., Meschini, F., Narayanam, J. M. R. & Stephenson, C. R. J. Friedel–Crafts amidoalkylation via thermolysis and oxidative photocatalysis. J. Org. Chem. 77, 4425–4431 (2012).
Po, H. N. Heterocyclic and macrocyclic amine complexes of silver(ii) and silver(iii). Coord. Chem. Rev. 20, 171–195 (1976).
Edwards, J. O. & Gallopo, A. R. Kinetics and mechanisms of the spontaneous and metal-modified oxidations of ethanol by peroxydisulfate ion. J. Org. Chem. 36, 4089–4096 (1971).
Tan, X. et al. Silver-catalyzed decarboxylative bromination of aliphatic carboxylic acids. Org. Lett. 19, 1634–1637 (2017).
Reddy, D. N. & Prabhakaran, E. N. Synthesis and isolation of 5,6-dihydro-4H-1,3-oxazine hydrobromides by autocyclization of N-(3-bromopropyl)amides. J. Org. Chem. 76, 680–683 (2011).
Bandarage, U. K. & Davies, R. J. A new synthesis of spiropyrrolidine–tetralones via an unexpected formal ring-contraction of 4-disubstituted piperidine to 3-disubstituted pyrrolidine. Tetrahedr. Lett. 51, 6415–6417 (2010).
Aldmairi, A. H., Griffiths-Jones, C., Dupauw, A., Henderson, L. & Knight, D. W. Piperidines from acid-catalysed cyclisations: pitfalls, solutions and a new ring contraction to pyrrolidines. Tetrahedr. Lett. 58, 3690–3694 (2017).
Sengupta, S. & Mehta, G. Late stage modification of peptides via C–H activation reactions. Tetrahedr. Lett. 58, 1357–1372 (2017).
Wang, F., He, Y., Tian, M., Zhang, X. & Fan, X. Synthesis of α-formylated N-heterocycles and their 1,1-diacetates from inactivated cyclic amines involving an oxidative ring contraction. Org. Lett. 20, 864–867 (2018).
Kolb, H. C., Finn, M. G. & Sharpless, K. B. Click chemistry: diverse chemical function from a few good reactions. Angew. Chem. Int. Ed. 40, 2004–2021 (2001).
Shechter, Y., Burstein, Y. & Patchornik, A. Selective oxidation of methionine residues in proteins. Biochemistry 14, 4497–4503 (1975).
Lin, S. et al. Redox-based reagents for chemoselective methionine bioconjugation. Science 355, 597–602 (2017).
Acknowledgements
This work was supported by the National Institutes of Health (NIH; NIGMS RO1 086374). J.B.R. thanks the NIH for a graduate diversity supplement fellowship (NIGMS RO1 086374). Y.K. thanks the Japan Society for the Promotion of Science (JSPS) for an Overseas Research Fellowship. L.T.G. thanks LMU PROSA and DAAD for financial support. We thank J. Derrick for assistance with electrochemical measurements.
Author information
Authors and Affiliations
Contributions
J.B.R. and Y.K. conceived the research and designed the experiments. J.B.R., Y.K. and L.T.G. performed the experiments. R.S. directed the project. J.B.R., Y.K. and R.S. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
J.B.R., Y.K., L.T.G. and R.S. are listed as inventors on an initial patent application describing the silver-mediated deconstructive halogenation of cyclic amines and subsequent transformations (052103-515P01US).
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
This file contains Supplementary Text and Data – see contents page for details
Rights and permissions
About this article
Cite this article
Roque, J.B., Kuroda, Y., Göttemann, L.T. et al. Deconstructive diversification of cyclic amines. Nature 564, 244–248 (2018). https://doi.org/10.1038/s41586-018-0700-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-018-0700-3
Keywords
This article is cited by
-
Synthesis of dienes from pyrrolidines using skeletal modification
Nature Communications (2023)
-
Carbon-to-nitrogen single-atom transmutation of azaarenes
Nature (2023)
-
Isolable iminium ions as a platform for N-(hetero)aryl piperidine synthesis
Nature Synthesis (2023)
-
Single-atom skeletal editing of 2H-indazoles enabled by difluorocarbene
Science China Chemistry (2023)
-
Decarboxylative oxidation-enabled consecutive C-C bond cleavage
Nature Communications (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.