Abstract
The latent viral reservoir is the critical barrier for the development of a cure for HIV-1 infection. Previous studies have shown direct antiviral activity of potent HIV-1 Env-specific broadly neutralizing antibodies (bNAbs) administered when antiretroviral therapy (ART) was discontinued, but it remains unclear whether bNAbs can target the viral reservoir during ART. Here we show that administration of the V3 glycan-dependent bNAb PGT121 together with the Toll-like receptor 7 (TLR7) agonist vesatolimod (GS-9620) during ART delayed viral rebound following discontinuation of ART in simian–human immunodeficiency virus (SHIV)-SF162P3-infected rhesus monkeys in which ART was initiated during early acute infection. Moreover, in the subset of monkeys that were treated with both PGT121 and GS-9620 and that did not show viral rebound after discontinuation of ART, adoptive transfer studies and CD8-depletion studies also did not reveal virus. These data demonstrate the potential of bNAb administration together with innate immune stimulation as a possible strategy for targeting the viral reservoir.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data generated and analysed in this study are available from the corresponding author upon reasonable request. Source data for figures from individual animals are available online.
Change history
05 November 2018
In Fig. 4b of this Article, the x-axis labels ‘PGT121’ and ‘GS-9620’ were inadvertently swapped in both graphs. In Fig. 5a, b, ‘TLR7’ should have been ‘GS-9620’. These figures have been corrected online.
References
Finzi, D. et al. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science 278, 1295–1300 (1997).
Persaud, D., Zhou, Y., Siliciano, J. M. & Siliciano, R. F. Latency in human immunodeficiency virus type 1 infection: no easy answers. J. Virol. 77, 1659–1665 (2003).
Chun, T. W. et al. Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proc. Natl Acad. Sci. USA 94, 13193–13197 (1997).
Ho, Y. C. et al. Replication-competent noninduced proviruses in the latent reservoir increase barrier to HIV-1 cure. Cell 155, 540–551 (2013).
Finzi, D. et al. Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nat. Med. 5, 512–517 (1999).
Chun, T. W., Davey, R. T., Jr, Engel, D., Lane, H. C. & Fauci, A. S. Re-emergence of HIV after stopping therapy. Nature 401, 874–875 (1999).
Barouch, D. H. & Deeks, S. G. Immunologic strategies for HIV-1 remission and eradication. Science 345, 169–174 (2014).
Deeks, S. G. et al. International AIDS Society global scientific strategy: towards an HIV cure 2016. Nat. Med. 22, 839–850 (2016).
Shan, L. et al. Stimulation of HIV-1-specific cytolytic T lymphocytes facilitates elimination of latent viral reservoir after virus reactivation. Immunity 36, 491–501 (2012).
Barouch, D. H. et al. Therapeutic efficacy of potent neutralizing HIV-1-specific monoclonal antibodies in SHIV-infected rhesus monkeys. Nature 503, 224–228 (2013).
Shingai, M. et al. Antibody-mediated immunotherapy of macaques chronically infected with SHIV suppresses viraemia. Nature 503, 277–280 (2013).
Julg, B. et al. Virological control by the CD4-binding site antibody N6 in simian-human immunodeficiency virus-infected rhesus monkeys. J. Virol. 91, e00498-17 (2017).
Caskey, M. et al. Viraemia suppressed in HIV-1-infected humans by broadly neutralizing antibody 3BNC117. Nature 522, 487–491 (2015).
Caskey, M. et al. Antibody 10-1074 suppresses viremia in HIV-1-infected individuals. Nat. Med. 23, 185–191 (2017).
Scheid, J. F. et al. HIV-1 antibody 3BNC117 suppresses viral rebound in humans during treatment interruption. Nature 535, 556–560 (2016).
Bar, K. J. et al. Effect of HIV antibody VRC01 on viral rebound after treatment interruption. N. Engl. J. Med. 375, 2037–2050 (2016).
Walker, L. M. et al. Broad neutralization coverage of HIV by multiple highly potent antibodies. Nature 477, 466–470 (2011).
Liu, J. et al. Antibody-mediated protection against SHIV challenge includes systemic clearance of distal virus. Science 353, 1045–1049 (2016).
Tsai, A. et al. Toll-like receptor 7 agonist GS-9620 induces HIV expression and HIV-specific immunity in cells from HIV-infected individuals on suppressive antiretroviral therapy. J. Virol. 91, e02166-16 (2017).
Borducchi, E. N. et al. Ad26/MVA therapeutic vaccination with TLR7 stimulation in SIV-infected rhesus monkeys. Nature 540, 284–287 (2016).
Barouch, D. H. et al. Protective efficacy of a global HIV-1 mosaic vaccine against heterologous SHIV challenges in rhesus monkeys. Cell 155, 531–539 (2013).
Kawai, T. et al. Interferon-α induction through Toll-like receptors involves a direct interaction of IRF7 with MyD88 and TRAF6. Nat. Immunol. 5, 1061–1068 (2004).
Hemmi, H. et al. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat. Immunol. 3, 196–200 (2002).
Whitney, J. B. et al. Rapid seeding of the viral reservoir prior to SIV viraemia in rhesus monkeys. Nature 512, 74–77 (2014).
Henrich, T. J. et al. Antiretroviral-free HIV-1 remission and viral rebound after allogeneic stem cell transplantation: report of 2 cases. Ann. Intern. Med. 161, 319–327 (2014).
Persaud, D. et al. Absence of detectable HIV-1 viremia after treatment cessation in an infant. N. Engl. J. Med. 369, 1828–1835 (2013).
Nishimura, Y. et al. Early antibody therapy can induce long-lasting immunity to SHIV. Nature 543, 559–563 (2017).
Acknowledgements
We thank K. Reimann and A. Hill for advice, assistance, and reagents. We acknowledge support from the Bill & Melinda Gates Foundation (OPP1107669), the American Foundation for AIDS Research (109219-58-RGRL), the National Institutes of Health (AI096040, AI124377, AI126603, AI129797, AI128751, OD024917), and the Ragon Institute of MGH, MIT, and Harvard.
Author information
Authors and Affiliations
Contributions
D.H.B. and R.G. designed the study. J.H. and R.G. developed the ART formulation and TLR7 agonist. E.B. conducted the cytokine analyses. E.N.B., J.L., J.P.N., A.M.C., P.A., N.B.M., A.C., D.J., L.P., K.M., and E.T.M. performed the immunologic and virologic assays. W.-H.Y., S.F., T.B., and G.A. led the computational modelling. W.L. led the statistical analysis. M.G.L. led the clinical care of the rhesus monkeys. D.H.B. led the study and wrote the paper with all co-authors.
Corresponding author
Ethics declarations
Competing interests
E.B., J.H., and R.G. are employees of Gilead Sciences. The other authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Study design.
Forty-four rhesus monkeys (n = 11 monkeys per group) were infected with SHIV-SF162P3 at week 0 and ART was initiated at week 1 (day 7). GS-9620 administrations and PGT121 infusions are shown from weeks 96 to 114. ART was discontinued at week 130.
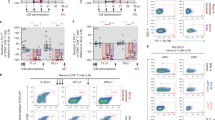
Extended Data Fig. 2 Immune activation following GS-9620 administration and before ART discontinuation.
a, Activation of CD4+ T cells was assessed by CD38 expression on days 0 and 1 following GS-9620 administration, supplementing the data shown in Fig. 2a (n = 11 monkeys per group). Representative data are shown following the fifth GS-9620 dose, which was comparable to the other doses. Red horizontal bars indicate median values. P values reflect two-sided Mann–Whitney tests. b, Plasma levels of IFNα, IL-1RA, I-TAC, eotaxin, MIG, MCP-1, IL-1β, IL-6, IP-10 are shown on day 1 following GS-9620 administration (n = 11 monkeys per group). Red bars represent mean values. Combined data from all GS-9620 administrations with pre-dose levels subtracted are shown.
Extended Data Fig. 3 Anti-drug antibody (ADA) assay before ART discontinuation.
ADA responses were assessed in the PGT121+GS-9620 and PGT121-alone groups every 2 weeks from weeks 106 to 124 using an electrochemoluminescence (ECL) assay with an anti-PGT121 idiotypic mAb (n = 11 monkeys per group). No ADA was detected. One monkey in the PGT121+GS-9620 group had background reactivity in this assay at week 106 before PGT121 exposure (green bars).
Extended Data Fig. 4 PGT121 pharmacokinetics in serum and tissues before ART discontinuation.
a, Peak serum PGT121 levels are shown (limit of detection 0.5 μg ml−1) 1 h following each of five infusions of PGT121 (weeks 106–114) and during the washout period (weeks 114–130) (n = 11 monkeys per group). b, PGT121 levels (limit of detection 0.5 μg ml−1) were assessed in cell lysates (L) and initial wash supernatants (S) from 106 lymph node (LN) and colorectal (CR) cells from week 120 (n = 11 monkeys per group). Positive controls (Ctrl) included lymph node samples from naive monkeys spiked with PGT121. Red bars represent median values.
Extended Data Fig. 5 IFNγ ELISPOT responses before ART discontinuation.
Gag-, Env-, and Pol-specific IFNγ ELISPOT responses in PBMCs are shown at week 4 (a), week 96 (b), and week 120 (c) (n = 11 monkeys per group). Spot-forming cells (SFCs) per million PBMCs are shown. Red horizontal bars indicate median values. The dotted line represents the assay limit of quantitation (55 SFCs per million PBMCs).
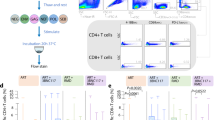
Extended Data Fig. 6 IFNγ intracellular cytokine staining (ICS) responses before ART discontinuation.
Gag-, Env-, and Pol-specific IFNγ ICS responses in PBMCs (a) and in LNMCs (b) are shown at week 120 (n = 11 monkeys per group). Per cent IFNγ-producing CD8+CD3+ T cells in PBMCs and per cent IFNγ-producing total CD8+CD3+ T cells (CD8) and follicular CXCR5+CD8+CD3+ T cells (fCD8) in LNMCs are shown. Red horizontal bars indicate median values. The dotted line represents the assay limit of quantitation (0.05% CD8+CD3+ T cells).
Extended Data Fig. 7 IFNγ ELISPOT responses following ART discontinuation and trends for viral rebound.
a, Gag-, Env-, and Pol-specific IFNγ ELISPOT responses in PBMCs are shown at day 140 following ART discontinuation (n = 11 monkeys per group). SFCs per million PBMCs are shown. Monkeys in each group that demonstrated viral rebound versus no rebound are shown separately. Red horizontal bars indicate median values. The dotted line represents the assay limit of quantitation (55 SFCs per million PBMCs). b, Trends for viral rebound are shown in the GS-9620 and PGT121 groups in relation to pre-ART week 1 viral loads, supplementing the data shown in Fig. 5c. Red horizontal bars indicate median values.
Extended Data Fig. 8 CD8 depletion efficiency.
CD8+ T cells per μl peripheral blood are shown in PGT121+GS-9620-treated monkeys before and after CD8 depletion in monkeys that exhibited viral rebound and post-rebound virologic control (n = 5, left, red lines) and in monkeys that exhibited no viral rebound following ART discontinuation (n = 5, right, black lines).
Extended Data Fig. 9 Computational model.
a, LASSO and PLSR model identifies the parameters that correlate with delayed viral rebound (n = 11 monkeys per group). Left, individual monkeys are shown distributed by latent variables 1 and 3 of the model. Timing of viral rebound is indicated by the colour gradient. R2 = 0.176, root mean square error (RMSE) = 0.917, P < 0.001 in two-sided permutation tests. Middle, the contribution of the selected features to model separation is displayed in variable importance in projection (VIP) scores, related to early (blue) or late (red) viral rebound. Right, correlation between viral rebound and latent variable 1. P value reflects a two-sided Spearman rank-correlation test. b, LASSO and PLSR model identifies the parameters that correlate with reduced total viral loads (n = 11 monkeys per group). Left, individual monkeys are showed distributed by latent variables 1 and 2. Total viral loads are indicated by the colour gradient. R2 = 0.282, root mean square error (RMSE) = 0.857, P < 0.001 in in two-sided permutation tests. Middle, the contribution of the selected features to model separation is displayed in VIP scores, related to high (blue) or low (red) total viral loads. Right, correlation between total viral loads and latent variable 1. P value reflects a two-sided Spearman rank-correlation test.
Extended Data Fig. 10 In vitro killing studies.
a, GS-9620 treatment led to CD69 upregulation of CD56+ NK cells and CD3+ T cells in vitro following incubation of human PBMCs with 1,000 nM GS-9620 for 5 days (n = 9). b, GS-9620 treatment augmented PGT121-mediated killing of PGT121 in vitro. Per cent p24 reduction in CD4+ T cells (n = 6) using an antibody-mediated killing assay (see Methods). Per cent killing was calculated as the per cent reduction in p24 in CD4+ T cells with PGT121 compared with no PGT121. P values reflect two-sided paired Student’s t-tests.
Supplementary information
Rights and permissions
About this article
Cite this article
Borducchi, E.N., Liu, J., Nkolola, J.P. et al. Antibody and TLR7 agonist delay viral rebound in SHIV-infected monkeys. Nature 563, 360–364 (2018). https://doi.org/10.1038/s41586-018-0600-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-018-0600-6
Keywords
This article is cited by
-
The cell biology of HIV-1 latency and rebound
Retrovirology (2024)
-
Inhibitory receptor CD47 binding to plasma TSP1 suppresses NK-cell IFN-γ production via activating the JAK/STAT3 pathway during HIV infection
Journal of Translational Medicine (2023)
-
Multimeric immunotherapeutic complexes activating natural killer cells towards HIV-1 cure
Journal of Translational Medicine (2023)
-
Prevention, treatment and cure of HIV infection
Nature Reviews Microbiology (2023)
-
AZD5582 plus SIV-specific antibodies reduce lymph node viral reservoirs in antiretroviral therapy-suppressed macaques
Nature Medicine (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.