Abstract
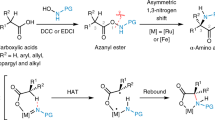
Quaternary amino acids, in which the α-carbon that bears the amino and carboxyl groups also carries two carbon substituents, have an important role as modifiers of peptide conformation and bioactivity and as precursors of medicinally important compounds1,2. In contrast to enantioselective alkylation at this α-carbon, for which there are several methods3,4,5,6,7,8, general enantioselective introduction of an aryl substituent at the α-carbon is synthetically challenging9. Nonetheless, the resultant α-aryl amino acids and their derivatives are valuable precursors to bioactive molecules10,11. Here we describe the synthesis of quaternary α-aryl amino acids from enantiopure amino acid precursors by α-arylation without loss of stereochemical integrity. Our approach relies on the temporary formation of a second stereogenic centre in an N′-arylurea adduct12 of an imidazolidinone derivative6 of the precursor amino acid, and uses readily available enantiopure amino acids both as a precursor and as a source of asymmetry. It avoids the use of valuable transition metals, and enables arylation with electron-rich, electron-poor and heterocyclic substituents. Either enantiomer of the product can be formed from a single amino acid precursor. The method is practical and scalable, and provides the opportunity to produce α-arylated quaternary amino acids in multi-gram quantities.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Toniolo, C., Crisma, M., Formaggio, F. & Peggion, C. Control of peptide conformation by the Thorpe–Ingold effect (Cα-tetrasubstitution). Biopolymers 60, 396–419 (2001).
Meusel, M. & Gütschow, M. Recent developments in hydantoin chemistry. A review. Org. Prep. Proced. Int. 36, 391–443 (2004).
Cativiela, C. & Díaz-de-Villegas, M. D. Recent progress on the stereoselective synthesis of acyclic quaternary α-amino acids. Tetrahedron Asymmetry 18, 569–623 (2007).
Hashimoto, T. & Maruoka, K. Recent development and application of chiral phase-transfer catalysts. Chem. Rev. 107, 5656–5682 (2007).
Schöllkopf, U. Enantioselective synthesis of non-proteinogenic amino acids via metallated bis-lactim ethers of 2,5-diketopiperazines. Tetrahedron 39, 2085–2091 (1983).
Seebach, D., Sting, A. R. & Hoffmann, M. Self-regeneration of stereocenters (SRS)—applications, limitations, and abandonment of a synthetic principle. Angew. Chem. Int. Edn Engl. 35, 2708–2748 (1996).
Kawabata, T. & Fuji, K. Memory of chirality: asymmetric induction based on the dynamic chirality of enolates. Top. Stereochem. 23, 175–205 (2003).
Branca, M. et al. Memory of chirality of tertiary aromatic amides: a simple and efficient method for the enantioselective synthesis of quaternary α-amino acids. J. Am. Chem. Soc. 131, 10711–10718 (2009).
Shirakawa, S., Yamamoto, K. & Maruoka, K. Phase-transfer-catalyzed asymmetric SNAr reaction of α-amino acid derivatives with arene chromium complexes. Angew. Chem. Int. Ed. 54, 838–840 (2015).
Ma, D. W. Conformationally constrained analogues of l-glutamate as subtype-selective modulators of metabotropic glutamate receptors. Bioorg. Chem. 27, 20–34 (1999).
Sonowal, H. et al. Aldose reductase inhibitor increases doxorubicin-sensitivity of colon cancer cells and decreases cardiotoxicity. Sci. Rep. 7, 3182 (2017).
Clayden, J., Dufour, J., Grainger, D. M. & Helliwell, M. Substituted diarylmethylamines by stereospecific intramolecular electrophilic arylation of lithiated ureas. J. Am. Chem. Soc. 129, 7488–7489 (2007).
Wang, X.-J. et al. Asymmetric synthesis of LFA-1 inhibitor BIRT2584 on metric ton scale. Org. Process Res. Dev. 15, 1185–1191 (2011).
Yee, N. K. et al. Practical synthesis of a cell adhesion inhibitor by self-regeneration of stereocenters. Tetrahedron Asymmetry 14, 3495–3501 (2003).
Grainger, D. M. et al. The mechanism of the stereospecific intramolecular arylation of lithiated ureas: the role of Li+ probed by electronic structure calculations, and by NMR and IR spectroscopy. Eur. J. Org. Chem. 4, 731–743 (2012).
Atkinson, R. C. et al. Intramolecular arylation of amino acid enolates. Chem. Commun. 49, 9734–9736 (2013).
Tomohara, K., Yoshimura, T., Hyakutake, R., Yang, P. & Kawabata, T. Asymmetric α-arylation of amino acid derivatives by Clayden rearrangement of ester enolates via memory of chirality. J. Am. Chem. Soc. 135, 13294–13297 (2013).
Atkinson, R. C., Fernández-Nieto, F., Mas Roselló, J. & Clayden, J. Pseudoephedrine-directed asymmetric α-arylation of α-amino acid derivatives. Angew. Chem. Int. Ed. 54, 8961–8965 (2015).
Nagib, D. A., Scott, M. E. & MacMillan, D. W. C. Enantioselective α-trifluoromethylation of aldehydes via photoredox organocatalysis. J. Am. Chem. Soc. 131, 10875–10877 (2009).
Wakeham, R. J., Taylor, J. E., Bull, S. D., Morris, J. A. & Williams, J. M. J. Iodide as an activating agent for acid chlorides in acylation reactions. Org. Lett. 15, 702–705 (2013).
Naef, R. & Seebach, D. Preparation of the enantiomercially pure cis-configurated and trans-configurated 2-(tert-butyl)-3-methylimidazolidin-4-ones from the amino-acids (S)-alanine, (S)-phenylalanine, (R)-phenylglycine, (S)-methionine, and (S)-valine. Helv. Chim. Acta 68, 135–143 (1985).
Holden, C. M. & Greaney, M. F. Modern aspects of the Smiles rearrangement. Chem Eur. J. 23, 8992–9008 (2017).
Snape, T. J. A truce on the Smiles rearrangement: revisiting an old reaction—the Truce–Smiles rearrangement. Chem. Soc. Rev. 37, 2452–2458 (2008).
Sung, R.-Y. et al. Kinetic studies on the nucleophilic substitution reaction of 4-X-substituted-2,6-dinitrochlorobenzene with pyridines in MeOH–MeCN mixtures. Bull. Korean Chem. Soc. 30, 1579–1582 (2009).
Bunnett, J. F. & Zahler, R. E. Aromatic nucleophilic substitution reactions. Chem. Rev. 49, 273–412 (1951).
Schimler, S. D. et al. Nucleophilic deoxyfluorination of phenols via aryl fluorosulfonate intermediates. J. Am. Chem. Soc. 139, 1452–1455 (2017).
Neumann, C. N. & Ritter, T. Facile C–F bond formation through a concerted nucleophilic aromatic substitution mediated by the PhenoFluor reagent. Acc. Chem. Res. 50, 2822–2833 (2017).
Kwan, E. E., Zeng, Y., Besser, H. A. & Jacobsen, E. N. Concerted nucleophilic aromatic substitutions. Nat. Chem. 10, 917–923 (2018).
Clayden, J., Hennecke, U., Vincent, M. A., Hillier, I. H. & Helliwell, M. The origin of the conformational preference of N,N′-diaryl-N,N′-dimethyl ureas. Phys. Chem. Chem. Phys. 12, 15056–15064 (2010).
Costil, R. et al. Heavily substituted atropisomeric diarylamines by unactivated Smiles rearrangement of N-aryl anthranilamides. Angew. Chem. Int. Ed. 56, 12533–12537 (2017).
Acknowledgements
We acknowledge funding from the EPSRC (GR/L018527) and ERC (Advanced Grant ROCOCO and Proof of Concept grant QUATERMAIN), and we are grateful to M. M. Amer for assistance with the synthesis of starting materials.
Reviewer information
Nature thanks T. Kawabata and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
D.J.L., J.W.W. and J.C. devised the experiments; D.J.L. and J.W.W. carried out the experiments; D.J.L., J.W.W. and J.C. analysed the results and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors have filed a patent on this work (GB1621512.1).
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Supplementary information
Supplementary Information
This file contains: General Information, General Procedures, Experimental Procedures and Characterisation Data, 1H and 13C NMR Spectra, HPLC and SFC Traces, X-Ray Crystallographic Data, Crossover Experiment, In situ IR (ReactIR), and References
Rights and permissions
About this article
Cite this article
Leonard, D.J., Ward, J.W. & Clayden, J. Asymmetric α-arylation of amino acids. Nature 562, 105–109 (2018). https://doi.org/10.1038/s41586-018-0553-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-018-0553-9
Keywords
This article is cited by
-
Challenges and recent advancements in the synthesis of α,α-disubstituted α-amino acids
Nature Communications (2024)
-
Making α-aryl quaternary stereocentres
Nature Synthesis (2024)
-
Modular α-tertiary amino ester synthesis through cobalt-catalysed asymmetric aza-Barbier reaction
Nature Chemistry (2024)
-
Nickel-catalysed regio- and stereoselective acylzincation of unsaturated hydrocarbons with organozincs and CO
Nature Synthesis (2023)
-
Ligand-enabled palladium-catalysed enantioselective synthesis of α-quaternary amino and glycolic acids derivatives
Nature Synthesis (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.