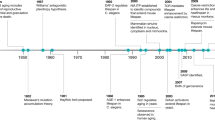
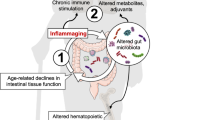
Abstract
Longer human lives have led to a global burden of late-life disease. However, some older people experience little ill health, a trait that should be extended to the general population. Interventions into lifestyle, including increased exercise and reduction in food intake and obesity, can help to maintain healthspan. Altered gut microbiota, removal of senescent cells, blood factors obtained from young individuals and drugs can all improve late-life health in animals. Application to humans will require better biomarkers of disease risk and responses to interventions, closer alignment of work in animals and humans, and increased use of electronic health records, biobank resources and cohort studies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Oeppen, J. & Vaupel, J. W. Broken limits to life expectancy. Science 296, 1029–1031 (2002).
Waite, L. J. Aging, Health, and Public Policy: Demographic and Economic Perspectives (Population Council, 2004).
Fogel, R. W. & Costa, D. L. A theory of technophysio evolution, with some implications for forecasting population, health care costs, and pension costs. Demography 34, 49–66 (1997).
Vaupel, J. W. et al. Biodemographic trajectories of longevity. Science 280, 855–860 (1998).
Dong, X., Milholland, B. & Vijg, J. Evidence for a limit to human lifespan. Nature 538, 257–259 (2016).
Kontis, V. et al. Future life expectancy in 35 industrialised countries: projections with a Bayesian model ensemble. Lancet 389, 1323–1335 (2017). Analysis of age-specific death rates in 35 industrialized countries shows that there is a high probability that life expectancy in these countries will continue to increase during the coming decades.
Christensen, K. et al. Physical and cognitive functioning of people older than 90 years: a comparison of two Danish cohorts born 10 years apart. Lancet 382, 1507–1513 (2013).
Zeng, Y., Feng, Q., Hesketh, T., Christensen, K. & Vaupel, J. W. Survival, disabilities in activities of daily living, and physical and cognitive functioning among the oldest-old in China: a cohort study. Lancet 389, 1619–1629 (2017).
Burger, O., Baudisch, A. & Vaupel, J. W. Human mortality improvement in evolutionary context. Proc. Natl Acad. Sci. USA 109, 18210–18214 (2012).
Poulain, M., Herm, A. & Pes, G. The blue zones: areas of exceptional longevity around the world. Vienna Yearb. Popul. Res. 11, 87–108 (2013).
Cooper, R., Strand, B. H., Hardy, R., Patel, K. V. & Kuh, D. Physical capability in mid-life and survival over 13 years of follow-up: British birth cohort study. Br. Med. J. 348, g2219 (2014).
Crimmins, E. M. Lifespan and healthspan: past, present, and promise. Gerontologist 55, 901–911 (2015).
Jagger, C. et al. Inequalities in healthy life years in the 25 countries of the European Union in 2005: a cross-national meta-regression analysis. Lancet 372, 2124–2131 (2008).
World Report on Ageing and Health. http://who.int/ageing/events/world-report-2015-launch/en/ (WHO, 2015).
Stenholm, S. et al. Body mass index as a predictor of healthy and disease-free life expectancy between ages 50 and 75: a multicohort study. Int. J. Obes. 41, 769–775 (2017).
Niccoli, T. & Partridge, L. Ageing as a risk factor for disease. Curr. Biol. 22, R741–R752 (2012).
Christensen, K., McGue, M., Petersen, I., Jeune, B. & Vaupel, J. W. Exceptional longevity does not result in excessive levels of disability. Proc. Natl Acad. Sci. USA 105, 13274–13279 (2008). A survey of a Danish cohort born in 1905 and followed for physical and cognitive independence from 1998 to 2005 across the age range of 92–100 years shows that lifespan extension of a population does not necessarily results in exceptional levels of disability at high ages.
Andersen, S. L., Sebastiani, P., Dworkis, D. A., Feldman, L. & Perls, T. T. Health span approximates life span among many supercentenarians: compression of morbidity at the approximate limit of life span. J. Gerontol. A 67A, 395–405 (2012).
Kenyon, C. J. The genetics of ageing. Nature 464, 504–512 (2010).
Fontana, L. & Partridge, L. Promoting health and longevity through diet: from model organisms to humans. Cell 161, 106–118 (2015).
Fontana, L., Partridge, L. & Longo, V. D. Extending healthy life span—from yeast to humans. Science 328, 321–326 (2010).
López-Otín, C., Blasco, M. A., Partridge, L., Serrano, M. & Kroemer, G. The hallmarks of aging. Cell 153, 1194–1217 (2013).
van den Berg, N., Beekman, M., Smith, K. R., Janssens, A. & Slagboom, P. E. Historical demography and longevity genetics: back to the future. Ageing Res. Rev. 38, 28–39 (2017).
Kaplanis, J. et al. Quantitative analysis of population-scale family trees with millions of relatives. Science 360, 171–175 (2018).
Hjelmborg, J. et al. Genetic influence on human lifespan and longevity. Hum. Genet. 119, 312–321 (2006).
Slagboom, P. E., van den Berg, N. & Deelen, J. Phenome and genome based studies into human ageing and longevity: An overview. Biochim. Biophys. Acta https://doi.org/10.1016/j.bbadis.2017.09.017 (2017).
Mahley, R. W. & Rall, S. C. Jr. Apolipoprotein E: far more than a lipid transport protein. Annu. Rev. Genomics Hum. Genet. 1, 507–537 (2000).
Sebastiani, P., Nussbaum, L., Andersen, S. L., Black, M. J. & Perls, T. T. Increasing sibling relative risk of survival to older and older ages and the importance of precise definitions of “aging,” “life span,” and “longevity”. J. Gerontol. A 71, 340–346 (2016).
Nygaard, M. et al. Birth cohort differences in the prevalence of longevity-associated variants in APOE and FOXO3A in Danish long-lived individuals. Exp. Gerontol. 57, 41–46 (2014).
Terry, D. F. et al. Lower all-cause, cardiovascular, and cancer mortality in centenarians’ offspring. J. Am. Geriatr. Soc. 52, 2074–2076 (2004).
Westendorp, R. G. et al. Nonagenarian siblings and their offspring display lower risk of mortality and morbidity than sporadic nonagenarians: The Leiden Longevity Study. J. Am. Geriatr. Soc. 57, 1634–1637 (2009).
Newman, A. B. et al. Health and function of participants in the Long Life Family Study: a comparison with other cohorts. Aging (Albany NY) 3, 63–76 (2011).
Deelen, J. et al. Employing biomarkers of healthy ageing for leveraging genetic studies into human longevity. Exp. Gerontol. 82, 166–174 (2016).
Ash, A. S. et al. Are members of long-lived families healthier than their equally long-lived peers? Evidence from the Long Life Family Study. J. Gerontol. A 70, 971–976 (2015).
Beekman, M. et al. Genome-wide association study (GWAS)-identified disease risk alleles do not compromise human longevity. Proc. Natl Acad. Sci. USA 107, 18046–18049 (2010).
Erikson, G. A. et al. Whole-genome sequencing of a healthy aging cohort. Cell 165, 1002–1011 (2016).
Bergman, A., Atzmon, G., Ye, K., MacCarthy, T. & Barzilai, N. Buffering mechanisms in aging: a systems approach toward uncovering the genetic component of aging. PLOS Comput. Biol. 3, e170 (2007).
Suh, Y. et al. Functionally significant insulin-like growth factor I receptor mutations in centenarians. Proc. Natl Acad. Sci. USA 105, 3438–3442 (2008).
Druley, T. E. et al. Candidate gene resequencing to identify rare, pedigree-specific variants influencing healthy aging phenotypes in the long life family study. BMC Geriatr. 16, 80 (2016).
Morris, B. J., Willcox, D. C., Donlon, T. A. & Willcox, B. J. FOXO3: a major gene for human longevity—a mini-review. Gerontology 61, 515–525 (2015).
Flachsbart, F. et al. Association of FOXO3A variation with human longevity confirmed in German centenarians. Proc. Natl Acad. Sci. USA 106, 2700–2705 (2009). A genetic variant in FOXO3A , rs2802292, is associated with longevity in humans.
Willcox, B. J. et al. FOXO3A genotype is strongly associated with human longevity. Proc. Natl Acad. Sci. USA 105, 13987–13992 (2008).
Belsky, D. W. et al. Quantification of biological aging in young adults. Proc. Natl Acad. Sci. USA 112, E4104–E4110 (2015).
Bektas, A., Schurman, S. H., Sen, R. & Ferrucci, L. Aging, inflammation and the environment. Exp. Gerontol. 105, 10–18 (2018).
Chahal, H. S. & Drake, W. M. The endocrine system and ageing. J. Pathol. 211, 173–180 (2007).
Lakatta, E. G. & Levy, D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises. Part II: the aging heart in health: links to heart disease. Circulation 107, 346–354 (2003).
Barnett, K. et al. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet 380, 37–43 (2012). Analysis of Scottish health registry data from 2007 to 2012 shows a high level of multimorbidity (two or more disorders) in those over age 65 and a 10–15 years earlier onset of multimorbidity in people living in socio-economically deprived areas, challenging the single-disease clinical framework and advocating personalized approaches.
Crimmins, E. M., Kim, J. K. & Seeman, T. E. Poverty and biological risk: the earlier “aging” of the poor. J. Gerontol. A 64A, 286–292 (2009).
McGuigan, F. E., Bartosch, P. & Åkesson, K. E. Musculoskeletal health and frailty. Best Pract. Res. Clin. Rheumatol. 31, 145–159 (2017).
Crimmins, E., Kim, J. K. & Vasunilashorn, S. Biodemography: new approaches to understanding trends and differences in population health and mortality. Demography 47, S41–S64 (2010).
Ensrud, K. E. et al. Frailty and risk of falls, fracture, and mortality in older women: the study of osteoporotic fractures. J. Gerontol. A 62, 744–751 (2007).
Marengoni, A. et al. Aging with multimorbidity: a systematic review of the literature. Ageing Res. Rev. 10, 430–439 (2011).
Guthrie, B., Makubate, B., Hernandez-Santiago, V. & Dreischulte, T. The rising tide of polypharmacy and drug–drug interactions: population database analysis 1995–2010. BMC Med. 13, 74 (2015).
Gu, Q., Dillon, C. F. & Burt, V. L. Prescription drug use continues to increase: U.S. prescription drug data for 2007–2008. NCHS Data Brief 42, 1–8 (2010).
Bushardt, R. L., Massey, E. B., Simpson, T. W., Ariail, J. C. & Simpson, K. N. Polypharmacy: misleading, but manageable. Clin. Interv. Aging 3, 383–389 (2008).
Parameswaran Nair, N. et al. Hospitalization in older patients due to adverse drug reactions — the need for a prediction tool. Clin. Interv. Aging 11, 497–505 (2016).
Marcum, Z. A. et al. Prevalence of unplanned hospitalizations caused by adverse drug reactions in older veterans. J. Am. Geriatr. Soc. 60, 34–41 (2012).
Howard, R. L. et al. Which drugs cause preventable admissions to hospital? A systematic review. Br. J. Clin. Pharmacol. 63, 136–147 (2007).
Stringhini, S. et al. Socioeconomic status and the 25 × 25 risk factors as determinants of premature mortality: a multicohort study and meta-analysis of 1·7 million men and women. Lancet 389, 1229–1237 (2017). A plea to include socio-economic factors into the initiative of high income WHO member states to cut mortality due to non-communicable diseases by 25% by 2025, showing in a very large prospective multi-cohort meta-analysis study the years-of-life-lost due to high alcohol intake, physical inactivity, current smoking, hypertension, diabetes, obesity and low socio-economic status.
Harvey, J. A., Chastin, S. F. & Skelton, D. A. How Sedentary are older people? A systematic review of the amount of sedentary behavior. J. Aging Phys. Act. 23, 471–487 (2015).
Global Action Plan for the Prevention and Control of NCDs 2013–2020. http://who.int/nmh/events/ncd_action_plan/en/ (WHO, 2013).
Diabetes Prevention Program Research Group. Long-term effects of lifestyle intervention or metformin on diabetes development and microvascular complications over 15-year follow-up: the Diabetes Prevention Program Outcomes Study. Lancet Diabetes Endocrinol. 3, 866–875 (2015).
Estruch, R. et al. Primary prevention of cardiovascular disease with a mediterranean diet supplemented with extra-virgin olive oil or nuts. N. Engl. J. Med. 378, e34 (2018).
Toledo, E. et al. Mediterranean diet and invasive breast cancer risk among women at high cardiovascular risk in the PREDIMED trial: a randomized clinical trial. JAMA Intern. Med. 175, 1752–1760 (2015).
Penedo, F. J. & Dahn, J. R. Exercise and well-being: a review of mental and physical health benefits associated with physical activity. Curr. Opin. Psychiatry 18, 189–193 (2005).
Heilbronn, L. K. et al. Effect of 6-month calorie restriction on biomarkers of longevity, metabolic adaptation, and oxidative stress in overweight individuals: a randomized controlled trial. J. Am. Med. Assoc. 295, 1539–1548 (2006).
Ngandu, T. et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet 385, 2255–2263 (2015). A two-year proof-of-concept randomized clinical trial in the Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER), in which positive results are found for a multi-domain approach (diet, exercise, cognitive training and vascular risk monitoring) compared to general health advice to prevent cognitive decline in at-risk elderly people (60–77 years of age) from the general population.
Most, J., Tosti, V., Redman, L. M. & Fontana, L. Calorie restriction in humans: an update. Ageing Res. Rev. 39, 36–45 (2017).
Mattson, M. P., Longo, V. D. & Harvie, M. Impact of intermittent fasting on health and disease processes. Ageing Res. Rev. 39, 46–58 (2017).
Laird, E. et al. The prevalence of vitamin D deficiency and the determinants of 25(OH)D concentration in older Irish adults: data from The Irish Longitudinal Study on Ageing (TILDA). J. Gerontol. A 73, 519–525 (2018).
Levine, M. E. et al. Low protein intake is associated with a major reduction in IGF-1, cancer, and overall mortality in the 65 and younger but not older population. Cell Metab. 19, 407–417 (2014).
Ettehad, D. et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet 387, 957–967 (2016).
Collins, R. et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet 388, 2532–2561 (2016).
Bibbins-Domingo, K. et al. Statin use for the primary prevention of cardiovascular disease in adults: US preventive services task force recommendation statement. J. Am. Med. Assoc. 316, 1997–2007 (2016).
Ahmadi, S. F. et al. Reverse epidemiology of traditional cardiovascular risk factors in the geriatric population. J. Am. Med. Dir. Assoc. 16, 933–939 (2015).
Conroy, S. P., Westendorp, R. G. J. & Witham, M. D. Hypertension treatment for older people—navigating between Scylla and Charybdis. Age Ageing (2018).
Vijg, J. Aging of the Genome: The Dual Role of DNA in Life and Death (Oxford Univ. Press, Oxford, 2007).
Yanai, H. & Fraifeld, V. E. The role of cellular senescence in aging through the prism of Koch-like criteria. Ageing Res. Rev. 41, 18–33 (2018).
Pomatto, L. C. D. & Davies, K. J. A. The role of declining adaptive homeostasis in ageing. J. Physiol. (Lond.) 595, 7275–7309 (2017).
Franceschi, C. & Campisi, J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J. Gerontol. A 69, S4–S9 (2014).
Mitchell, S. J., Scheibye-Knudsen, M., Longo, D. L. & de Cabo, R. Animal models of aging research: implications for human aging and age-related diseases. Annu. Rev. Anim. Biosci. 3, 283–303 (2015).
Tiku, V. et al. Small nucleoli are a cellular hallmark of longevity. Nat. Commun. 8, 16083 (2016).
Heidinger, B. J. et al. Telomere length in early life predicts lifespan. Proc. Natl Acad. Sci. USA 109, 1743–1748 (2012).
Varela, E., Muñoz-Lorente, M. A., Tejera, A. M., Ortega, S. & Blasco, M. A. Generation of mice with longer and better preserved telomeres in the absence of genetic manipulations. Nat. Commun. 7, 11739 (2016).
Kapahi, P., Kaeberlein, M. & Hansen, M. Dietary restriction and lifespan: lessons from invertebrate models. Ageing Res. Rev. 39, 3–14 (2017).
Colman, R. J. et al. Caloric restriction reduces age-related and all-cause mortality in rhesus monkeys. Nat. Commun. 5, 3557 (2014).
Mattison, J. A. et al. Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study. Nature 489, 318–321 (2012).
Vaughan, K. L. et al. Caloric restriction study design limitations in rodent and nonhuman primate studies. J. Gerontol. A 73, 48–53 (2018).
Pan, H. & Finkel, T. Key proteins and pathways that regulate lifespan. J. Biol. Chem. 292, 6452–6460 (2017).
Pawlikowska, L. et al. Association of common genetic variation in the insulin/IGF1 signaling pathway with human longevity. Aging Cell 8, 460–472 (2009).
Deelen, J. et al. Gene set analysis of GWAS data for human longevity highlights the relevance of the insulin/IGF-1 signaling and telomere maintenance pathways. Age (Dordr.) 35, 235–249 (2013).
Passtoors, W. M. et al. Gene expression analysis of mTOR pathway: association with human longevity. Aging Cell 12, 24–31 (2013).
Johnson, S. C., Rabinovitch, P. S. & Kaeberlein, M. mTOR is a key modulator of ageing and age-related disease. Nature 493, 338–345 (2013).
Fruman, D. A. et al. The PI3K pathway in human disease. Cell 170, 605–635 (2017).
Longo, V. D. et al. Interventions to slow aging in humans: are we ready? Aging Cell 14, 497–510 (2015).
Ingram, D. K. & de Cabo, R. Calorie restriction in rodents: caveats to consider. Ageing Res. Rev. 39, 15–28 (2017).
Racette, S. B. et al. One year of caloric restriction in humans: feasibility and effects on body composition and abdominal adipose tissue. J. Gerontol. A 61, 943–950 (2006).
Solon-Biet, S. M. et al. The ratio of macronutrients, not caloric intake, dictates cardiometabolic health, aging, and longevity in ad libitum-fed mice. Cell Metab. 19, 418–430 (2014).
Grandison, R. C., Piper, M. D. & Partridge, L. Amino-acid imbalance explains extension of lifespan by dietary restriction in Drosophila. Nature 462, 1061–1064 (2009).
Manoogian, E. N. C. & Panda, S. Circadian rhythms, time-restricted feeding, and healthy aging. Ageing Res. Rev. 39, 59–67 (2017).
Acosta-Rodriguez, V. A., de Groot, M. H. M., Rijo-Ferreira, F., Green, C. B. & Takahashi, J. S. Mice under caloric restriction self-impose a temporal restriction of food intake as revealed by an automated feeder system. Cell Metab. 26, 267–277 (2017).
Xie, K. et al. Every-other-day feeding extends lifespan but fails to delay many symptoms of aging in mice. Nat. Commun. 8, 155 (2017).
Fontana, L. The science of nutritional modulation of aging. Ageing Res. Rev. 39, 1–2 (2017).
Wei, M. et al. Fasting-mimicking diet and markers/risk factors for aging, diabetes, cancer, and cardiovascular disease. Sci. Transl. Med. 9, eaai8700 (2017). Healthy human subjects randomly allocated to a diet that mimics fasting, which is low in calories, sugars and protein, but high in unsaturated fats, as opposed to unrestricted food consumption, showed reduced body weight, trunk and total body fat, had lower blood pressure and decreased levels of IGF-1, with more marked effects in participants at risk of disease.
Longo, V. D. & Panda, S. Fasting, circadian rhythms, and time-restricted feeding in healthy lifespan. Cell Metab. 23, 1048–1059 (2016).
Newman, J. C. et al. Strategies and challenges in clinical trials targeting human aging. J. Gerontol. A 71, 1424–1434 (2016).
Blenis, J.TOR, the gateway to cellular metabolism, cell growth, and disease. Cell 171, 10–13 (2017).
Slack, C. Ras signaling in aging and metabolic regulation. Nutr. Healthy Aging 4, 195–205 (2017).
Bitto, A. et al. Transient rapamycin treatment can increase lifespan and healthspan in middle-aged mice. eLife 5, e16351 (2016).
Mannick, J. B. et al. mTOR inhibition improves immune function in the elderly. Sci. Transl. Med. 6, 268ra179 (2014). At doses that were well-tolerated, the mTOR inhibitor RAD001 enhanced the response to the influenza vaccine in elderly volunteers by about 20% and reduced the percentage of CD4 + and CD8 + T lymphocytes that expressed the programmed death-1 receptor, which inhibits T cell signalling and shows higher expression with increasing age.
Strong, R. et al. Longer lifespan in male mice treated with a weakly estrogenic agonist, an antioxidant, an α-glucosidase inhibitor or a Nrf2-inducer. Aging Cell 15, 872–884 (2016).
Barzilai, N., Crandall, J. P., Kritchevsky, S. B. & Espeland, M. A. Metformin as a tool to target aging. Cell Metab. 23, 1060–1065 (2016).
Espeland, M. A. et al. Clinical trials targeting aging and age-related multimorbidity. J. Gerontol. A 72, 355–361 (2017).
Arriola Apelo, S. I., Pumper, C. P., Baar, E. L., Cummings, N. E. & Lamming, D. W. Intermittent administration of rapamycin extends the life span of female C57BL/6J mice. J. Gerontol. A 71, 876–881 (2016).
Johnson, S. C. & Kaeberlein, M. Rapamycin in aging and disease: maximizing efficacy while minimizing side effects. Oncotarget 7, 44876–44878 (2016).
Muñoz-Espín, D. et al. Programmed cell senescence during mammalian embryonic development. Cell 155, 1104–1118 (2013).
Demaria, M. et al. An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev. Cell 31, 722–733 (2014).
Childs, B. G. et al. Senescent cells: an emerging target for diseases of ageing. Nat. Rev. Drug Discov. 16, 718–735 (2017).
Jeon, O. H. et al. Local clearance of senescent cells attenuates the development of post-traumatic osteoarthritis and creates a pro-regenerative environment. Nat. Med. 23, 775–781 (2017). Transection of the anterior cruciate ligament in mice caused accumulation of senescent cells in the articular cartilage and synovium, and selective elimination of these cells or injection of a senolytic molecule attenuated the development of osteoarthritis, reduced pain and increased cartilage development.
Childs, B. G., Durik, M., Baker, D. J. & van Deursen, J. M. Cellular senescence in aging and age-related disease: from mechanisms to therapy. Nat. Med. 21, 1424–1435 (2015).
McHugh, D. & Gil, J. Senescence and aging: causes, consequences, and therapeutic avenues. J. Cell Biol. 217, 65–77 (2018).
van Willigenburg, H., de Keizer, P. L. J. & de Bruin, R. W. F. Cellular senescence as a therapeutic target to improve renal transplantation outcome. Pharmacol. Res. 130, 322–330 (2018).
Rando, T. A. & Chang, H. Y. Aging, rejuvenation, and epigenetic reprogramming: resetting the aging clock. Cell 148, 46–57 (2012).
Ocampo, A. et al. In vivo amelioration of age-associated hallmarks by partial reprogramming. Cell 167, 1719–1733 (2016).
Clark, R. I. & Walker, D. W. Role of gut microbiota in aging-related health decline: insights from invertebrate models. Cell. Mol. Life Sci. 75, 93–101 (2018).
Kundu, P., Blacher, E., Elinav, E. & Pettersson, S. Our gut microbiome: the evolving inner self. Cell 171, 1481–1493 (2017).
Schmidt, T. S. B., Raes, J. & Bork, P. The Human gut microbiome: from association to modulation. Cell 172, 1198–1215 (2018).
Smith, P. et al. Regulation of life span by the gut microbiota in the short-lived African turquoise killifish. eLife 6, e27014 (2017). Recolonizing the gut of middle-age turquoise killifish with bacteria from young, rather than middle-aged, donors extends lifespan, delays behavioural decline and prevents the changes in the microbiome associated with host ageing.
O’Toole, P. W. & Jeffery, I. B. Gut microbiota and aging. Science 350, 1214–1215 (2015).
Kootte, R. S. et al. Improvement of insulin sensitivity after lean donor feces in metabolic syndrome is driven by baseline intestinal microbiota composition. Cell Metab. 26, 611–619 (2017).
Plovier, H. et al. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat. Med. 23, 107–113 (2017).
Castellano, J. M. et al. Human umbilical cord plasma proteins revitalize hippocampal function in aged mice. Nature 544, 488–492 (2017). Treatment with human umbilical cord plasma revitalizes the hippocampus and improves cognitive function in mice, which is (partially) driven by TIMP2.
Casey, J. A., Schwartz, B. S., Stewart, W. F. & Adler, N. E. Using electronic health records for population health research: a review of methods and applications. Annu. Rev. Public Health 37, 61–81 (2016).
Stott, D. J. et al. Thyroid hormone therapy for older adults with subclinical hypothyroidism. N. Engl. J. Med. 376, 2534–2544 (2017).
Mertens, J. et al. Directly reprogrammed human neurons retain aging-associated transcriptomic signatures and reveal age-related nucleocytoplasmic defects. Cell Stem Cell 17, 705–718 (2015). Direct conversion of human fibroblasts into induced neurons shows that these cells retain their age-related transcriptional profiles, and demonstrates the potential of direct reprogramming for in vitro modelling of ageing.
Hu, J. L., Todhunter, M. E., LaBarge, M. A. & Gartner, Z. J. Opportunities for organoids as new models of aging. J. Cell Biol. 217, 39–50 (2018).
Swerdlow, D. I. et al. Selecting instruments for Mendelian randomization in the wake of genome-wide association studies. Int. J. Epidemiol. 45, 1600–1616 (2016).
Ainsworth, H. F., Shin, S. Y. & Cordell, H. J. A comparison of methods for inferring causal relationships between genotype and phenotype using additional biological measurements. Genet. Epidemiol. 41, 577–586 (2017).
Finan, C. et al. The druggable genome and support for target identification and validation in drug development. Sci. Transl. Med. 9, eaag1166 (2017).
Schrauwen-Hinderling, V. B. & Schols, A. M. W. J. Imaging in metabolic research: challenges and opportunities. J. Appl. Physiol. 124, 160–161 (2018).
Sebastiani, P. et al. Four genome-wide association studies identify new extreme longevity variants. J. Gerontol. A 72, 1453–1464 (2017).
Deelen, J. et al. Genome-wide association meta-analysis of human longevity identifies a novel locus conferring survival beyond 90 years of age. Hum. Mol. Genet. 23, 4420–4432 (2014).
Pilling, L. C. et al. Human longevity: 25 genetic loci associated in 389,166 UK biobank participants. Aging (Albany NY) 9, 2504–2520 (2017).
Joshi, P. K. et al. Genome-wide meta-analysis associates HLA-DQA1/DRB1 and LPA and lifestyle factors with human longevity. Nat. Commun. 8, 910 (2017).
Zeng, Y. et al. Novel loci and pathways significantly associated with longevity. Sci. Rep. 6, 21243 (2016).
Broer, L. et al. GWAS of longevity in CHARGE consortium confirms APOE and FOXO3 candidacy. J. Gerontol. A 70, 110–118 (2015).
Flachsbart, F. et al. Immunochip analysis identifies association of the RAD50/IL13 region with human longevity. Aging Cell 15, 585–588 (2016).
Anderson, R., Richardson, G. D. & Passos, J. F. Mechanisms driving the ageing heart. Exp. Gerontol. https://doi.org/10.1016/j.exger.2017.10.015 (2017).
Meiners, S., Eickelberg, O. & Königshoff, M. Hallmarks of the ageing lung. Eur. Respir. J. 45, 807–827 (2015).
van Dongen, J. et al. Genetic and environmental influences interact with age and sex in shaping the human methylome. Nat. Commun. 7, 11115 (2016).
Slieker, R. C. et al. Age-related accrual of methylomic variability is linked to fundamental ageing mechanisms. Genome Biol. 17, 191 (2016).
Declerck, K. & Vanden Berghe, W. Back to the future: epigenetic clock plasticity towards healthy aging. Mech. Ageing Dev. https://doi.org/10.1016/j.mad.2018.01.002 (2018).
Frake, R. A., Ricketts, T., Menzies, F. M. & Rubinsztein, D. C. Autophagy and neurodegeneration. J. Clin. Invest. 125, 65–74 (2015).
Sharples, A. P. et al. Longevity and skeletal muscle mass: the role of IGF signalling, the sirtuins, dietary restriction and protein intake. Aging Cell 14, 511–523 (2015).
Wahl, D. et al. Nutritional strategies to optimise cognitive function in the aging brain. Ageing Res. Rev. 31, 80–92 (2016).
Sterky, F. H., Lee, S., Wibom, R., Olson, L. & Larsson, N. G. Impaired mitochondrial transport and Parkin-independent degeneration of respiratory chain-deficient dopamine neurons in vivo. Proc. Natl Acad. Sci. USA 108, 12937–12942 (2011).
Timmers, S. et al. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab. 14, 612–622 (2011).
Beijers, R. J. H. C. G., Gosker, H. R. & Schols, A. M. W. J. Resveratrol for patients with chronic obstructive pulmonary disease: hype or hope? Curr. Opin. Clin. Nutr. Metab. Care 21, 138–144 (2018).
Robinson, M. M. et al. Enhanced protein translation underlies improved metabolic and physical adaptations to different exercise training modes in young and old humans. Cell Metab. 25, 581–592 (2017).
Sturmlechner, I., Durik, M., Sieben, C. J., Baker, D. J. & van Deursen, J. M. Cellular senescence in renal ageing and disease. Nat. Rev. Nephrol. 13, 77–89 (2017).
Goodell, M. A. & Rando, T. A. Stem cells and healthy aging. Science 350, 1199–1204 (2015).
de Haan, G. & Lazare, S. S. Aging of hematopoietic stem cells. Blood 131, 479–487 (2018).
Čamernik, K. et al. Mesenchymal stem cells in the musculoskeletal system: from animal models to human tissue regeneration? Stem Cell Rev. 14, 346–369 (2018).
van den Akker, E. B. et al. Uncompromised 10-year survival of oldest old carrying somatic mutations in DNMT3A and TET2. Blood 127, 1512–1515 (2016).
WHO. Disease Burden and Mortality Estimates: Disease Burden, 2000–2016 http://www.who.int/healthinfo/global_burden_disease/estimates/en/index1.html (WHO, 2018).
Walter, S. et al. A genome-wide association study of aging. Neurobiol. Aging 32, 2109.e15–2109.e28 (2011).
Schächter, F. et al. Genetic associations with human longevity at the APOE and ACE loci. Nat. Genet. 6, 29–32 (1994). Genetic variants in ApoE are associated with longevity in humans, one of these variants, the ApoE ε4 allele, shows a deleterious effect whereas the other, the ApoE ε2 allele, has a protective effect.
Budovsky, A. et al. LongevityMap: a database of human genetic variants associated with longevity. Trends Genet. 29, 559–560 (2013).
Jeck, W. R., Siebold, A. P. & Sharpless, N. E. Review: a meta-analysis of GWAS and age-associated diseases. Aging Cell 11, 727–731 (2012).
Tazearslan, C., Huang, J., Barzilai, N. & Suh, Y. Impaired IGF1R signaling in cells expressing longevity-associated human IGF1R alleles. Aging Cell 10, 551–554 (2011).
Johnson, T. E. Recent results: biomarkers of aging. Exp. Gerontol. 41, 1243–1246 (2006).
Lara, J. et al. A proposed panel of biomarkers of healthy ageing. BMC Med. 13, 222 (2015).
Fried, L. P. et al. Frailty in older adults: evidence for a phenotype. J. Gerontol. A 56, M146–M157 (2001). The Fried criteria were among the earliest multi-domain definitions to index frailty, including shrinking (weight loss), muscle weakness (handgrip strength), poor endurance (self-reported exhaustion), slowness (gait speed) and low physical activity (kcal expended per week), estimated in community-dwelling people over 65 years, and this definition of frailty is now widely used in large epidemiological and clinical studies and formed the basis for the development of novel indexes to predict elderly people who have a higher risk of incidences of disease, hospitalization, falls, disability and mortality.
Mitnitski, A. B., Mogilner, A. J. & Rockwood, K. Accumulation of deficits as a proxy measure of aging. Sci. World J. 1, 323–336 (2001).
Peters, M. J. et al. The transcriptional landscape of age in human peripheral blood. Nat. Commun. 6, 8570 (2015).
Horvath, S. DNA methylation age of human tissues and cell types. Genome Biol. 14, R115 (2013). A combination of CpG methylation sites (epigenetic clock) is associated with chronological ageing of multiple tissues and cell types and can be used to estimate the biological age of a person.
Hannum, G. et al. Genome-wide methylation profiles reveal quantitative views of human aging rates. Mol. Cell 49, 359–367 (2013).
Hertel, J. et al. Measuring biological age via metabonomics: the metabolic age score. J. Proteome Res. 15, 400–410 (2016).
Cole, J. H. et al. Brain age predicts mortality. Mol. Psychiatry 23, 1385–1392 (2018).
Jylhävä, J., Pedersen, N. L. & Hägg, S. Biological age predictors. EBioMedicine 21, 29–36 (2017).
Acknowledgements
We thank N. van den Berg for help with the preparation of Fig. 1 and N. Chaturvedi and B. J. Zwaan for their critical reading of the manuscript. L.P. acknowledges support from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (no. 741989) and a Wellcome Trust Strategic Award. J.D. acknowledges support from the Alexander von Humboldt Foundation. We apologize to the authors of many relevant studies for not citing their work owing to space limitations.
Reviewer information
Nature thanks V. D. Longo and J. Vijg for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
All authors contributed to the design and writing of the Review.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
About this article
Cite this article
Partridge, L., Deelen, J. & Slagboom, P.E. Facing up to the global challenges of ageing. Nature 561, 45–56 (2018). https://doi.org/10.1038/s41586-018-0457-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-018-0457-8
Keywords
This article is cited by
-
An aging-related immune landscape in the hematopoietic immune system
Immunity & Ageing (2024)
-
Clarifying the biological and statistical assumptions of cross-sectional biological age predictors: an elaborate illustration using synthetic and real data
BMC Medical Research Methodology (2024)
-
PICLS with human cells is the first high throughput screening method for identifying novel compounds that extend lifespan
Biology Direct (2024)
-
Cortistatin prevents glucocorticoid-associated osteonecrosis of the femoral head via the GHSR1a/Akt pathway
Communications Biology (2024)
-
A single-cell atlas of lung homeostasis reveals dynamic changes during development and aging
Communications Biology (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.