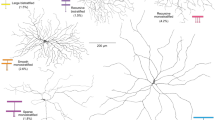
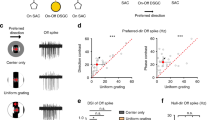
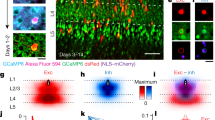
Abstract
To encode specific sensory inputs, cortical neurons must generate selective responses for distinct stimulus features. In principle, a variety of factors can contribute to the response selectivity of a cortical neuron: the tuning and strength of excitatory1,2,3 and inhibitory synaptic inputs4,5,6, dendritic nonlinearities7,8,9 and spike threshold10,11. Here we use a combination of techniques including in vivo whole-cell recording, synaptic- and cellular-resolution in vivo two-photon calcium imaging, and GABA (γ-aminobutyric acid) neuron-selective optogenetic manipulation to dissect the factors that contribute to the direction-selective responses of layer 2/3 neurons in ferret visual cortex (V1). Two-photon calcium imaging of dendritic spines12,13 revealed that each neuron receives a mixture of excitatory synaptic inputs selective for the somatic preferred or null direction of motion. The relative number of preferred- and null-tuned excitatory inputs predicted a neuron’s somatic direction preference, but failed to account for the degree of direction selectivity. By contrast, in vivo whole-cell patch-clamp recordings revealed a notable degree of direction selectivity in subthreshold responses that was significantly correlated with spiking direction selectivity. Subthreshold direction selectivity was predicted by the magnitude and variance of the response to the null direction of motion, and several lines of evidence, including conductance measurements, demonstrate that differential tuning of excitation and inhibition suppresses responses to the null direction of motion. Consistent with this idea, optogenetic inactivation of GABAergic neurons in layer 2/3 reduced direction selectivity by enhancing responses to the null direction. Furthermore, by optogenetically mapping connections of inhibitory neurons in layer 2/3 in vivo, we find that layer 2/3 inhibitory neurons make long-range, intercolumnar projections to excitatory neurons that prefer the opposite direction of motion. We conclude that intracortical inhibition exerts a major influence on the degree of direction selectivity in layer 2/3 of ferret V1 by suppressing responses to the null direction of motion.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Cossell, L. et al. Functional organization of excitatory synaptic strength in primary visual cortex. Nature 518, 399–403 (2015).
Ko, H. et al. Functional specificity of local synaptic connections in neocortical networks. Nature 473, 87–91 (2011).
Lee, W. C. et al. Anatomy and function of an excitatory network in the visual cortex. Nature 532, 370–374 (2016).
Tan, A. Y., Brown, B. D., Scholl, B., Mohanty, D. & Priebe, N. J. Orientation selectivity of synaptic input to neurons in mouse and cat primary visual cortex. J. Neurosci. 31, 12339–12350 (2011).
Monier, C., Chavane, F., Baudot, P., Graham, L. J. & Frégnac, Y. Orientation and direction selectivity of synaptic inputs in visual cortical neurons: a diversity of combinations produces spike tuning. Neuron 37, 663–680 (2003).
Liu, B. H. et al. Broad inhibition sharpens orientation selectivity by expanding input dynamic range in mouse simple cells. Neuron 71, 542–554 (2011).
Smith, S. L., Smith, I. T., Branco, T. & Häusser, M. Dendritic spikes enhance stimulus selectivity in cortical neurons in vivo. Nature 503, 115–120 (2013).
Palmer, L. M. et al. NMDA spikes enhance action potential generation during sensory input. Nat. Neurosci. 17, 383–390 (2014).
Lavzin, M., Rapoport, S., Polsky, A., Garion, L. & Schiller, J. Nonlinear dendritic processing determines angular tuning of barrel cortex neurons in vivo. Nature 490, 397–401 (2012).
Priebe, N. J. & Ferster, D. Inhibition, spike threshold, and stimulus selectivity in primary visual cortex. Neuron 57, 482–497 (2008).
Anderson, J. S., Carandini, M. & Ferster, D. Orientation tuning of input conductance, excitation, and inhibition in cat primary visual cortex. J. Neurophysiol. 84, 909–926 (2000).
Wilson, D. E., Whitney, D. E., Scholl, B. & Fitzpatrick, D. Orientation selectivity and the functional clustering of synaptic inputs in primary visual cortex. Nat. Neurosci. 19, 1003–1009 (2016).
Chen, T. W. et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 499, 295–300 (2013).
Priebe, N. J. & Ferster, D. Direction selectivity of excitation and inhibition in simple cells of the cat primary visual cortex. Neuron 45, 133–145 (2005).
Torre, V. & Poggio, T. A synaptic mechanism possibly underlying directional selectivity to motion. Proc. R. Soc. Lond. B 202, 409–416 (1978).
Sillito, A. M. Inhibitory processes underlying the directional specificity of simple, complex and hypercomplex cells in the cat’s visual cortex. J. Physiol. (Lond.) 271, 699–720 (1977).
Prescott, S. A. & De Koninck, Y. Gain control of firing rate by shunting inhibition: roles of synaptic noise and dendritic saturation. Proc. Natl Acad. Sci. USA 100, 2076–2081 (2003).
Chance, F. S., Abbott, L. F. & Reyes, A. D. Gain modulation from background synaptic input. Neuron 35, 773–782 (2002).
Katzner, S., Busse, L. & Carandini, M. GABAA inhibition controls response gain in visual cortex. J. Neurosci. 31, 5931–5941 (2011).
Isaacson, J. S. & Scanziani, M. How inhibition shapes cortical activity. Neuron 72, 231–243 (2011).
Wehr, M. & Zador, A. M. Balanced inhibition underlies tuning and sharpens spike timing in auditory cortex. Nature 426, 442–446 (2003).
Atallah, B. V., Bruns, W., Carandini, M. & Scanziani, M. Parvalbumin-expressing interneurons linearly transform cortical responses to visual stimuli. Neuron 73, 159–170 (2012).
Crook, J. M., Kisvárday, Z. F. & Eysel, U. T. Evidence for a contribution of lateral inhibition to orientation tuning and direction selectivity in cat visual cortex: reversible inactivation of functionally characterized sites combined with neuroanatomical tracing techniques. Eur. J. Neurosci. 10, 2056–2075 (1998).
Wilson, D. E. et al. GABAergic neurons in ferret visual cortex participate in functionally specific networks. Neuron 93, 1058–1065.e4 (2017).
Packer, A. M. & Yuste, R. Dense, unspecific connectivity of neocortical parvalbumin-positive interneurons: a canonical microcircuit for inhibition? J. Neurosci. 31, 13260–13271 (2011).
Hofer, S. B. et al. Differential connectivity and response dynamics of excitatory and inhibitory neurons in visual cortex. Nat. Neurosci. 14, 1045–1052 (2011).
Levy, R. B. & Reyes, A. D. Spatial profile of excitatory and inhibitory synaptic connectivity in mouse primary auditory cortex. J. Neurosci. 32, 5609–5619 (2012).
Martin, K. A., Somogyi, P. & Whitteridge, D. Physiological and morphological properties of identified basket cells in the cat’s visual cortex. Exp. Brain Res. 50, 193–200 (1983).
Roerig, B. & Chen, B. Relationships of local inhibitory and excitatory circuits to orientation preference maps in ferret visual cortex. Cereb. Cortex 12, 187–198 (2002).
Dhawale, A. K., Hagiwara, A., Bhalla, U. S., Murthy, V. N. & Albeanu, D. F. Non-redundant odor coding by sister mitral cells revealed by light addressable glomeruli in the mouse. Nat. Neurosci. 13, 1404–1412 (2010).
Li, Y. T., Liu, B. H., Chou, X. L., Zhang, L. I. & Tao, H. W. Strengthening of direction selectivity by broadly tuned and spatiotemporally slightly offset inhibition in mouse visual cortex. Cereb. Cortex 25, 2466–2477 (2015).
Borst, A. & Helmstaedter, M. Common circuit design in fly and mammalian motion vision. Nat. Neurosci. 18, 1067–1076 (2015).
Dimidschstein, J. et al. A viral strategy for targeting and manipulating interneurons across vertebrate species. Nat. Neurosci. 19, 1743–1749 (2016).
Govorunova, E. G., Sineshchekov, O. A., Janz, R., Liu, X. & Spudich, J. L. Natural light-gated anion channels: A family of microbial rhodopsins for advanced optogenetics. Science 349, 647–650 (2015).
Peirce, J. W. PsychoPy—Psychophysics software in Python. J. Neurosci. Methods 162, 8–13 (2007).
Pologruto, T. A., Sabatini, B. L. & Svoboda, K. ScanImage: flexible software for operating laser scanning microscopes. Biomed. Eng. Online 2, 13 (2003).
Baker, C. A., Elyada, Y. M., Parra, A. & Bolton, M. M. Cellular resolution circuit mapping with temporal-focused excitation of soma-targeted channelrhodopsin. eLife 5, e14193 (2016).
Wu, C., Ivanova, E., Zhang, Y. & Pan, Z. H. rAAV-mediated subcellular targeting of optogenetic tools in retinal ganglion cells in vivo. PLoS ONE 8, e66332 (2013).
Pnevmatikakis, E. A. & Giovannucci, A. NoRMCorre: An online algorithm for piecewise rigid motion correction of calcium imaging data. J. Neurosci. Methods 291, 83–94 (2017).
Sage, D., Prodanov, D., Tinevez, J. Y. & Schindelin, J. in ImageJ User & Developer Conference (Luxembourg, 2012).
Sobczyk, A., Scheuss, V. & Svoboda, K. NMDA receptor subunit-dependent [Ca2+] signaling in individual hippocampal dendritic spines. J. Neurosci. 25, 6037–6046 (2005).
Purushothaman, G., Khaytin, I. & Casagrande, V. A. Quantification of optical images of cortical responses for inferring functional maps. J. Neurophysiol. 101, 2708–2724 (2009).
Acknowledgements
We thank D. Whitney for help with analysis, A. Banerjee and F. Albeanu for help with patterned photostimulation setup, J. Chang for discussions about optogenetic stimulation, D. Ouimet for surgical assistance, N. Shultz and R. Satterfield for histology, T. Laviv for discussions about CyRFP, C. Baker and M. Bolton for cloning and the gift of the ChR2 construct, and the GENIE project for access to GCaMP6. This work was supported by EY011488, the Max Planck Florida Institute for Neuroscience, and the Max Planck Society.
Reviewer information
Nature thanks A. Huberman and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
D.E.W., B.S. and D.F. conceived experiments. D.E.W. and B.S. performed experiments and analysed data with guidance from D.F. D.E.W., B.S. and D.F. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Summed spine inputs fail to predict somatic direction selectivity, regardless of the method used to compute the sum.
a, No significant correlation between the DSI of summed spine inputs (with amplitude included) and somatic DSI. Spearman’s r = −0.11, P = 0.68, n = 17. b, No significant correlation between the fraction of spines that respond more strongly to the preferred direction and somatic DSI. Spearman’s r = −0.082, P = 0.75, n = 17.
Extended Data Fig. 2 Distribution of spiking DSI.
Dashed line indicates cutoff of DSI > 0.3; n = 69 cells with spiking responses.
Extended Data Fig. 3 Example of noise suppression at null stimulus relative to blank.
Figure shows responses to preferred, null and blank.
Extended Data Fig. 4 Direction tuning fits for excitatory and inhibitory conductances.
a, Difference in direction preference of excitation and inhibition are significantly greater than chance; Monte Carlo significance test, P = 0.023; difference in direction preference, 135° ± 95°, median ± IQR, n = 10 cells from 7 animals. b, FWHM of excitation and inhibition were not significantly different. FWHM 61° ± 46° and 61° ± 110° for excitation and inhibition, respectively; median ± IQR, n = 10, Wilcoxon sign-rank P = 0.70. c, Individual (grey) and population average (coloured) tuning curves for Ge, Gi and predicted Vm, peak-aligned to excitation.
Extended Data Fig. 5 I/E ratio at preferred direction is not correlated with simulated subthreshold direction selectivity.
Spearman’s r = 0.0061, P = 1, n = 10 cells from 7 animals.
Extended Data Fig. 6 Putative GABAergic neuron directly suppressed by blue light.
Error bars, mean ± s.e.m.
Extended Data Fig. 7 Additional data related to blue light photoinhibition of GABAergic neurons.
a, Optogenetic suppression of GABAergic neurons significantly reduces spiking direction selectivity; Wilcoxon sign-rank, n = 14 cells with spiking responses, P = 0.0049. Black line, mean; grey lines, single cells. b, Absolute Vm depolarization induced by blue light is not related to optogenetic changes in Vm direction selectivity (computed as the difference in DSI between light off and light on conditions); Spearman’s r = 0.11, P = 0.70, n = 14 cells with spiking responses from 4 animals.
Extended Data Fig. 8 Alignment of GABAergic neurons with intrinsic signal polar direction map.
a, Underlying intrinsic signal polar direction map with direction-tuned GABAergic neurons overlaid. b, Direction preferences of inhibitory neurons and intrinsic signal direction preference map are significantly more similar than chance; P < 0.001, Monte Carlo significance test, n = 76 direction-selective neurons from 3 planes in 1 animal.
Extended Data Fig. 9 Reversal potential of optogenetically evoked PSPs is consistent with inhibition.
Grey points are individual data points; black is mean ± s.e.m. Data come from individual stimulation trials from one cell.
Extended Data Fig. 10 Relationship of IPSP amplitude and distance.
Grey points are individual data points; black is binned mean ± s.e.m. Data come from trial-averaged stimulation responses from n = 21 cells from 7 animals.
Supplementary information
Rights and permissions
About this article
Cite this article
Wilson, D.E., Scholl, B. & Fitzpatrick, D. Differential tuning of excitation and inhibition shapes direction selectivity in ferret visual cortex. Nature 560, 97–101 (2018). https://doi.org/10.1038/s41586-018-0354-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-018-0354-1
This article is cited by
-
Synaptic wiring motifs in posterior parietal cortex support decision-making
Nature (2024)
-
Acute exercise as a modifier of neocortical plasticity and aperiodic activity in the visual cortex
Scientific Reports (2023)
-
Development of visual response selectivity in cortical GABAergic interneurons
Nature Communications (2022)
-
Inhibition for gain modulation in the motor system
Experimental Brain Research (2022)
-
Recurrent network dynamics shape direction selectivity in primary auditory cortex
Nature Communications (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.