Abstract
Controlled transport of water molecules through membranes and capillaries is important in areas as diverse as water purification and healthcare technologies1,2,3,4,5,6,7. Previous attempts to control water permeation through membranes (mainly polymeric ones) have concentrated on modulating the structure of the membrane and the physicochemical properties of its surface by varying the pH, temperature or ionic strength3,8. Electrical control over water transport is an attractive alternative; however, theory and simulations9,10,11,12,13,14 have often yielded conflicting results, from freezing of water molecules to melting of ice14,15,16 under an applied electric field. Here we report electrically controlled water permeation through micrometre-thick graphene oxide membranes17,18,19,20,21. Such membranes have previously been shown to exhibit ultrafast permeation of water17,22 and molecular sieving properties18,21, with the potential for industrial-scale production. To achieve electrical control over water permeation, we create conductive filaments in the graphene oxide membranes via controllable electrical breakdown. The electric field that concentrates around these current-carrying filaments ionizes water molecules inside graphene capillaries within the graphene oxide membranes, which impedes water transport. We thus demonstrate precise control of water permeation, from ultrafast permeation to complete blocking. Our work opens up an avenue for developing smart membrane technologies for artificial biological systems, tissue engineering and filtration.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Karnik, R. et al. Electrostatic control of ions and molecules in nanofluidic transistors. Nano Lett. 5, 943–948 (2005).
Gravelle, S. et al. Optimizing water permeability through the hourglass shape of aquaporins. Proc. Natl Acad. Sci. USA 110, 16367–16372 (2013).
Liu, Z., Wang, W., Xie, R., Ju, X.-J. & Chu, L.-Y. Stimuli-responsive smart gating membranes. Chem. Soc. Rev. 45, 460–475 (2016).
Xiao, K. et al. Electrostatic-charge- and electric-field-induced smart gating for water transportation. ACS Nano 10, 9703–9709 (2016).
Wang, Z. et al. Polarity-dependent electrochemically controlled transport of water through carbon nanotube membranes. Nano Lett. 7, 697–702 (2007).
Hetherington, A. M. & Woodward, F. I. The role of stomata in sensing and driving environmental change. Nature 424, 901–908 (2003).
Borgnia, M. J., Nielsen, S., Engel, A. & Agre, P. Cellular and molecular biology of the aquaporin water channels. Annu. Rev. Biochem. 68, 425–458 (1999).
Zhao, C., Nie, S., Tang, M. & Sun, S. Polymeric pH-sensitive membranes—a review. Prog. Polym. Sci. 36, 1499–1520 (2011).
Kou, J. et al. Electromanipulating water flow in nanochannels. Angew. Chem. Int. Ed. 54, 2351–2355 (2015).
Li, J. et al. Electrostatic gating of a nanometer water channel. Proc. Natl Acad. Sci. USA 104, 3687–3692 (2007).
Gong, X. et al. A charge-driven molecular water pump. Nat. Nanotechnol. 2, 709–712 (2007).
Vaitheeswaran, S., Rasaiah, J. C. & Hummer, G. Electric field and temperature effects on water in the narrow nonpolar pores of carbon nanotubes. J. Chem. Phys. 121, 7955–7965 (2004).
Saitta, A. M., Saija, F. & Giaquinta, P. V. Ab initio molecular dynamics study of dissociation of water under an electric field. Phys. Rev. Lett. 108, 207801 (2012).
Qiu, H. & Guo, W. Electromelting of confined monolayer ice. Phys. Rev. Lett. 110, 195701 (2013).
Choi, E.-M., Yoon, Y.-H., Lee, S. & Kang, H. Freezing transition of interfacial water at room temperature under electric fields. Phys. Rev. Lett. 95, 085701 (2005).
Diallo, S. O., Mamontov, E., Nobuo, W., Inagaki, S. & Fukushima, Y. Enhanced translational diffusion of confined water under electric field. Phys. Rev. E 86, 021506 (2012).
Nair, R. R., Wu, H. A., Jayaram, P. N., Grigorieva, I. V. & Geim, A. K. Unimpeded permeation of water through helium-leak–tight graphene-based membranes. Science 335, 442–444 (2012).
Joshi, R. K. et al. Precise and ultrafast molecular sieving through graphene oxide membranes. Science 343, 752–754 (2014).
Sun, P., Wang, K. & Zhu, H. Recent developments in graphene-based membranes: structure, mass-transport mechanism and potential applications. Adv. Mater. 28, 2287–2310 (2016).
Liu, G., Jin, W. & Xu, N. Graphene-based membranes. Chem. Soc. Rev. 44, 5016–5030 (2015).
Abraham, J. et al. Tunable sieving of ions using graphene oxide membranes. Nat. Nanotechnol. 12, 546–550 (2017).
Radha, B. et al. Molecular transport through capillaries made with atomic-scale precision. Nature 538, 222–225 (2016).
Kao, K.-C. Dielectric Phenomena in Solids: With Emphasis on Physical Concepts of Electronic Processes Ch. 8 (Academic Press, Amsterdam, 2004).
Acik, M. et al. The role of oxygen during thermal reduction of graphene oxide studied by infrared absorption spectroscopy. J. Phys. Chem. C 115, 19761–19781 (2011).
Hontoria-Lucas, C., López-Peinado, A. J., López-González, J. D., Rojas-Cervantes, M. L. & Martín-Aranda, R. M. Study of oxygen-containing groups in a series of graphite oxides: physical and chemical characterization. Carbon 33, 1585–1592 (1995).
Konkena, B. & Vasudevan, S. Understanding aqueous dispersibility of graphene oxide and reduced graphene oxide through pKa measurements. J. Phys. Chem. Lett. 3, 867–872 (2012).
Jackson, J. D. Surface charges on circuit wires and resistors play three roles. Am. J. Phys. 64, 855–870 (1996).
Marcus, A. The electric field associated with a steady current in long cylindrical conductor. Am. J. Phys. 9, 225–226 (1941).
Chen, L. et al. Ion sieving in graphene oxide membranes via cationic control of interlayer spacing. Nature 550, 380–383 (2017).
Tielrooij, K. J., Garcia-Araez, N., Bonn, M. & Bakker, H. J. Cooperativity in ion hydration. Science 328, 1006–1009 (2010).
Siegel, J., Lyutakov, O., Rybka, V., Kolská, Z. & Svorčík, V. Properties of gold nanostructures sputtered on glass. Nanoscale Res. Lett. 6, 96 (2011).
O’Dwyer, J. J. Dielectric breakdown in solids. Adv. Phys. 7, 349–394 (1958).
Kim, S. K. et al. Conductive graphitic channel in graphene oxide-based memristive devices. Adv. Funct. Mater. 26, 7406–7414 (2016).
Eda, G. et al. Graphene oxide gate dielectric for graphene-based monolithic field effect transistors. Appl. Phys. Lett. 102, 133108 (2013).
Standley, B., Mendez, A., Schmidgall, E. & Bockrath, M. Graphene-graphite oxide field-effect transistors. Nano Lett. 12, 1165–1169 (2012).
Lee, J. S., Lee, S. & Noh, T. W. Resistive switching phenomena: a review of statistical physics approaches. Appl. Phys. Rev. 2, 031303 (2015).
Qin, S. et al. A physics/circuit-based switching model for carbon-based resistive memory with sp2/sp3 cluster conversion. Nanoscale 4, 6658–6663 (2012).
Chen, C. et al. Annealing a graphene oxide film to produce a free standing high conductive graphene film. Carbon 50, 659–667 (2012).
Borini, S. et al. Ultrafast graphene oxide humidity sensors. ACS Nano 7, 11166–11173 (2013).
Pei, S. & Cheng, H. The reduction of graphene oxide. Carbon 50, 3210–3228 (2012).
Park, S. et al. Colloidal suspensions of highly reduced graphene oxide in a wide variety of organic solvents. Nano Lett. 9, 1593–1597 (2009).
Ganguly, A., Sharma, S., Papakonstantinou, P. & Hamilton, J. Probing the thermal deoxygenation of graphene oxide using high-resolution in situ X-ray-based spectroscopies. J. Phys. Chem. C 115, 17009–17019 (2011).
Müller, R. A semiquantitative treatment of surface charges in DC circuits. Am. J. Phys. 80, 782–788 (2012).
Jackson, J. D. Classical Electrodynamics 3rd edn, Ch. 1, 12–14 (John Wiley & Sons, New York, 1999).
Geissler, P. L., Dellago, C., Chandler, D., Hutter, J. & Parrinello, M. Autoionization in liquid water. Science 291, 2121–2124 (2001).
Mafé, S., Ramírez, P. & Alcaraz, A. Electric field-assisted proton transfer and water dissociation at the junction of a fixed-charge bipolar membrane. Chem. Phys. Lett. 294, 406–412 (1998).
Pinkerton, T. D. et al. Electric field effects in ionization of water–ice layers on platinum. Langmuir 15, 851–856 (1999).
Wilson, N. R. et al. Graphene oxide: structural analysis and application as a highly transparent support for electron microscopy. ACS Nano 3, 2547–2556 (2009).
Loh, K. P., Bao, Q., Eda, G. & Chhowalla, M. Graphene oxide as a chemically tunable platform for optical applications. Nat. Chem. 2, 1015–1024 (2010).
Plimpton, S. Fast parallel algorithms for short-range molecular dynamics. J. Comput. Phys. 117, 1–19 (1995).
Vácha, R., Buch, V., Milet, A., Devlin, J. P. & Jungwirth, P. Autoionization at the surface of neat water: is the top layer pH neutral, basic, or acidic? Phys. Chem. Chem. Phys. 9, 4736–4747 (2007).
Vácha, R., Horinek, D., Berkowitz, M. L. & Jungwirth, P. Hydronium and hydroxide at the interface between water and hydrophobic media. Phys. Chem. Chem. Phys. 10, 4975–4980 (2008).
Mills, R. Self-diffusion in normal and heavy water in the range 1-45.deg. J. Phys. Chem. 77, 685–688 (1973).
Meyer, B. et al. Partial dissociation of water leads to stable superstructures on the surface of zinc oxide. Angew. Chem. Int. Ed. 43, 6641–6645 (2004).
Brodskaya, E., Alexander, P. L. & Aatto, L. Investigation of water clusters containing OH- and H3O+ ions in atmospheric conditions. A molecular dynamics simulation study. J. Phys. Chem. B 106, 6479–6487 (2002).
Huang, H. et al. Salt concentration, pH and pressure controlled separation of small molecules through lamellar graphene oxide membranes. Chem. Commun. 49, 5963–5965 (2013).
Acknowledgements
This work was supported by the Royal Society, Engineering and Physical Sciences Research Council, UK (EP/K016946/1, EP/N013670/1 and EP/P00119X/1), British Council (award reference number 279336045), European Research Council (contract 679689) and Lloyd’s Register Foundation. We thank J. Waters for assisting with X-ray measurements and G. Yu for electrical measurements.
Reviewer information
Nature thanks H. Fang, N. Koratkar, B. Mi and H. B. Park for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
R.R.N. initiated and supervised the project. K.-G.Z. performed the experiment and analysed the data with help from K.S.V. and R.R.N.; K.S.V. carried out the PF TUNA and Raman characterization and analysis. C.T.C. carried out the mass spectroscopy. K.H., J.A. and Y.S. helped in sample preparation, characterization and data analysis. K.S.V., M.N.-A., H.G.-K., K.S.N. and F.M.P. performed the theoretical modelling and simulations. J.C.Z. and A.P. performed the XPS characterizations. O.P.M., V.G.K. and A.N.G. performed the infrared characterizations. A.K.G. contributed to theoretical discussions. R.R.N., K.S.V., K.-G.Z. and K.S.N. co-wrote the paper. All authors contributed to discussions.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
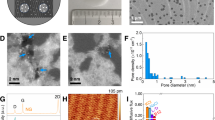
Extended Data Fig. 1 Metal–GO–metal sandwiched membranes.
a, Fabrication procedure for the metal–GO–metal sandwich membrane. b, Photograph of one of our metal–GO–metal sandwich membranes attached to the PET sheet (step 2 in a). Scale bar, 6 mm. This was further attached onto another plastic disk to seal the metal container for gravimetric testing. c, SEM image showing the discontinuities and voids in a 10-nm gold thin film on a GO membrane. Scale bar, 150 nm. d, Water permeation rate of metal–GO–metal sandwiched membranes as a function of gold electrode thickness. The dotted line is a guide to the eye. Water permeation rates of a bare porous silver (Ag) support (red filled circle) and a commercial polyamide nanofiltration membrane (green filled circle) are provided for comparison. Inset, SEM image of a 50-nm-thick gold thin film on a GO membrane. Scale bar, 150 nm.
Extended Data Fig. 2 Conducting filament formation in a GO membrane and its electrical characterization.
a, I–V characteristics during the first voltage sweep show a sudden increase in the current for membranes exposed to humid conditions, suggesting partial electrical breakdown of the GO membrane and conducting filament formation. b, In-plane and out-of-plane I–V characteristics of the GO membrane after filament formation. Inset, out-of-plane I–V characteristics of the GO membrane at 100% RH and vacuum.
Extended Data Fig. 3 Raman and AFM characterization of conducting filaments in GO membranes.
a, Topographical SEM image of a GO membrane after the formation of conducting filaments. b–d, Raman intensity ratio (ID/IG) mapping of D and G bands for a pristine GO membrane (b) and a GO membrane after conducting filaments have formed (c, d). c, Raman imaging from the membrane surface close to the positive electrode (about 200 nm away). d, Raman imaging from the membrane surface close to the negative electrode (about 100 nm away). e, f, The Raman spectra from the dark blue and green regions in c, respectively. g–j, Topography and the corresponding TUNA current image of pristine GO (g and h, respectively) and conducting GO (i and j) membranes (filament formed at 100% RH) exfoliated on a gold-thin-film-coated Si substrate. The conducting filaments are marked by red circles. Scale bars, 1 μm. k, Out-of-plane I–V characteristics of a conducting GO membrane with a diameter of about 7 mm before (parent membrane) and after dividing into four equal pieces. Inset, schematic of the structure of conducting carbon filaments in the GO membrane.
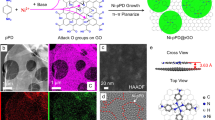
Extended Data Fig. 4 Influence of intercalated water and oxygen content on conducting filament formation in GO membranes.
a, b, Topography and the corresponding TUNA current images of GO membranes after filament formation at 40% RH (a and b, respectively) and inside liquid water (c and d). e, I–V characteristics of pristine and partially reduced (‘pr’) GO membranes during the first voltage sweep at 100% RH show partial breakdown. f, TUNA current image of a partially reduced GO membrane after filament formation. The conducting filaments are marked by red circles. Scale bars, 2 μm.
Extended Data Fig. 5 XPS characterization of GO membranes.
a, C 1s spectrum from a pristine GO membrane. b, c, C 1s spectra from GO membranes used for the electrically controlled permeation experiments after filament formation, from a freshly cleaved membrane surface close to the inner middle region (b) and close to the positive electrode (c). Black lines, raw data; red lines, the fitting envelope; blue lines, deconvolved peaks attributed to the chemical environments indicated.
Extended Data Fig. 6 Mass spectrometry to probe electrically controlled water permeation.
a, Schematic of the experimental set-up for mass spectrometry measurements. A throttle valve (TV) controls the gas inlet with a capacitance gauge (CG) used to measure the upstream pressure. An isolation valve (IV) isolates upstream and downstream sides of the membrane. A rotary pump (RP1) evacuates the feed and the permeate side to 1 mbar. The quadrupole mass spectrometer (QMS) measures the downstream partial pressure. A turbomolecular pump (TP) backed by a rotary pump (RP2) evacuates the high-vacuum chamber of the mass spectrometer. An active ion gauge (IG) measures the pressure down to 1 × 10−9 torr in the high-vacuum side. b, The partial pressure of He, H2, O2 and H2O at the permeate side as a function of time at different currents through the membrane. No detectable change is observed in the partial pressures of He, H2 and O2 under different currents through the membrane. c, The partial pressure of H2O as a function of the current across the GO membrane and the corresponding I–V characteristics (colour-coded axes).The dotted lines are guides to the eye.
Extended Data Fig. 7 Electrically controlled liquid water permeation in GO membranes.
a, Schematic of the experimental set-up. b, I–V characteristics of a Au/GO/Ag membrane during the first voltage sweep while it is immersed in liquid water in the experimental set-up. c, Liquid water permeation rate as a function of current across the membrane after filament formation and the corresponding I–V characteristics (colour-coded axes). Sample-to-sample variation in the permeation is less than 30% (three samples measured).
Extended Data Fig. 8 In situ membrane temperature and water absorption measurements.
a, Measured membrane temperature as a function of the current flowing across the membrane during the electrically controlled water permeation experiment. Error bars, standard deviation from 10 different measurements across the sample. b, The weight intake of a Au/GO/Ag membrane (1-μm-thick GO) at different humidity and electric current values. Weight intake is calculated with respect to the weight of the membrane at 0% RH. The shaded areas show the time during humidity sweeps.
Extended Data Fig. 9 Electric field around a current-carrying conductor.
a, Schematic of the application of a voltage V across an electrically conducting wire with radius a and length L; b is the point at which the potential decays to zero; r represents any point between a and b where the electric field E is calculated. b, Magnitude of E and its spatial distribution as a function of r and z around a conductive filament with 1-V potential difference across the ends and with 1-nA current flow.
Extended Data Fig. 10 Molecular dynamics simulations.
a, Side view of our molecular dynamics simulation set-up used to study the flow of water mixed with H3O+ and OH− ions in the graphene capillary. The model contains two boxes connected by a graphene capillary. At the beginning of the simulation, water was mixed with H3O+ and OH− ions (red and white dots). By moving the left wall (subjected to external pressure) of the box towards the capillary, the water flow is created and the right box is gradually filled. The arrow indicates the direction of the external pressure applied on the left wall of the box. b, c, Number of water molecules in the capillary (b) and number of water molecules in the right box (c) for pure water and water with ions once pressure is applied to the left box (colour-coded labels). d, Water flow rate as a function of the concentration of ions inside the capillary.
Rights and permissions
About this article
Cite this article
Zhou, KG., Vasu, K.S., Cherian, C.T. et al. Electrically controlled water permeation through graphene oxide membranes. Nature 559, 236–240 (2018). https://doi.org/10.1038/s41586-018-0292-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-018-0292-y
This article is cited by
-
Bio-inspired design of next-generation ultrapermeable membrane systems
npj Clean Water (2024)
-
Nanowire-assisted electrochemical perforation of graphene oxide nanosheets for molecular separation
Nature Communications (2024)
-
A hybrid approach to water potability prediction: leveraging artificial fish swarm algorithm and convolutional neural networks
Asian Journal of Civil Engineering (2024)
-
Molecular dynamics simulations of the effect of starch on transport of water and ions through graphene nanopores
Journal of Molecular Modeling (2024)
-
Proton and molecular permeation through the basal plane of monolayer graphene oxide
Nature Communications (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.