Abstract
The ability of the taste system to identify a tastant (what it tastes like) enables animals to recognize and discriminate between the different basic taste qualities1,2. The valence of a tastant (whether it is appetitive or aversive) specifies its hedonic value and elicits the execution of selective behaviours. Here we examine how sweet and bitter are afforded valence versus identity in mice. We show that neurons in the sweet-responsive and bitter-responsive cortex project to topographically distinct areas of the amygdala, with strong segregation of neural projections conveying appetitive versus aversive taste signals. By manipulating selective taste inputs to the amygdala, we show that it is possible to impose positive or negative valence on a neutral water stimulus, and even to reverse the hedonic value of a sweet or bitter tastant. Remarkably, mice with silenced neurons in the amygdala no longer exhibit behaviour that reflects the valence associated with direct stimulation of the taste cortex, or with delivery of sweet and bitter chemicals. Nonetheless, these mice can still identify and discriminate between tastants, just as wild-type controls do. These results help to explain how the taste system generates stereotypic and predetermined attractive and aversive taste behaviours, and support the existence of distinct neural substrates for the discrimination of taste identity and the assignment of valence.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Yarmolinsky, D. A., Zuker, C. S. & Ryba, N. J. Common sense about taste: from mammals to insects. Cell 139, 234–244 (2009).
Scott, K. Taste recognition: food for thought. Neuron 48, 455–464 (2005).
Spector, A. C. & Travers, S. P. The representation of taste quality in the mammalian nervous system. Behav. Cogn. Neurosci. Rev. 4, 143–191 (2005).
Accolla, R., Bathellier, B., Petersen, C. C. & Carleton, A. Differential spatial representation of taste modalities in the rat gustatory cortex. J. Neurosci. 27, 1396–1404 (2007).
Yoshimura, H., Sugai, T., Fukuda, M., Segami, N. & Onoda, N. Cortical spatial aspects of optical intrinsic signals in response to sucrose and NaCl stimuli. Neuroreport 15, 17–20 (2004).
Chen, X., Gabitto, M., Peng, Y., Ryba, N. J. & Zuker, C. S. A gustotopic map of taste qualities in the mammalian brain. Science 333, 1262–1266 (2011).
Peng, Y. et al. Sweet and bitter taste in the brain of awake behaving animals. Nature 527, 512–515 (2015).
Susaki, E. A. et al. Whole-brain imaging with single-cell resolution using chemical cocktails and computational analysis. Cell 157, 726–739 (2014).
Zingg, B. et al. AAV-mediated anterograde transsynaptic tagging: mapping corticocollicular input-defined neural pathways for defense behaviors. Neuron 93, 33–47 (2017).
Cai, H., Haubensak, W., Anthony, T. E. & Anderson, D. J. Central amygdala PKC-δ+ neurons mediate the influence of multiple anorexigenic signals. Nat. Neurosci. 17, 1240–1248 (2014).
Janak, P. H. & Tye, K. M. From circuits to behaviour in the amygdala. Nature 517, 284–292 (2015).
Paton, J. J., Belova, M. A., Morrison, S. E. & Salzman, C. D. The primate amygdala represents the positive and negative value of visual stimuli during learning. Nature 439, 865–870 (2006).
Douglass, A. M. et al. Central amygdala circuits modulate food consumption through a positive-valence mechanism. Nat. Neurosci. 20, 1384–1394 (2017).
Kim, J., Pignatelli, M., Xu, S., Itohara, S. & Tonegawa, S. Antagonistic negative and positive neurons of the basolateral amygdala. Nat. Neurosci. 19, 1636–1646 (2016).
Namburi, P. et al. A circuit mechanism for differentiating positive and negative associations. Nature 520, 675–678 (2015).
Gore, F. et al. Neural representations of unconditioned stimuli in basolateral amygdala mediate innate and learned responses. Cell 162, 134–145 (2015).
Han, W. et al. Integrated control of predatory hunting by the central nucleus of the amygdala. Cell 168, 311–324 (2017).
Kim, J., Zhang, X., Muralidhar, S., LeBlanc, S. A. & Tonegawa, S. Basolateral to central amygdala neural circuits for appetitive behaviors. Neuron 93, 1464–1479 (2017).
Beyeler, A. C. et al. Organization of valence-encoding and projection-defined neurons in the basolateral amygdala. Cell Rep. 22, 905–918 (2018).
Boyden, E. S., Zhang, F., Bamberg, E., Nagel, G. & Deisseroth, K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat. Neurosci. 8, 1263–1268 (2005).
Tye, K. M. et al. Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature 471, 358–362 (2011).
Stachniak, T. J., Ghosh, A. & Sternson, S. M. Chemogenetic synaptic silencing of neural circuits localizes a hypothalamus→midbrain pathway for feeding behavior. Neuron 82, 797–808 (2014).
Maren, S. Neurobiology of Pavlovian fear conditioning. Annu. Rev. Neurosci. 24, 897–931 (2001).
Cardinal, R. N., Parkinson, J. A., Hall, J. & Everitt, B. J. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci. Biobehav. Rev. 26, 321–352 (2002).
Schoenbaum, G., Chiba, A. A. & Gallagher, M. Neural encoding in orbitofrontal cortex and basolateral amygdala during olfactory discrimination learning. J. Neurosci. 19, 1876–1884 (1999).
Lein, E. S. et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature 445, 168–176 (2007).
Bakker, R., Tiesinga, P. & Kötter, R. The scalable brain atlas: instant web-based access to public brain atlases and related content. Neuroinformatics 13, 353–366 (2015).
Armbruster, B. N., Li, X., Pausch, M. H., Herlitze, S. & Roth, B. L. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc. Natl Acad. Sci. USA 104, 5163–5168 (2007).
Grill, H. J. & Norgren, R. The taste reactivity test. II. Mimetic responses to gustatory stimuli in chronic thalamic and chronic decerebrate rats. Brain Res. 143, 281–297 (1978).
Madisen, L. et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci. 13, 133–140 (2010).
Susaki, E. A. et al. Advanced CUBIC protocols for whole-brain and whole-body clearing and imaging. Nat. Protoc. 10, 1709–1727 (2015).
Avants, B. B. et al. A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage 54, 2033–2044 (2011).
Zhang, Y. et al. Coding of sweet, bitter, and umami tastes: different receptor cells sharing similar signaling pathways. Cell 112, 293–301 (2003).
Kim, C. K. et al. Molecular and circuit-dynamical identification of top-down neural mechanisms for restraint of reward seeking. Cell 170, 1013–1027 (2017).
Ilango, A. et al. Similar roles of substantia nigra and ventral tegmental dopamine neurons in reward and aversion. J. Neurosci. 34, 817–822 (2014).
Krettek, J. E. & Price, J. L. A description of the amygdaloid complex in the rat and cat with observations on intra-amygdaloid axonal connections. J. Comp. Neurol. 178, 255–279 (1978).
Acknowledgements
We thank M. Tessier-Lavigne, N. Renier and P. Ariel for help with CUBIC; members of the Zuker laboratory and R. Axel for helpful discussions. We also acknowledge the Bio-Imaging Resource Center at Rockefeller University. Research reported in this publication was supported by the National Institute on Drug Abuse of the National Institutes of Health under award number R01DA035025 (C.S.Z). N.J.P.R. is supported by the Intramural Research Program of the NIH, NIDCR; and C.D.S. was supported by R01 MH082017 from NIMH. C.S.Z. is an investigator of the Howard Hughes Medical Institute and a Senior Fellow at Janelia Farm Research Campus.
Reviewer information
Nature thanks I. de Araujo and P. Kenny for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
L.W. and S.G.-S. designed the study, carried out the experiments and analysed data, Y.P. and J.Z. performed behavioural experiments. C.S.Z., X.C., N.J.P.R. and C.D.S. designed the study and analysed data. C.S.Z., L.W. and N.J.P.R. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Projections from the sweet and the bitter cortex terminate in distinct targets in the amygdala.
a, The cartoon illustrates the imaging planes of the optical horizontal sections at different depths of the amygdala. The brain diagrams were rendered by the scalable brain composer (https://scalablebrainatlas.incf.org/services/sba-composer.php?template=ABA_v3) based on the Allen Mouse Brain Common Coordinate Framework version 326,27. b, Segregation of sweet and bitter cortical projections in the amygdala. Sweet cortical neurons project to anterior BLA (green), whereas bitter cortical neurons predominantly innervate the CEA (red) and a portion of the posterior BLA (red). Top, optical horizontal sections at different dorsal–ventral positions are shown (sections 1–4; see a). Bottom, the boundaries of amygdala nuclei were determined by aligning fluorescence images to the Allen Brain Institute atlas26 (http://brain-map.org/). Scale bar, 500 μm. Similar results were observed in six independently labelled and imaged animals.
Extended Data Fig. 2 Activity-dependent labelling of sweet and bitter cortical targets in the amygdala.
Fos expression16 in response to optogenetic activation of the sweet and bitter cortical fields was used to label activated neurons. a, Schematic of optogenetic stimulation strategy in the bitter cortex for Fos induction. b, Fos expression in the amygdala in response to photostimulation of the bitter cortex. The majority of Fos+ neurons are localized in the CEA. c, Fos expression in a control mouse without light stimulation. d–f, Photostimulation of the sweet cortex induces Fos expression in the amygdala. Note that the CEA, as a major local output of the BLA11,36, also shows strong Fos labelling in response to photostimulation. Scale bars, 200 μm. Similar results were obtained in three animals for each experiment.
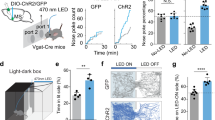
Extended Data Fig. 3 Place preference by photostimulation of cortico-amgydalar projections.
a, Representative tracking of a mouse during the 15-min place-preference test in a two-chamber arena; the left chamber in the diagram was coupled to stimulation of sweet cortico-amygdalar projections (see Methods for details); this animal spent over 80% of the test time in the chamber linked to stimulation of sweet projections to the amygdala. b, Quantification of preference index before (Pre) and during light stimulation. n = 8 mice, two-tailed paired t-test, P = 0.0156. c, d, Place-preference test with stimulation of bitter cortical projections in the amygdala. n = 5 mice, two-tailed paired t-test, P = 0.0207. e, f, Animals used in d were also tested in a licking assay with similar light stimulation intensity, demonstrating strong suppression of licking responses. n = 5 mice, two-tailed paired t-test, P = 0.0056. Values are mean ± s.e.m. We note that we have examined multiple independent behavioural experiments activating sweet projections to the BLA and have never observed the induction of motor patterns or consummatory behaviour. Strong stimulation of bitter cortico-amygdalar projections (20 Hz, 10–15 mW) often elicited prototypical orofacial rejection behaviour (Supplementary Video 1).
Extended Data Fig. 4 Activation of bitter cortical projections to posterior BLA is aversive.
As shown in Fig. 1 and Extended Data Fig. 1, a fraction of the bitter cortico-amygdalar projections terminate in the posterior BLA. As expected, stimulation of these projections elicits aversive responses. a, Representative histograms showing licking events in the presence (blue) or absence (grey) of photostimulation of bitter cortical projections to the posterior BLA. AAV-ChR2 was injected into the bitter cortex, and the stimulating fibre was targeted above the posterior BLA (coordinates: bregma −2 mm; lateral 3.4 mm; ventral 4.3 mm). b, Quantification of licking responses. n = 3 mice, two-tailed paired t-test, P = 0.0121. Values are mean ± s.e.m.
Extended Data Fig. 5 Control experiments for silencing amygdala with DREADD and NBQX.
a, Quantification of licking response before and after saline administration in mice that expressed inhibitory DREADD (hM4Di; see Fig. 5). n = 6 mice, two-tailed paired t-test; pre, P = 0.0011; saline, P < 0.0001; post, P = 0.0014. b, Quantification of licking response before and after CNO administration (10 mg kg−1) in wild-type (WT) non-DREADD-expressing animals. n = 6 mice, two-tailed paired t-test; pre, P = 0.0025; CNO, P = 0.0008; post, P = 0.0021. c, Controls with saline infusion instead of NBQX for Fig. 5c. n = 6 mice, two-tailed paired t-test; pre, P = 0.0080; saline, P = 0.0054; post, P = 0.0046. Values are mean ± s.e.m.
Extended Data Fig. 6 Chemogenetic silencing of neurons in the taste cortex impairs tastant recognition.
a, Quantification of the error rate for sweet taste recognition (2 mM AceK) in a three-port assay before and after silencing neurons in the sweet taste cortex with inhibitory DREADD (hM4Di) and CNO (10 mg kg−1). n = 5 mice, repeated-measures one-way ANOVA followed by Bonferroni post hoc test, F2,8 = 46.84, P < 0.0001. Pharmacological silencing data using NBQX can be found in a previously published study7. b, Quantification of the error rate of sweet taste recognition (1 mM AceK) before and after silencing of the amygdala with inhibitory DREADD (hM4Di) and CNO (10 mg kg−1). n = 4 mice, two-tailed paired t-test; pre versus CNO, P = 0.7888. See also Fig. 6. Note that sweet recognition was only affected by silencing the taste cortex, but not the amygdala. All tested animals recognized sweet taste with the correct behavioural choice (before silencing) in at least 90% of the trials. Values are mean ± s.e.m. See Supplementary Table 1 for detailed statistics.
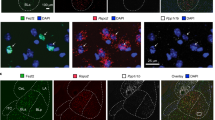
Extended Data Fig. 7 Retrograde labelling of gustatory thalamic neurons.
a, b, Injection of the retrograde tracer cholera toxin subunit B–Alexa Fluor 594 in the taste cortex (bitter cortical field; shown in red in a) selectively labels neurons in the taste thalamus (VPMpc; b). Similar results were observed in two animals. c, d, Diagrams of the corresponding brain regions, adapted from the Allen Brain Institute atlas. Scale bars, 500 μm.
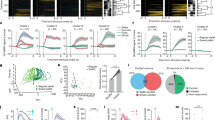
Extended Data Fig. 8 Licking responses to photostimulation of intermediate regions between sweet and bitter cortex.
Behavioural responses (see Fig. 3) in water-only trials linked to contact-driven self-stimulation are shown for mice expressing ChR2 between the sweet and bitter cortical fields. Note that a positive index means attraction, whereas a negative index means aversion to light stimulation. n = 14 mice. Data points indicate the behavioural test of individual animals at different stimulation sites relative to bregma position.
Supplementary information
Supplementary Table
This file contains Supplementary Table 1, which shows statistical presentation of data from Figures 3-6 and Extended Data Figures 3–6.
Video 1: Behavioral responses to ChR2 stimulation of bitter cortico-amygdalar projections
The video shows a mouse expressing ChR2-YFP in the bitter cortical field before and after stimulation of projections to CEA. Trial 1 shows a control experiment with the animal drinking water. Trial 2 shows orofacial responses to high concentrations of an orally applied bitter tastant (>30 mM Quinine); note gaping responses. Trial 3 shows responses elicited by direct stimulation of bitter cortico-amygdalar projections (see Methods for details). Note robust gaping responses; under these strong stimulating conditions (20 Hz, 10-12 mW) similar results were observed in all tested animals (n=3).
Rights and permissions
About this article
Cite this article
Wang, L., Gillis-Smith, S., Peng, Y. et al. The coding of valence and identity in the mammalian taste system. Nature 558, 127–131 (2018). https://doi.org/10.1038/s41586-018-0165-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-018-0165-4
This article is cited by
-
(R)-ketamine restores anterior insular cortex activity and cognitive deficits in social isolation-reared mice
Molecular Psychiatry (2024)
-
The anterior insula and its projection to amygdala nuclei modulate the abstinence-exacerbated expression of conditioned place preference
Psychopharmacology (2024)
-
Experience-dependent changes in affective valence of taste in male mice
Molecular Brain (2023)
-
Cocaine induces locomotor sensitization through a dopamine-dependent VTA-mPFC-FrA cortico-cortical pathway in male mice
Nature Communications (2023)
-
Linking emotional valence and anxiety in a mouse insula-amygdala circuit
Nature Communications (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.