Abstract
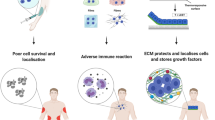
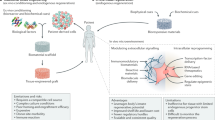
Although only a few stem cell-based therapies are currently available to patients, stem cells hold tremendous regenerative potential, and several exciting clinical applications are on the horizon. Biomaterials with tuneable mechanical and biochemical properties can preserve stem cell function in culture, enhance survival of transplanted cells and guide tissue regeneration. Rapid progress with three-dimensional hydrogel culture platforms provides the opportunity to grow patient-specific organoids, and has led to the discovery of drugs that stimulate endogenous tissue-specific stem cells and enabled screens for drugs to treat disease. Therefore, bioengineering technologies are poised to overcome current bottlenecks and revolutionize the field of regenerative medicine.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Hirsch, T. et al. Regeneration of the entire human epidermis using transgenic stem cells. Nature 551, 327–332 (2017).
Schwartz, S. D. et al. Embryonic stem cell trials for macular degeneration: a preliminary report. Lancet 379, 713–720 (2012).
Schwartz, S. D. et al. Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt’s macular dystrophy: follow-up of two open-label phase 1/2 studies. Lancet 385, 509–516 (2015).
Mandai, M. et al. Autologous induced stem-cell-derived retinal cells for macular degeneration. N. Engl. J. Med. 376, 1038–1046 (2017).
Trounson, A. & McDonald, C. Stem cell therapies in clinical trials: progress and challenges. Cell Stem Cell 17, 11–22 (2015).
FDA warns about stem cell therapies. US Food & Drug Administration https://www.fda.gov/ForConsumers/ConsumerUpdates/ucm286155.htm (FDA, 2017).
Anderson, A. J., Piltti, K. M., Hooshmand, M. J., Nishi, R. A. & Cummings, B. J. Preclinical efficacy failure of human CNS-derived stem cells for use in the pathway study of cervical spinal cord injury. Stem Cell Reports 8, 249–263 (2017).
Marsh, S. E. et al. HuCNS-SC Human NSCs fail to differentiate, form ectopic clusters, and provide no cognitive benefits in a transgenic model of Alzheimer’s disease. Stem Cell Reports 8, 235–248 (2017).
Rodin, S. et al. Long-term self-renewal of human pluripotent stem cells on human recombinant laminin-511. Nat. Biotechnol. 28, 611–615 (2010).
Melkoumian, Z. et al. Synthetic peptide-acrylate surfaces for long-term self-renewal and cardiomyocyte differentiation of human embryonic stem cells. Nat. Biotechnol. 28, 606–610 (2010).
Klim, J. R., Li, L., Wrighton, P. J., Piekarczyk, M. S. & Kiessling, L. L. A defined glycosaminoglycan-binding substratum for human pluripotent stem cells. Nat. Methods 7, 989–994 (2010).
Villa-Diaz, L. G. et al. Synthetic polymer coatings for long-term growth of human embryonic stem cells. Nat. Biotechnol. 28, 581–583 (2010).
Gobaa, S. et al. Artificial niche microarrays for probing single stem cell fate in high throughput. Nat. Methods 8, 949–955 (2011).
Gefen, A. & Margulies, S. S. Are in vivo and in situ brain tissues mechanically similar? J. Biomech. 37, 1339–1352 (2004).
Rho, J. Y., Ashman, R. B. & Turner, C. H. Young’s modulus of trabecular and cortical bone material: ultrasonic and microtensile measurements. J. Biomech. 26, 111–119 (1993).
Gilbert, P. M. et al. Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science 329, 1078–1081 (2010).This study demonstrated that muscle stem cells best maintained their stem cell phenotype and regenerative potential when cultured on substrates with stiffness approximating that of healthy muscle.
Cosgrove, B. D. et al. Rejuvenation of the muscle stem cell population restores strength to injured aged muscles. Nat. Med. 20, 255–264 (2014).
Yang, C., Tibbitt, M. W., Basta, L. & Anseth, K. S. Mechanical memory and dosing influence stem cell fate. Nat. Mater. 13, 645–652 (2014).This study used hydrogel substrates that were dynamically softened by light to demonstrate that mesenchymal stem cells can ‘remember’ the stiffness of the substrates on which they were cultured.
Li, C. X. et al. MicroRNA-21 preserves the fibrotic mechanical memory of mesenchymal stem cells. Nat. Mater. 16, 379–389 (2017).
Holst, J. et al. Substrate elasticity provides mechanical signals for the expansion of hemopoietic stem and progenitor cells. Nat. Biotechnol. 28, 1123–1128 (2010).
Choi, J. S. & Harley, B. A. C. Marrow-inspired matrix cues rapidly affect early fate decisions of hematopoietic stem and progenitor cells. Sci. Adv. 3, e1600455 (2017).
Chowdhury, F. et al. Soft substrates promote homogeneous self-renewal of embryonic stem cells via downregulating cell-matrix tractions. PLoS ONE 5, e15655 (2010).
Madl, C. M. et al. Maintenance of neural progenitor cell stemness in 3D hydrogels requires matrix remodelling. Nat. Mater. 16, 1233–1242 (2017).These studies 23,24,42 identified mechanisms by which matrix degradation can modulate stem cell fate.
Gjorevski, N. et al. Designer matrices for intestinal stem cell and organoid culture. Nature 539, 560–564 (2016).
McMurray, R. J. et al. Nanoscale surfaces for the long-term maintenance of mesenchymal stem cell phenotype and multipotency. Nat. Mater. 10, 637–644 (2011).
Chen, W. et al. Nanotopography influences adhesion, spreading, and self-renewal of human embryonic stem cells. ACS Nano 6, 4094–4103 (2012).
Lei, Y. & Schaffer, D. V. A fully defined and scalable 3D culture system for human pluripotent stem cell expansion and differentiation. Proc. Natl Acad. Sci. USA 110, E5039–E5048 (2013).
Zweigerdt, R., Andree, B., Kropp, C. & Kempf, H. in Bioreactors: Design, Operation and Novel Applications (ed. Mandenius, C.-F.) (Wiley-VCH, Weinheim, 2016).
Li, Y. et al. Engineering-derived approaches for iPSC preparation, expansion, differentiation and applications. Biofabrication 9, 032001 (2017).
Nie, Y., Bergendahl, V., Hei, D. J., Jones, J. M. & Palecek, S. P. Scalable culture and cryopreservation of human embryonic stem cells on microcarriers. Biotechnol. Prog. 25, 20–31 (2009).
Kehoe, D. E., Jing, D., Lock, L. T. & Tzanakakis, E. S. Scalable stirred-suspension bioreactor culture of human pluripotent stem cells. Tissue Eng. Part A 16, 405–421 (2010).
Tabata, Y., Horiguchi, I., Lutolf, M. P. & Sakai, Y. Development of bioactive hydrogel capsules for the 3D expansion of pluripotent stem cells in bioreactors. Biomater. Sci. 2, 176–183 (2014).
Weaver, V. M. et al. Reversion of the malignant phenotype of human breast cells in three-dimensional culture and in vivo by integrin blocking antibodies. J. Cell Biol. 137, 231–245 (1997).
Paszek, M. J. et al. Tensional homeostasis and the malignant phenotype. Cancer Cell 8, 241–254 (2005).
Engler, A. J., Sen, S., Sweeney, H. L. & Discher, D. E. Matrix elasticity directs stem cell lineage specification. Cell 126, 677–689 (2006).This study identified substrate stiffness as a potent regulator of stem cell differentiation in 2D culture systems.
Huebsch, N. et al. Harnessing traction-mediated manipulation of the cell/matrix interface to control stem-cell fate. Nat. Mater. 9, 518–526 (2010).
Saha, K. et al. Substrate modulus directs neural stem cell behavior. Biophys. J. 95, 4426–4438 (2008).
Przybyla, L., Lakins, J. N. & Weaver, V. M. tissue mechanics orchestrate Wnt-dependent human embryonic stem cell differentiation. Cell Stem Cell 19, 462–475 (2016).
Cameron, A. R., Frith, J. E. & Cooper-White, J. J. The influence of substrate creep on mesenchymal stem cell behaviour and phenotype. Biomaterials 32, 5979–5993 (2011).These studies 39,40,41 demonstrated that the viscoelastic properties of engineered extracellular matrices can modulate stem cell differentiation.
Chaudhuri, O. et al. Hydrogels with tunable stress relaxation regulate stem cell fate and activity. Nat. Mater. 15, 326–334 (2016).
Das, R. K., Gocheva, V., Hammink, R., Zouani, O. F. & Rowan, A. E. Stress-stiffening-mediated stem-cell commitment switch in soft responsive hydrogels. Nat. Mater. 15, 318–325 (2016).
Khetan, S. et al. Degradation-mediated cellular traction directs stem cell fate in covalently crosslinked three-dimensional hydrogels. Nat. Mater. 12, 458–465 (2013).
Cosgrove, B. D. et al. N-cadherin adhesive interactions modulate matrix mechanosensing and fate commitment of mesenchymal stem cells. Nat. Mater. 15, 1297–1306 (2016).
Kloxin, A. M., Kasko, A. M., Salinas, C. N. & Anseth, K. S. Photodegradable hydrogels for dynamic tuning of physical and chemical properties. Science 324, 59–63 (2009).
Freeman, R. et al. Instructing cells with programmable peptide DNA hybrids. Nat. Commun. 8, 15982 (2017).
Lam, J., Carmichael, S. T., Lowry, W. E. & Segura, T. Hydrogel design of experiments methodology to optimize hydrogel for iPSC-NPC culture. Adv. Healthc. Mater. 4, 534–539 (2015).
Downing, T. L. et al. Biophysical regulation of epigenetic state and cell reprogramming. Nat. Mater. 12, 1154–1162 (2013).
Caiazzo, M. et al. Defined three-dimensional microenvironments boost induction of pluripotency. Nat. Mater. 15, 344–352 (2016).
Aguado, B. A., Mulyasasmita, W., Su, J., Lampe, K. J. & Heilshorn, S. C. Improving viability of stem cells during syringe needle flow through the design of hydrogel cell carriers. Tissue Eng. Part A 18, 806–815 (2012).This study identified shear-thinning hydrogels as material carriers to protect cells from mechanical damage during injection.
Cai, L., Dewi, R. E. & Heilshorn, S. C. Injectable hydrogels with in situ double network formation enhance retention of transplanted stem cells. Adv. Funct. Mater. 25, 1344–1351 (2015).
Yan, C. et al. Injectable solid peptide hydrogel as a cell carrier: effects of shear flow on hydrogels and cell payload. Langmuir 28, 6076–6087 (2012).
Gaffey, A. C. et al. Injectable shear-thinning hydrogels used to deliver endothelial progenitor cells, enhance cell engraftment, and improve ischemic myocardium. J. Thorac. Cardiovasc. Surg. 150, 1268–1277 (2015).
Führmann, T. et al. Injectable hydrogel promotes early survival of induced pluripotent stem cell-derived oligodendrocytes and attenuates longterm teratoma formation in a spinal cord injury model. Biomaterials 83, 23–36 (2016).
Vegas, A. J. et al. Long-term glycemic control using polymer-encapsulated human stem cell-derived beta cells in immune-competent mice. Nat. Med. 22, 306–311 (2016).
Lam, J., Lowry, W. E., Carmichael, S. T. & Segura, T. Delivery of iPS-NPCs to the stroke cavity within a hyaluronic acid matrix promotes the differentiation of transplanted cells. Adv. Funct. Mater. 24, 7053–7062 (2014).
Huebsch, N. et al. Matrix elasticity of void-forming hydrogels controls transplanted-stem-cell-mediated bone formation. Nat. Mater. 14, 1269–1277 (2015).This study demonstrated that hydrogel stiffness can modulate stem cell behaviour in vivo.
Darnell, M. et al. Substrate stress-relaxation regulates scaffold remodeling and bone formation in vivo. Adv. Healthc. Mater. 6, 1601185 (2017).
Silva, G. A. et al. Selective differentiation of neural progenitor cells by high-epitope density nanofibers. Science 303, 1352–1355 (2004).
Sleep, E. et al. Injectable biomimetic liquid crystalline scaffolds enhance muscle stem cell transplantation. Proc. Natl Acad. Sci. USA 114, E7919–E7928 (2017).
Lovett, M., Lee, K., Edwards, A. & Kaplan, D. L. Vascularization strategies for tissue engineering. Tissue Eng. Part B Rev. 15, 353–370 (2009).
Richardson, T. P., Peters, M. C., Ennett, A. B. & Mooney, D. J. Polymeric system for dual growth factor delivery. Nat. Biotechnol. 19, 1029–1034 (2001).
Levenberg, S. et al. Engineering vascularized skeletal muscle tissue. Nat. Biotechnol. 23, 879–884 (2005).
Miller, J. S. et al. Rapid casting of patterned vascular networks for perfusable engineered three-dimensional tissues. Nat. Mater. 11, 768–774 (2012).
Arakawa, C. K., Badeau, B. A., Zheng, Y. & DeForest, C. A. Multicellular vascularized engineered tissues through user-programmable biomaterial photodegradation. Adv. Mater. 29, 1703156 (2017).
Suuronen, E. J. et al. Functional innervation in tissue engineered models for in vitro study and testing purposes. Toxicol. Sci. 82, 525–533 (2004).
Shvartsman, D. et al. Sustained delivery of VEGF maintains innervation and promotes reperfusion in ischemic skeletal muscles via NGF/GDNF signaling. Mol. Ther. 22, 1243–1253 (2014).
DiMarco, R. L., Dewi, R. E., Bernal, G., Kuo, C. & Heilshorn, S. C. Protein-engineered scaffolds for in vitro 3D culture of primary adult intestinal organoids. Biomater. Sci. 3, 1376–1385 (2015).
Cruz-Acuña, R. et al. Synthetic hydrogels for human intestinal organoid generation and colonic wound repair. Nat. Cell Biol. 19, 1326–1335 (2017).
Lancaster, M. A. et al. Guided self-organization and cortical plate formation in human brain organoids. Nat. Biotechnol. 35, 659–666 (2017).
Shao, Y. et al. Self-organized amniogenesis by human pluripotent stem cells in a biomimetic implantation-like niche. Nat. Mater. 16, 419–425 (2017).
Qian, X. et al. Brain-region-specific organoids using mini-bioreactors for modeling ZIKV exposure. Cell 165, 1238–1254 (2016).
Ma, Z. et al. Three-dimensional filamentous human diseased cardiac tissue model. Biomaterials 35, 1367–1377 (2014).
Burridge, P. W. et al. Human induced pluripotent stem cell-derived cardiomyocytes recapitulate the predilection of breast cancer patients to doxorubicin-induced cardiotoxicity. Nat. Med. 22, 547–556 (2016).
Lind, J. U. et al. Instrumented cardiac microphysiological devices via multimaterial three-dimensional printing. Nat. Mater. 16, 303–308 (2017).
Ribeiro, A. J. S. et al. Contractility of single cardiomyocytes differentiated from pluripotent stem cells depends on physiological shape and substrate stiffness. Proc. Natl Acad. Sci. USA 112, 12705–12710 (2015).
Nguyen, E. H. et al. Versatile synthetic alternatives to Matrigel for vascular toxicity screening and stem cell expansion. Nat. Biomed. Eng. 1, 0096 (2017).
Musah, S. et al. Mature induced-pluripotent-stem-cell-derived human podocytes reconstitute kidney glomerular-capillary-wall function on a chip. Nat. Biomed. Eng. 1, 0069 (2017).
Dekkers, J. F. et al. Characterizing responses to CFTR-modulating drugs using rectal organoids derived from subjects with cystic fibrosis. Sci. Transl. Med. 8, 344ra84 (2016).
McLean, W. J. et al. Clonal expansion of Lgr5-positive cells from mammalian cochlea and high-purity generation of sensory hair cells. Cell Rep. 18, 1917–1929 (2017).
Saini, A. Cystic fibrosis patients benefit from mini guts. Cell Stem Cell 19, 425–427 (2016).
Lyon, J. Hearing restoration: a step closer? J. Am. Med. Assoc. 318, 319–320 (2017).
Ho, A. T. V. et al. Prostaglandin E2 is essential for efficacious skeletal muscle stem-cell function, augmenting regeneration and strength. Proc. Natl Acad. Sci. USA 114, 6675–6684 (2017).
Rosales, A. M., Vega, S. L., DelRio, F. W., Burdick, J. A. & Anseth, K. S. Hydrogels with reversible mechanics to probe dynamic cell microenvironments. Angew. Chem. Int. Ed. 56, 12132–12136 (2017).
DeForest, C. A. & Tirrell, D. A. A photoreversible protein-patterning approach for guiding stem cell fate in three-dimensional gels. Nat. Mater. 14, 523–531 (2015).
Lee, T. T. et al. Light-triggered in vivo activation of adhesive peptides regulates cell adhesion, inflammation and vascularization of biomaterials. Nat. Mater. 14, 352–360 (2015). This study demonstrated the feasibility of using light as a stimulus to dynamically modify biomaterial properties in vivo.
Cambria, E. et al. Covalent modification of synthetic hydrogels with bioactive proteins via sortase-mediated ligation. Biomacromolecules 16, 2316–2326 (2015).
Guvendiren, M. & Burdick, J. A. Stiffening hydrogels to probe short- and long-term cellular responses to dynamic mechanics. Nat. Commun. 3, 792 (2012).
Turner, M. et al. Toward the development of a global induced pluripotent stem cell library. Cell Stem Cell 13, 382–384 (2013).
Rice, J. J. et al. Engineering the regenerative microenvironment with biomaterials. Adv. Healthc. Mater. 2, 57–71 (2013).
Vishwakarma, A. et al. Engineering immunomodulatory biomaterials to tune the inflammatory response. Trends Biotechnol. 34, 470–482 (2016).
Ali, O. A., Emerich, D., Dranoff, G. & Mooney, D. J. In situ regulation of DC subsets and T cells mediates tumor regression in mice. Sci. Transl. Med. 1, 8ra19 (2009).
Hori, Y., Stern, P. J., Hynes, R. O. & Irvine, D. J. Engulfing tumors with synthetic extracellular matrices for cancer immunotherapy. Biomaterials 30, 6757–6767 (2009).
Getts, D. R. et al. Microparticles bearing encephalitogenic peptides induce T-cell tolerance and ameliorate experimental autoimmune encephalomyelitis. Nat. Biotechnol. 30, 1217–1224 (2012).
Yoon, Y. M. et al. A combination hydrogel microparticle-based vaccine prevents type 1 diabetes in non-obese diabetic mice. Sci. Rep. 5, 13155 (2015).
Pompano, R. R. et al. Titrating T-cell epitopes within self-assembled vaccines optimizes CD4+ helper T cell and antibody outputs. Adv. Healthc. Mater. 3, 1898–1908 (2014).
Spiller, K. L. et al. Sequential delivery of immunomodulatory cytokines to facilitate the M1-to-M2 transition of macrophages and enhance vascularization of bone scaffolds. Biomaterials 37, 194–207 (2015).This study demonstrated that regulation of the host immune response can enhance regeneration in response to engineered constructs.
Acknowledgements
C.M.M. is supported by the Stanford ChEM-H Interdisciplinary Postdoctoral Training Program in Quantitative Mechanobiology. S.C.H. acknowledges support from the National Institutes of Health (NIH) (U19 AI116484 and R21 HL13804201), the National Science Foundation (DMR 1508006) and the California Institute for Regenerative Medicine (CIRM) (RT3-07948). H.M.B. acknowledges support from the NIH (R01 AG020961, R01 AR063963, R01 NS089533, and R01 HG00967401), CIRM (DISC1-10036), the American Heart Association (17CSA33590101), the Baxter Foundation, and the Li Ka Shing Foundation.
Author information
Authors and Affiliations
Contributions
C.M.M., S.C.H. and H.M.B. all participated in the planning, writing and editing of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Madl, C.M., Heilshorn, S.C. & Blau, H.M. Bioengineering strategies to accelerate stem cell therapeutics. Nature 557, 335–342 (2018). https://doi.org/10.1038/s41586-018-0089-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-018-0089-z
This article is cited by
-
Programmable synthetic receptors: the next-generation of cell and gene therapies
Signal Transduction and Targeted Therapy (2024)
-
Exosomes from adipose-derived stem cells activate sebocytes through the PI3K/AKT/SREBP-1 pathway to accelerate wound healing
Cell and Tissue Research (2024)
-
Application of biomimetic three-dimensional scaffolds in bone tissue repairing
Macromolecular Research (2024)
-
Engineering considerations of iPSC-based personalized medicine
Biomaterials Research (2023)
-
Bioinspired nanotopographical design of drug delivery systems
Nature Reviews Bioengineering (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.