Abstract
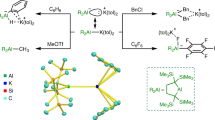
The reactivity of aluminium compounds is dominated by their electron deficiency and consequent electrophilicity; these compounds are archetypal Lewis acids (electron-pair acceptors). The main industrial roles of aluminium, and classical methods of synthesizing aluminium–element bonds (for example, hydroalumination and metathesis), draw on the electron deficiency of species of the type AlR3 and AlCl31,2. Whereas aluminates, [AlR4]−, are well known, the idea of reversing polarity and using an aluminium reagent as the nucleophilic partner in bond-forming substitution reactions is unprecedented, owing to the fact that low-valent aluminium anions analogous to nitrogen-, carbon- and boron-centred reagents of the types [NX2]−, [CX3]− and [BX2]− are unknown3,4,5. Aluminium compounds in the +1 oxidation state are known, but are thermodynamically unstable with respect to disproportionation. Compounds of this type are typically oligomeric6,7,8, although monomeric systems that possess a metal-centred lone pair, such as Al(Nacnac)Dipp (where (Nacnac)Dipp = (NDippCR)2CH and R = tBu, Me; Dipp = 2,6-iPr2C6H3), have also been reported9,10. Coordination of these species, and also of (η5-C5Me5)Al, to a range of Lewis acids has been observed11,12,13, but their primary mode of reactivity involves facile oxidative addition to generate Al(iii) species6,7,8,14,15,16. Here we report the synthesis, structure and reaction chemistry of an anionic aluminium(i) nucleophile, the dimethylxanthene-stabilized potassium aluminyl [K{Al(NON)}]2 (NON = 4,5-bis(2,6-diisopropylanilido)-2,7-di-tert-butyl-9,9-dimethylxanthene). This species displays unprecedented reactivity in the formation of aluminium–element covalent bonds and in the C–H oxidative addition of benzene, suggesting that it could find further use in both metal–carbon and metal–metal bond-forming reactions.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Change history
04 June 2018
In Fig. 1 of this Letter, the hydrogen (H) atoms attached to each of the two nitrogen (N) atoms in the chemical structure of (NON)H2 were inadvertently missing. The original figure has been corrected online.
References
Aldridge, S. & Downs, A. J. (eds) The Group 13 Metals Aluminium, Gallium, Indium and Thallium: Chemical Patterns and Peculiarities (Wiley, Chichester, 2011).
Helmboldt, O. et al. Ullmann’s Encyclopedia of Industrial Chemistry: Aluminum Compounds, Inorganic (Wiley VCH, Weinheim, 2007).
Lappert, M., Protchenko, A., Power, P. & Seeber, A. Metal Amide Chemistry (Wiley, Chichester, 2009).
Rappoport, Z. & Marek, I. (eds) The Chemistry of Organolithium Compounds (Wiley–Blackwell, Chichester, 2004).
Segawa, Y., Yamashita, M. & Nozaki, K. Boryllithium: isolation, characterization, and reactivity as a boryl anion. Science 314, 113–115 (2006).
Dohmeier, C., Loos, D. & Schnöckel, H. Aluminum(i) and gallium(i) compounds: syntheses, structures, and reactions. Angew. Chem. Int. Ed. 35, 129–149 (1996).
Nagendran, S. & Roesky, H. The chemistry of aluminum(i), silicon(ii) and germanium(ii). Organometallics 27, 457–492 (2008).
Jones, C. & Stasch, A. in The Group 13 Metals Aluminium, Gallium, Indium and Thallium: Chemical Patterns and Peculiarities (eds Aldridge, S. & Downs, A. J.) 285–341 (Wiley, Chichester, 2011).
Cui, C. et al. Synthesis and structure of a monomeric aluminum(i) compound [{HC(CMeNAr)2}Al] (Ar = 2,6-iPr2C6H3): a stable aluminum analogue of a carbene. Angew. Chem. Int. Ed. 39, 4274–4276 (2000).
Li, X., Cheng, X., Song, H. & Cui, C. Synthesis of HC[(CBut)(NAr)]2Al (Ar = 2,6-Pri 2C6H3) and its reaction with isocyanides, a bulky azide, and H2O. Organometallics 26, 1039–1043 (2007).
Linti, G. & Schnöckel, H. Low valent aluminium and gallium compounds — structural variety and coordination modes to transition metal fragments. Coord. Chem. Rev. 206–207, 285–319 (2000).
Asay, M., Jones, C. & Driess, M. N-heterocyclic carbene analogues with low-valent group 13 and group 14 elements: syntheses, structures, and reactivities of a new generation of multitalented ligands. Chem. Rev. 111, 354–396 (2011).
González-Gallardo, S., Bollermann, T., Fischer, R. A. & Murugavel, R. Cyclopentadiene based low-valent group 13 metal compounds: ligands in coordination chemistry and link between metal rich molecules and intermetallic materials. Chem. Rev. 112, 3136–3170 (2012).
Chu, T., Korobkov, I. & Nikonov, G. I. Oxidative addition of σ bonds to an Al(i) center. J. Am. Chem. Soc. 136, 9195–9202 (2014).
Crimmin, M. R., Butler, M. J. & White, A. J. P. Oxidative addition of carbon–fluorine and carbon–oxygen bonds to Al(i). Chem. Commun. 51, 15994–15996 (2015).
Chu, T., Boyko, Y., Korobkov, I. & Nikonov, G. I. Transition metal-like oxidative addition of C–F and C–O bonds to an aluminum(i) center. Organometallics 34, 5363–5365 (2015).
Sundermann, A., Reiher, M. & Schoeller, W. W. Isoelectronic Arduengo-type carbene analogues with the group IIIa elements boron, aluminum, gallium, and indium. Eur. J. Inorg. Chem. 1998, 305–310 (1998).
Tuononen, H. M., Roesler, R., Dutton, J. L. & Ragogna, P. J. Electronic structures of main-group carbene analogues. Inorg. Chem. 46, 10693–10706 (2007).
Westrum, L. J. & Rakita, P. E. (eds) Handbook of Grignard Reagents 2nd edn (CRC Press, Boca Raton, 2015).
Uhl, W. Organoelement compounds possessing Al–Al, Ga–Ga, In–In, and Tl–Tl single bonds. Adv. Organomet. Chem. 51, 53–108 (2004).
Twamley, B. & Power, P. P. Synthesis of the square-planar gallium species K2[Ga4(C6H3-2,6-Trip2)2] (Trip = C6H2-2,4,6-iPr3): the role of aryl–alkali metal ion interactions in the structure of gallium clusters. Angew. Chem. Int. Ed. 39, 3500–3503 (2000).
Cordero, B. et al. Covalent radii revisited. Dalton Trans. 21, 2832–2838 (2008).
Bakewell, C., Ward, B. J., White, A. J. P. & Crimmin, M. R. A combined experimental and computational study on the reaction of fluoroarenes with Mg–Mg, Mg–Zn, Mg–Al and Al–Zn bonds. Chem. Sci. 9, 2348–2356 (2018).
Green, S. P., Jones, C. & Stasch, A. Stable magnesium(i) compounds with Mg–Mg bonds. Science 318, 1754–1757 (2007).
Martínez-Martínez, A. J., Kennedy, A. R., Mulvey, R. E. & O’Hara, C. T. Directed ortho-meta′- and meta-meta′-dimetalations: a template base approach to deprotonation. Science 346, 834–837 (2014).
Ohsato, T. et al. A potassium diboryllithate: synthesis, bonding properties, and the deprotonation of benzene. Angew. Chem. Int. Ed. 55, 11426–11430 (2016).
Cruz, C. A., Emslie, D. J. H., Harrington, L. E., Britten, J. F. & Robertson, C. M. Extremely stable thorium(iv) dialkyl complexes supported by rigid tridentate 4,5-bis(anilido)xanthene and 2,6-bis(anilidomethyl)pyridine ligands. Organometallics 26, 692–701 (2007).
Bonyhady, S. J. et al. β-diketiminate-stabilized magnesium(i) dimers and magnesium(ii) hydride complexes: synthesis, characterization, adduct formation, and reactivity studies. Chem. Eur. J. 16, 938–955 (2010).
Zakharkin, L. I. & Gavrilenko, V. V. Mutual conversions in the alumohydrides of lithium, sodium, and potassium. Russ. Chem. Bull. 11, 1076–1078 (1962).
Cosier, J. & Glazer, A. M. A nitrogen-gas-stream cryostat for general X-ray diffraction studies. J. Appl. Crystallogr. 19, 105–107 (1986).
CrysAlisPro v.1.171.35.8 (Agilent Technologies, 2011).
Sheldrick, G. M. SHELX-2014 (2014).
Hübschle, C. B., Sheldrick, G. M. & Dittrich, B. ShelXle: a Qt graphical user interface for SHELXL. J. Appl. Crystallogr. 44, 1281–1284 (2011).
Frisch, M. J. et al. Gaussian 09 Rev. D.01, (Gaussian Inc., 2009).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Perdew, J. P., Ernzerhof, M. & Burke, K. Rationale for mixing exact exchange with density functional approximations. J. Chem. Phys. 105, 9982–9985 (1996).
Adamo, C. & Barone, V. Toward reliable density functional methods without adjustable parameters: the PBE0 model. J. Chem. Phys. 110, 6158–6170 (1999).
Schäfer, A., Huber, C. & Ahlrichs, R. Fully optimized contracted Gaussian basis sets of triple zeta valence quality for atoms Li to Kr. J. Chem. Phys. 100, 5829–5835 (1994).
Grimme, S., Antony, J., Ehrlich, S. & Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H–Pu. J. Chem. Phys. 132, 154104–154119 (2010).
Grimme, S., Ehrlich, S. & Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 32, 1456–1465 (2011).
Glendening, E. D. et al. NBO v. 5.9 (2011).
Dennington, R., Keith, T. A. & Millam, J. M. GaussView v. 5.0 (Semichem Inc., 2009).
Acknowledgements
This work was supported by the SCG-Oxford Centre of Excellence. P.V. thanks the Magnus Ehrnrooth and Emil Aaltonen Foundations for postdoctoral funding. We thank the University of Oxford Advanced Research Computing facility, and N. Rees and H. Tuononen for assistance with NMR and quantum chemical studies, respectively.
Author contributions J.H. carried out the synthetic and reaction studies, P.V. carried out the computational analyses, J.H. and J.M.G. conducted the crystallographic studies, and J.M.G. and S.A. wrote the manuscript and managed the project.
Competing interests: The authors declare no competing interests.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Molecular structure of (NON)AlI as determined by X-ray crystallography.
Hydrogen atoms have been omitted and selected carbon atoms shown in wireframe format for clarity; thermal ellipsoids have been drawn at the 35% probability level. Key bond lengths (Å) and angles (°): Al(1)–I(1) 2.497(1), Al(1)–N(1) 1.846(2), Al(1)–N(2) 1.846(2), Al(1)–O(1) 1.967(2), N(1)–Al(1)–N(2) 143.0(1).
Extended Data Fig. 2 Molecular structure of [Al(NON)]2 as determined by X-ray crystallography.
Hydrogen atoms have been omitted and selected carbon atoms shown in wireframe format for clarity; thermal ellipsoids have been drawn at the 35% probability level. Key bond lengths (Å) and angles (°): Al(1)–Al(2) 2.646(1), Al(1)–N(1) 1.902(2), Al(1)–N(2) 1.895(2), Al(2)–N(3) 1.901(2), Al(2)–N(4) 1.900(2), Al(1)–O(1) 1.976(2), Al(2)–O(2) 1.981(2), N(1)–Al(1)–N(2) 119.0(1), N(3)–Al(2)–N(4) 118.6(1).
Extended Data Fig. 3 Molecular structure of one of the molecules in the asymmetric unit of [K{H2Al(NON)}]2 as determined by X-ray crystallography.
Second (essentially identical) component, benzene solvate molecules and carbon-bound hydrogen atoms have been omitted and selected carbon atoms shown in wireframe format for clarity; thermal ellipsoids have been drawn at the 35% probability level. Key distances (Å) and angles (°): Al(1)–N(1/2) 1.933(2)/1.921(2), Al(2)–N(3/4) 1.934(2)/1.917(2), Al(1)–O(1) 2.131(1), Al(2)–O(2) 2.124(2), Al(1)…Al(2) 6.356(1), Al(1)…K(1/2) 3.648(1)/4.065(1), Al(2)…K(1/2) 3.580(1)/4.039(1), Al(1)–H(1 A/1B) 1.69(4)/1.55(4), Al(2)–H(2 A/2B) 1.71(4)/1.58(4), N(1)–Al(1)–N(2) 130.3(1), N(3)–Al(2)–N(4) 131.1(1).
Extended Data Fig. 4 Infrared spectra of [K{Al(NON)}]2 and [K{H2Al(NON)}]2.
a, [K{Al(NON)}]2. b, [K{H2Al(NON)}]2. Both spectra have been measured on samples as Nujol mulls; the blue asterisk highlights the Al–H stretching band of [K{H2Al(NON)}]2.
Extended Data Fig. 5 Molecular structure of [K{Ga(NON)}]2 as determined by X-ray crystallography.
Hydrogen atoms have been omitted and selected carbon atoms shown in wireframe format for clarity; thermal ellipsoids have been drawn at the 35% probability level. Key bond lengths and distances (Å) and angles (°): Ga(1)…Ga(1′) 6.134(1), Ga(1)…K(1) 3.970(1), Ga(1)…K(1′) 3.784(1), Ga(1)–N(1) 2.093(2), Ga(1)–N(2) 2.106(2), Ga(1)–O(1) 2.542(2), N(1)–Ga(1)–N(2) 126.0(1).
Extended Data Fig. 6 Molecular structure of (NON)AlH·toluene as determined by X-ray crystallography.
Most hydrogen atoms have been omitted and selected carbon atoms shown in wireframe format for clarity; thermal ellipsoids have been drawn at the 35% probability level. Key bond lengths (Å) and angles (°): Al(1)–N(1) 1.873(1), Al(1)–N(2) 1.872(1), Al(1)–O(1) 1.944(1), Al(1)–H(1) 1.49(2), N(1)–Al(1)–N(2) 134.1(1).
Extended Data Fig. 7 Molecular structure of [K{Ph(H)Al(NON)}]2 as determined by X-ray crystallography.
Most hydrogen atoms and benzene solvate molecules have been omitted, and selected carbon atoms shown in wireframe format for clarity; thermal ellipsoids have been drawn at the 35% probability level. Key bond lengths (Å) and angles (°): Al(1)–N(1) 1.945(2), Al(1)–N(2) 1.944(2), Al(1)–O(1) 2.122(1), Al(1)–C(48) 2.007(1), Al(1)–H(1) 1.82(3), N(1)–Al(1)–N(2) 132.2(1).
Supplementary information
Supplementary Tables
This file contains Supplementary Tables 1-4
Supplementary Data
This file contains Supplementary Data files 1-10 and a guide.
Rights and permissions
About this article
Cite this article
Hicks, J., Vasko, P., Goicoechea, J.M. et al. Synthesis, structure and reaction chemistry of a nucleophilic aluminyl anion. Nature 557, 92–95 (2018). https://doi.org/10.1038/s41586-018-0037-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-018-0037-y
This article is cited by
-
Anions featuring an aluminium–silicon core with alumanyl silanide and aluminata-silene characteristics
Nature Chemistry (2023)
-
Synthesis of a low-valent Al4+ cluster cation salt
Nature Chemistry (2022)
-
Strongly reducing magnesium(0) complexes
Nature (2021)
-
Hydroarylation of olefins catalysed by a dimeric ytterbium(II) alkyl
Nature Communications (2021)
-
Charge frustration in ligand design and functional group transfer
Nature Reviews Chemistry (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.