Abstract
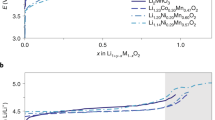
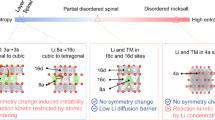
There is an urgent need for low-cost, resource-friendly, high-energy-density cathode materials for lithium-ion batteries to satisfy the rapidly increasing need for electrical energy storage. To replace the nickel and cobalt, which are limited resources and are associated with safety problems, in current lithium-ion batteries, high-capacity cathodes based on manganese would be particularly desirable owing to the low cost and high abundance of the metal, and the intrinsic stability of the Mn4+ oxidation state. Here we present a strategy of combining high-valent cations and the partial substitution of fluorine for oxygen in a disordered-rocksalt structure to incorporate the reversible Mn2+/Mn4+ double redox couple into lithium-excess cathode materials. The lithium-rich cathodes thus produced have high capacity and energy density. The use of the Mn2+/Mn4+ redox reduces oxygen redox activity, thereby stabilizing the materials, and opens up new opportunities for the design of high-performance manganese-rich cathodes for advanced lithium-ion batteries.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Whittingham, M. S. Lithium batteries and cathode materials. Chem. Rev. 104, 4271–4302 (2004).
Olivetti, E. A., Ceder, G., Gaustad, G. G. & Fu, X. Lithium-ion battery supply chain considerations: analysis of potential bottlenecks in critical metals. Joule 1, 229–243 (2017).
Lu, Z., MacNeil, D. D. & Dahn, J. R. Layered Li[Ni x Co1−2x Mn x ]O2 cathode materials for lithium-ion batteries. Electrochem. Solid-State Lett 4, A200–A203 (2001).
Thackeray, M. M., Johnson, P. J., de Picciotto, L. A., Bruce, P. G. & Goodenough, J. B. Electrochemical extraction of lithium from LiMn2O4. Mater. Res. Bull. 19, 179–187 (1984).
Kang, B. & Ceder, G. Battery materials for ultrafast charging and discharging. Nature 458, 190–193 (2009).
Nitta, N., Wu, F., Lee, J. T. & Yushin, G. Li-ion battery materials: present and future. Mater. Today 18, 252–264 (2015).
Thackeray, M. M. et al. Li2MnO3-stabilized LiMO2 (M = Mn, Ni, Co) electrodes for lithium-ion batteries. J. Mater. Chem. 17, 3112–3125 (2007).
Yabuuchi, N. et al. High-capacity electrode materials for rechargeable lithium batteries: Li3NbO4-based system with cation-disordered rocksalt structure. Proc. Natl Acad. Sci. USA 112, 7650–7655 (2015).
Armstrong, A. R. et al. Demonstrating oxygen loss and associated structural reorganization in the lithium battery cathode Li[Ni0.2Li0.2Mn0.6]O2. J. Am. Chem. Soc. 128, 8694–8698 (2006).
Hy, S. et al. Performance and design considerations for lithium excess layered oxide positive electrode materials for lithium ion batteries. Energy Environ. Sci. 9, 1931–1954 (2016).
Freire, M. et al. A new active Li–Mn–O compound for high energy density Li-ion batteries. Nat. Mater. 15, 173–177 (2016).
Kim, J. & Manthiram, A. A manganese oxyiodide cathode for rechargeable lithium batteries. Nature 390, 265–267 (1997).
Lee, J. et al. Unlocking the potential of cation-disordered oxides for rechargeable lithium batteries. Science 343, 519–522 (2014).
Urban, A., Lee, J. & Ceder, G. The configurational space of rocksalt-type oxides for high-capacity lithium battery electrodes.Adv. Energy Mater. 4, 1400478 (2014).
Richards, W. D., Dacek, S. T., Kitchaev, D. A. & Ceder, G. Fluorination of lithium-excess transition metal oxide cathode materials. Adv. Energy Mater. 343, 1701533 (2017).
Chen, R. et al. Disordered lithium-rich oxyfluoride as a stable host for enhanced Li+ intercalation storage. Adv. Energy Mater. 5, 1401814 (2015).
Takeda, N. et al. Reversible Li storage for nanosize cation/anion-disordered rocksalt-type oxyfluorides: LiMoO2–x LiF (0≤x≤2) binary system. J. Power Sources 367, 122–129 (2017).
Urban, A., Abdellahi, A., Dacek, S., Artrith, N. & Ceder, G. Electronic-structure origin of cation disorder in transition-metal oxides. Phys. Rev. Lett. 119, 176402 (2017).
Etcheri, V., Marom, R., Elazi, R., Salitra, G. & Aurbach, D. Challenges in the development of advanced Li-ion batteries: a review. Energy Environ. Sci. 4, 3243–3262 (2011).
Wang, R. et al. A disordered rock-salt Li-excess cathode materials with high capacity and substantial oxygen redox activity: Li1.25Nb0.25Mn0.5O2. Electrochem. Commun. 60, 70–73 (2015).
Yabuuchi, N. et al. Origin of stabilization and destabilization in solid-state redox reaction of oxide ions for lithium-ion batteries. Nat. Commun. 7, 13814 (2016).
Lee, J. et al. A new class of high capacity cation-disordered oxides for rechargeable lithium batteries: Li–Ni–Ti–Mo oxides. Energy Environ. Sci. 8, 3255–3265 (2015).
Manceau, A., Marcus, M. A. & Grangeon, S. Determination of Mn valence states in mixed-valent manganates by XANES spectroscopy. Am. Mineral. 97, 816–827 (2012).
de Groot, F. M. F. et al. Oxygen 1s X-ray-absorption edges of transition-metal oxides. Phys. Rev. B 40, 5715–5723 (1989).
Kurata, H., Lefèvre, E. & Colliex, C. Electron-energy-loss near-edge structures in the oxygen K-edge spectra of transition-metal oxides. Phys. Rev. B 47, 13763–13768 (1993).
Luo, K. et al. Charge-compensation in 3d-transition-metal-oxide intercalation cathodes through the generation of localized electron holes on oxygen. Nat. Chem. 8, 684–691 (2016).
Seo, D.-H. et al. The structural and chemical origin of the oxygen redox activity in layered and cation-disordered Li-excess cathode materials. Nat. Chem. 8, 692–697 (2016).
Xie, Y., Saubanère, M. & Doublet, M.-L. Requirements for reversible extra-capacity in Li-rich layered oxides for Li-ion batteries. Energy Environ. Sci. 10, 266–274 (2017).
Lee, J. et al. Mitigating oxygen loss to improve the cycling performance of high capacity cation-disordered cathode materials. Nat. Commun. 8, 981 (2017).
Abdellahi, A., Urban, A., Dacek, S. & Ceder, G. Understanding the effect of cation disorder on the voltage profile of lithium transition-metal oxides. Chem. Mater. 28, 5373–5383 (2016).
Cho, J., Kim, T.-J., Kim, Y. J. & Park, B. Complete blocking of Mn3+ ion dissolution from a LiMn2O4 spinel intercalation compound by Co3O4 coating. Chem. Commun. 1074–1075 (2001).
Xu, K. Electrolytes and interphases in Li-ion batteries and beyond. Chem. Rev. 114, 11503–11618 (2014).
Wang, Y. et al. Design principles for solid-state lithium superionic conductors. Nat. Mater. 14, 1026–1031 (2015).
Ravel, B. & Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 12, 537–541 (2005).
Yang, W. et al. Key electronic states in lithium battery materials probed by soft X-ray spectroscopy. J. Electron Spectrosc. Relat. Phenom. 190, 64–74 (2013).
McCloskey, B. D., Bethune, D. S., Shelby, R. M., Girishkumar, G. & Luntz, A. C. Solvents’ critical role in nonaqueous lithium–oxygen battery electrochemistry. J. Phys. Chem. Lett. 2, 1161–1166 (2011).
McCloskey, B. D. et al. On the efficacy of electrocatalysis in nonaqueous Li-O2 batteries. J. Am. Chem. Soc. 133, 18038–18041 (2011).
McCloskey, B. D. et al. Twin problems of interfacial carbonate formation in nonaqueous Li-O2 batteries. J. Phys. Chem. Lett. 3, 997–1001 (2012).
Sananes, M. T., Tuel, A., Hutchings, G. J. & Volta, J. C. Characterization of different precursors and activated vanadium phosphate catalysis by31P NMR spin echo mapping. J. Catal. 148, 395–398 (1994).
O’Dell, L. A. & Schurko, R. W. QCPMG using adiabatic pulses for faster acquisition of ultra-wideline NMR spectra. Chem. Phys. Lett 464, 97–102 (2008).
Pell, A. J., Clèment, R. J., Grey, C. P., Emsley, L. & Pintacuda, G. Frequency-stepped acquisition in nuclear magnetic resonance spectroscopy under magic angle spinning. J. Chem. Phys. 138, 114201 (2013).
Massiot, D. et al.71Ga and69Ga nuclear magnetic resonance study of β-Ga2O3: resolution of four- and six-fold coordinated Ga sites in static conditions. Solid State Nucl. Magn. Reson. 4, 241–248 (1995).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Richards, W. D., Wang, Y., Miara, L., Kim, J. C. & Ceder, G. Design of Li1+2x Zn1−x PS4, a new lithium ion conductor. Energy Environ. Sci. 9, 3272–3278 (2016).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Dudarev, S., Botton, G. A., Savrasov, S. Y. & Sutton, A. P. Electron-energy-loss spectra and the structural stability of nickel oxide: an LSDA+U study. Phys. Rev. B 57, 1505–1509 (1998).
Wang, L., Maxisch, T. & Ceder, G. Oxidation energies of transition metal oxides within the GGA+U framework. Phys. Rev. B 73, 8627 (1994).
Heyd, J., Scuseria, G. E. & Ernzerhof, M. Hybrid functionals based on a screened coulomb potential. J. Chem. Phys. 118, 8207 (2003).
Seo, D.-H., Urban, A. & Ceder, G. Calibrating transition-metal energy levels and oxygen bands in first-principles calculations: accurate prediction of redox potentials and charge transfer in lithium transition-metal oxides. Phys. Rev. B 92, 115118 (2015).
Raebiger, L. S. & Zunger, A. Charge self-regulation upon changing the oxidation state of transition metals in insulators. Nature 453, 763–766 (2008).
Dupré, N., Cuisinier, M. & Guyomard, D. Electrode/electrolyte interface studies in lithium batteries using NMR. Electrochem. Soc. Interface 20, 61–67 (2011).
Nakajima, M. & Yabuuchi, N. Lithium-excess cation-disordered rocksalt-type oxide with nanoscale phase segregation: Li1.25Nb0.25V0.5O2. Chem. Mater. 29, 6927–6935 (2017).
Middlemiss, D. S., Iliott, A. J., Clément, R. J., Strobridge, F. C. & Grey, C. P. Density functional theory-based bond pathway decompositions of hyperfine shifts: equipping solid-state NMR to characterize atomic environments in paramagnetic materials. Chem. Mater. 25, 1723–1734 (2013).
McCloskey, B. D. et al. Combining accurate O2 and Li2O2 assays to separate discharge and charge stability limitations in nonaqueous Li–O2 batteries. J. Phys. Chem. Lett. 4, 2989–2993 (2013).
Renfrew, S. & McCloskey, B. D. Residual lithium carbonate predominantly accounts for first cycle CO2 and CO outgassing of Li-stoichiometric and Li-rich layered transition-metal oxides. J. Am. Chem. Soc. 139, 17853–17860 (2017).
Yabuuchi, N., Yoshii, J., Myung, S.-T., Nakai, I. & Komaba, S. Detailed studies of a high-capacity electrode material for rechargeable batteries, Li2MnO3–LiCo1/3Ni1/3Mn1/3O2. J. Am. Chem. Soc. 133, 4404–4419 (2011).
Bettge, M. et al. Voltage fade of layered oxides: its measurement and impact on energy density. J. Electrochem. Soc. 160, A2046–A2055 (2013).
Chalmin, E., Farges, F. & Brown, G. E. Jr. A pre-edge analysis of Mn K-edge XANES spectra to help determine the speciation of manganese in minerals and glasses. Contrib. Mineral. Petrol. 157, 111–126 (2009).
Farges, F., Brown, G. E. Jr & Rehr, J. J. Coordination chemistry of Ti(iv) silicate glasses and melts: XAFS study of titanium coordination in oxide model compounds. Geochim. Cosmochim. Acta 60, 3023–3038 (1996).
Piilonen, P. C. et al. Structural environment of Nb5+ in dry and fluid-rich (H2O, F) silicate glasses: a combined XANES and EXAFS study. Can. Mineral. 44, 775–794 (2006).
Acknowledgements
Work by J.L., D.A.K., D.-H.K., Z.L., R.J.C. and G.C. was supported by Robert Bosch LLC, Umicore Specialty Oxides and Chemicals, and the Assistant Secretary for Energy Efficiency and Renewable Energy, Vehicle Technologies Office, of the U.S. Department of Energy under Contract No. DE-AC02-05CH11231, under the Advanced Battery Materials Research (BMR) Program. This research, in part, used resources of the Advanced Photon Source, a US Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under contract no. DE-AC02-06CH11357. Work at the Advanced Light Source is supported by DOE Office of Science User Facility under contract no. DE-AC02-05CH11231. Work at the Molecular Foundry was supported by the Office of Science, Office of Basic Energy Sciences, of the US DOE under contract no. DE-AC02-05CH11231. The computational work relied on resources provided by the Extreme Science and Engineering Discovery Environment (XSEDE), which is supported by National Science Foundation grant no. ACI-1548562. J.K.P. acknowledges NSF Graduate Research Fellowship (grant no. DGE-1106400). B.D.M. acknowledges support from the Assistant Secretary for Energy Efficiency and Renewable Energy, Vehicle Technologies Office, of the US DOE under contract no. DEAC02-05CH11231, under the Advanced Battery Materials Research (BMR) Program. The authors thank S.-H. Hsieh for assistance in the soft XAS experiments and the California NanoSystems Institute (CNSI) at the University of California Santa Barbara (UCSB) for experimental time on the 500 MHz NMR spectrometer. The NMR experimental work reported here made use of the shared facilities of the UCSB MRSEC (NSF DMR 1720256), a member of the Material Research Facilities Network.
Author information
Authors and Affiliations
Contributions
J.L. and G.C. planned the project. G.C. supervised all aspects of the research. J.L. designed, synthesized, characterized (XRD) and electrochemically tested the proposed compounds. D.A.K. performed density functional theory calculations and analysed the data with J.L. D.-H.K. acquired and analysed TEM data. C.-W.L. and M.B. acquired and analysed hard XAS data. J.K.P. acquired and analysed DEMS data with input from B.D.M. Y.-S.L. and J.G. performed soft XAS measurements and analysed the data with J.L. Z.L. performed supportive electrochemical measurements. R.J.C. acquired and analysed the NMR data. T.S. performed SEM. The manuscript was written by J.L. and G.C. and was revised by D.A.K. and R.J.C. with the help of the other authors. All authors contributed to discussions.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Solid-state NMR spectroscopy results.
a, b, 7Li spin echo NMR spectra acquired on as-synthesized Li2Mn2/3Nb1/3O2F (a) and Li2Mn1/2Ti1/2O2F (b) powders at 50 kHz MAS at a field B 0 = 11.7 T. The data have been fitted with a minimal number of Li sites: Li1, Li2 and Li3. Spinning sidebands of the three Li signals are indicated with asterisks. c, 19F spin echo sum spectra acquired on as-synthesized Li2Mn2/3Nb1/3O2F and Li2Mn1/2Ti1/2O2F powders at 50 kHz MAS at a field B 0 = 11.7 T. The spectra are compared to the spin echo spectrum collected on LiF under similar conditions. Spinning sidebands of the sharp LiF-like signals are indicated with asterisks. Detailed explanations of the results are given in Methods section ‘Supplementary Note 1’.
Extended Data Fig. 2 Structural characterization of Li2Mn2/3Nb1/3O2F.
a, TEM image of as-synthesized Li2Mn2/3Nb1/3O2F particles. Scale bar, 50 nm. b, A high-magnification TEM image of the area enclosed in a square in a. Scale bar, 10 nm. The yellow circle indicates the boundary of one of the many grains in the polycrystalline Li2Mn2/3Nb1/3O2F particle. c, An electron diffraction pattern of the Li2Mn2/3Nb1/3O2F particle. Scale bar, 5 nm−1. d, Fast Fourier-transformed (FFT) images of the dotted squared areas in b. e, The high magnification image across the squared areas 1, 2 and 3 in b. Scale bar, 5 nm. We can clearly observe lattice fringes and FFT peaks throughout the particle, indicating that our particles are made of small crystalline grains instead of amorphous phases.
Extended Data Fig. 3 Additional electrochemical data from Li2Mn2/3Nb1/3O2F.
a, b, Voltage profiles of the 60:30:10 electrode (that is, 60 wt% Li2Mn2/3Nb1/3O2F: 30 wt% carbon black: 10 wt% PTFE) when cycled between 2.0 V and 4.8 V (a), and 2.3 V and 4.6 V (b) at 20 mA g−1. c, d, Voltage profiles of the 70:20:10 (c) and the 80:15:5 (d) electrodes, when cycled between 1.5 V and 5.0 V at 20 mA g−1. e, Voltage profiles of the 80:15:5 electrode when cycled between 2.0 V and 4.8 V at 20 mA g−1. f, A comparison of the first discharge profiles of the 60:30:10, 70:20:10 and 80:15:5 Li2Mn2/3Nb1/3O2F electrodes (1.5–5.0 V, 20 mA g−1). The specific capacity was calculated on the amount of the Li2Mn2/3Nb1/3O2F powder in the cathode film.
Extended Data Fig. 4 Discharge capacity retention.
The 60:30:10 Li2Mn2/3Nb1/3O2F: carbon black:PTFE electrode was cycled between 1.5 V and 5.0 V at room temperature at 10, 20, 40, 100, 200, 400 and 1,000 mA g−1. A faster rate leads to less capacity fading during the initial 25 cycles. This is likely to be due to electrolyte decomposition per cycle occurring more (less) at a high voltage in a slower (faster) cycling test, which increases the impedance of a cell by creating a resistive surface layer and decreasing the ionic conductivity of the electrolyte.
Extended Data Fig. 5 Gas evolution measurements.
a, b, Initial voltage profiles (black solid line) of Li2Mn2/3Nb1/3O2F (a) and Li2Mn1/2Ti1/2O2F (b), when charged to 5.0 V at a rate of 20 mA g−1. DEMS results for O2 (red circles) and CO2 (blue triangles) evolution are also shown. c, Cumulative CO2 evolution from shaker-mixed Li2Mn1/2Ti1/2O2F and carbon black powder mixture, as a function of time during an acid titration test using 1 M H2SO4. Detailed explanations of the results are given in Methods section ‘Supplementary Note 2’. 1st c, first charge.
Extended Data Fig. 6 Evolution of the charge and discharge voltages.
Average charge voltage (triangles), discharge voltage (stars), and half of the charge–discharge voltage (circles) are shown when Li2Mn2/3Nb1/3O2F is cycled between 1.5 V and 4.6 V, 1.5 V and 4.8 V, and 1.5 V and 5.0 V, at 20 mA g−1. Detailed explanations of the results are given in Methods section ‘Supplementary Note 3’. c, charge; dc, discharge.
Extended Data Fig. 7 XANES of Li2Mn2/3Nb1/3O2F.
a, b, Manganese K-edge XANES spectra of Li2Mn2/3Nb1/3O2F: before cycle, after first charging to 135 mAh g−1, 270 mAh g−1 and 360 mAh g−1, and after first charging to 375 mAh g−1 then discharging to 320 mAh g−1. c–e, First derivatives of normalized absorbance at the pre-edge region of Mn K-edge spectra of Li2Mn2/3Nb1/3O2F: c, before cycle and after first charging to 375 mAh g−1 then discharging to 320 mAh g−1; d, after first charging to 135 mAh g−1; and e, to 270 mAh g−1 and 360 mAh g−1. Data from MnO, Mn2O3 and MnO2 are presented for comparison. Detailed explanations of the results are given in Methods section ‘Supplementary Note 4’.
Extended Data Fig. 8 Niobium K-edge XANES spectra of Li2Mn2/3Nb1/3O2F obtained by hard XAS.
Results are shown before cycle, after charging to 135 mAh g−1, 270 mAh g−1 and 360 mAh g−1, and after charging to 375 mAh g−1 then discharging to 320 mAh g−1. The Nb K-edge XANES spectra of the Li2Mn2/3Nb1/3O2F samples are similar to that of Nb2O5 (Nb5+ reference), indicating that Nb in the compound stays as Nb5+ during cycling. The observable small shape changes are likely to be related to changes in local disorder and distortion60.
Extended Data Fig. 9 Structural characterization of Li2Mn1/2Ti1/2O2F.
a, TEM image of as-synthesized Li2Mn1/2Ti1/2O2F particles. Scale bar, 50 nm. b, A high-magnification TEM image of the area enclosed in a square in a. Scale bar, 10 nm. The yellow circle indicates the boundary of one of the many grains in the polycrystalline Li2Mn1/2Ti1/2O2F particle. c, An electron diffraction pattern of the Li2Mn1/2Ti1/2O2F particle. Scale bar, 5 nm−1. d, FFT images of the dotted squared areas in b. e, The high magnification image across the squared areas 1, 2 and 3 in b. Scale bar, 5 nm. We can clearly observe lattice fringes and FFT peaks throughout the particle, indicating that our particles are made of small crystalline grains instead of amorphous phases.
Extended Data Fig. 10 Electrochemical properties of Li2Mn1/2Ti1/2O2F.
a–c, Voltage profiles and capacity retention of the 60:30:10 Li2Mn1/2Ti1/2O2F:carbon black:PTFE electrode when cycled at 20 mA g−1 at room temperature between 1.6 V and 5.0 V (a), 2.0 V and 4.8 V (b), and 2.3 V and 4.6 V (c). d, The initial charge–discharge profile of the 60:30:10 electrode when cycled between 1.6 V and 5.0 V at room temperature at 20, 40, 100, 200, 400 and 1,000 mA g−1. e,The discharge capacities during initial 25 cycles. f, Voltage profiles and capacity retention of the 80:15:5 electrode when cycled at 20 mA g−1 at room temperature between 2.0 V and 4.8 V. The specific capacity was calculated on the amount of the Li2Mn1/2Ti1/2O2F powder in the cathode film. Detailed explanations of the results are given in Methods section ‘Supplementary Note 5’.
Extended Data Fig. 11 XANES of Li2Mn1/2Ti1/2O2F.
a, b, Manganese K-edge XANES spectra of Li2Mn1/2Ti1/2O2F: before cycle (black), 120 mAh g−1 charged (navy), 240 mAh g−1 charged (wine), 400 mAh g−1 charged (grey), 330 mAh g−1 discharged after a 400 mAh g−1 charge (dark yellow). c–e, First derivatives of normalized absorbance at the pre-edge region of Mn K-edge spectra of Li2Mn1/2Ti1/2O2F: c, before cycle and after first charging to 400 mAh g−1 then discharging to 330 mAh g−1; d, after first charging to 120 mAh g−1; and e, to 240 mAh g−1 and 400 mAh g−1. f, Titanium K-edge XANES spectra of Li2Mn1/2Ti1/2O2F during the initial cycle. Data from MnO, Mn2O3, MnO2, Ti2O3 and TiO2 are presented for comparison. Detailed explanations of the results are given in Methods section ‘Supplementary Note 6’.
Rights and permissions
About this article
Cite this article
Lee, J., Kitchaev, D.A., Kwon, DH. et al. Reversible Mn2+/Mn4+ double redox in lithium-excess cathode materials. Nature 556, 185–190 (2018). https://doi.org/10.1038/s41586-018-0015-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-018-0015-4
This article is cited by
-
Efficient direct repairing of lithium- and manganese-rich cathodes by concentrated solar radiation
Nature Communications (2024)
-
Structural and electrochemical properties of LiFePO4 cathode synthesized by solid-state reaction using FeC2O4.2H2O precursor from local Indonesian ilmenite mineral
Emergent Materials (2024)
-
Realizing high initial Coulombic efficiency in manganese-based layered oxide cathodes for sodium-ion batteries via P2/O′3 biphasic structure optimization
Rare Metals (2024)
-
Interfacial cerium modification promotes the electrochemical properties of self-assembled Li1.2Mn0.54Ni0.13Co0.13O2 hollow microspheres
Ionics (2024)
-
Enhanced cyclic stability of partially disordered spinel cathodes through direct fluorination with gaseous fluorine
Rare Metals (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.