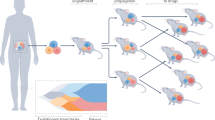
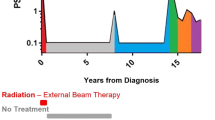
Abstract
Patient-derived xenografts (PDXs) are generated by engrafting human tumours into mice. Serially transplantable PDXs are used to study tumour biology and test therapeutics, linking the laboratory to the clinic. Although few prostate cancer PDXs are available in large repositories, over 330 prostate cancer PDXs have been established, spanning broad clinical stages, genotypes and phenotypes. Nevertheless, more PDXs are needed to reflect patient diversity, and to study new treatments and emerging mechanisms of resistance. We can maximize the use of PDXs by exchanging models and datasets, and by depositing PDXs into biorepositories, but we must address the impediments to accessing PDXs, such as institutional, ethical and legal agreements. Through collaboration, researchers will gain greater access to PDXs representing diverse features of prostate cancer.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Sung, H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249 (2021).
Sandhu, S. et al. Prostate cancer. Lancet 398, 1075–1090 (2021).
Centenera, M. M. et al. A patient‐derived explant (PDE) model of hormone‐dependent cancer. Mol. Oncol. 12, 1608–1622 (2018).
Arriaga, J. M. & Abate-Shen, C. Genetically engineered mouse models of prostate cancer in the postgenomic era. Cold Spring Harb. Perspect. Med. 9, a030528 (2019).
Drost, J. et al. Organoid culture systems for prostate epithelial and cancer tissue. Nat. Protoc. 11, 347–358 (2016).
Navone, N. M. et al. Movember GAP1 PDX project: an international collection of serially transplantable prostate cancer patient-derived xenograft (PDX) models. Prostate 78, 1262–1282 (2018).
Gleave, A. M., Ci, X., Lin, D. & Wang, Y. A synopsis of prostate organoid methodologies, applications, and limitations. Prostate 80, 518–526 (2020).
Risbridger, G. P., Toivanen, R. & Taylor, R. A. Preclinical models of prostate cancer: patient-derived xenografts, organoids, and other explant models. Cold Spring Harb. Perspect. Med. 8, a030536 (2018).
Davies, A. H., Wang, Y. & Zoubeidi, A. Patient-derived xenografts: a platform for accelerating translational research in prostate cancer. Mol. Cell. Endocrinol. 462, 17–24 (2018).
van de Merbel, A. F., van der Horst, G. & van der Pluijm, G. Patient-derived tumour models for personalized therapeutics in urological cancers. Nat. Rev. Urol. 18, 33–45 (2021).
Inoue, T., Terada, N., Kobayashi, T. & Ogawa, O. Patient-derived xenografts as in vivo models for research in urological malignancies. Nat. Rev. Urol. 14, 267–283 (2017).
Risbridger, G. P., Lawrence, M. G. & Taylor, R. A. PDX: moving beyond drug screening to versatile models for research discovery. J. Endocr. Soc. 4, bvaa132 (2020).
Toivanen, R. et al. A preclinical xenograft model identifies castration-tolerant cancer-repopulating cells in localized prostate tumors. Sci. Transl. Med. 5, 187ra71 (2013).
Priolo, C. et al. Establishment and genomic characterization of mouse xenografts of human primary prostate tumors. Am. J. Pathol. 176, 1901–1913 (2010).
Risbridger, G. P. et al. The MURAL collection of prostate cancer patient-derived xenografts enables discovery through preclinical models of uro-oncology. Nat. Commun. 12, 5049 (2021).
Wang, Y. et al. An orthotopic metastatic prostate cancer model in SCID mice via grafting of a transplantable human prostate tumor line. Lab. Invest. 85, 1392–1404 (2005).
Palanisamy, N. et al. The MD anderson prostate cancer patient-derived xenograft series (MDA PCa PDX) captures the molecular landscape of prostate cancer and facilitates marker-driven therapy development. Clin. Cancer Res. 26, 4933–4946 (2020).
Nguyen, H. M. et al. LuCaP prostate cancer patient‐derived xenografts reflect the molecular heterogeneity of advanced disease and serve as models for evaluating cancer therapeutics. Prostate 77, 654–671 (2017).
Woo, X. Y. et al. Conservation of copy number profiles during engraftment and passaging of patient-derived cancer xenografts. Nat. Genet. 53, 86–99 (2021).
Gao, H. et al. High-throughput screening using patient-derived tumor xenografts to predict clinical trial drug response. Nat. Med. 21, 1318–1325 (2015).
Conte, N. et al. PDX Finder: a portal for patient-derived tumor xenograft model discovery. Nucleic Acids Res. 47, D1073–D1079 (2019).
Krupke, D. M. et al. The mouse tumor biology database: a comprehensive resource for mouse models of human cancer. Cancer Res. 77, e67–e70 (2017).
Byrne, A. T. et al. Interrogating open issues in cancer precision medicine with patient-derived xenografts. Nat. Rev. Cancer 17, 254 (2017).
Lin, D. et al. High fidelity patient-derived xenografts for accelerating prostate cancer discovery and drug development. Cancer Res. 74, 1272–1283 (2014).
Marques, R. B. et al. The human PC346 xenograft and cell line panel: a model system for prostate cancer progression. Eur. Urol. 49, 245–257 (2006).
Brennen, W. N. et al. Resistance to androgen receptor signaling inhibition does not necessitate development of neuroendocrine prostate cancer. JCI Insight 6, e146827 (2021).
Stone, K. R., Mickey, D. D., Wunderli, H., Mickey, G. H. & Paulson, D. F. Isolation of a human prostate carcinoma cell line (DU145). Int. J. Cancer 21, 274–281 (1978).
Kaighn, M. E., Narayan, K. S., Ohnuki, Y., Lechner, J. F. & Jones, L. W. Establishment and characterization of a human prostatic carcinoma cell line (PC-3). Invest. Urol. 17, 16–23 (1979).
Horoszewicz, J. et al. The LNCaP cell line — a new model for studies on human prostatic carcinoma. Prog. Clin. Biol. Res. 37, 115–132 (1980).
Sobel, R. E. & Sadar, M. D. Cell lines used in prostate cancer research: a compendium of old and new lines — part 2. J. Urol. 173, 360–372 (2005).
Sobel, R. E. & Sadar, M. D. Cell lines used in prostate cancer research: a compendium of old and new lines — part 1. J. Urol. 173, 342–359 (2005).
Ghandi, M. et al. Next-generation characterization of the cancer cell line encyclopedia. Nature 569, 503–508 (2019).
Vargas, R. et al. Case study: patient-derived clear cell adenocarcinoma xenograft model longitudinally predicts treatment response. NPJ Precis. Oncol. 2, 14 (2018).
Wensink, G. E. et al. Patient-derived organoids as a predictive biomarker for treatment response in cancer patients. NPJ Precis. Oncol. 5, 30 (2021).
Gao, D. et al. Organoid cultures derived from patients with advanced prostate cancer. Cell 159, 176–187 (2014).
Puca, L. et al. Patient derived organoids to model rare prostate cancer phenotypes. Nat. Commun. 9, 2404 (2018).
Lawrence, M. G. et al. A preclinical xenograft model of prostate cancer using human tumors. Nat. Protoc. 8, 836–848 (2013).
Zhao, H., Nolley, R., Chen, Z. & Peehl, D. M. Tissue slice grafts: an in vivo model of human prostate androgen signaling. Am. J. Pathol. 177, 229–239 (2010).
Erickson, A. et al. Spatially resolved clonal copy number alterations in benign and malignant tissue. Nature 608, 360–367 (2022).
Porter, L. H. et al. Intraductal carcinoma of the prostate can evade androgen deprivation, with emergence of castrate‐tolerant cells. BJU Int. 121, 971–978 (2018).
Risbridger, G. P. et al. Patient-derived xenografts reveal that intraductal carcinoma of the prostate is a prominent pathology in BRCA2 mutation carriers with prostate cancer and correlates with poor prognosis. Eur. Urol. 67, 496–503 (2015).
Pauli, C. et al. Personalized in vitro and in vivo cancer models to guide precision medicine. Cancer Discov. 7, 462–477 (2017).
Servant, R. et al. Prostate cancer patient-derived organoids: detailed outcome from a prospective cohort of 81 clinical specimens. J. Pathol. 254, 543–555 (2021).
Welti, J. et al. Targeting bromodomain and extra-terminal (BET) family proteins in castration-resistant prostate cancer (CRPC). Clin. Cancer Res. 24, 3149–3162 (2018).
Nguyen, H. G. et al. Development of a stress response therapy targeting aggressive prostate cancer. Sci. Transl. Med. 10, eaar2036 (2018).
Mout, L. et al. Generating human prostate cancer organoids from leukapheresis enriched circulating tumour cells. Eur. J. Cancer 150, 179–189 (2021).
Cyrta, J. et al. Role of specialized composition of SWI/SNF complexes in prostate cancer lineage plasticity. Nat. Commun. 11, 5549 (2020).
Lee, S. et al. Establishment and analysis of three-dimensional (3D) organoids derived from patient prostate cancer bone metastasis specimens and their xenografts. J. Vis. Exp. 156, e60367 (2020).
Tang, F. et al. Chromatin profiles classify castration-resistant prostate cancers suggesting therapeutic targets. Science 376, eabe1505 (2022).
Faugeroux, V. et al. Genetic characterization of a unique neuroendocrine transdifferentiation prostate circulating tumor cell-derived explant model. Nat. Commun. 11, 1884 (2020).
Karkampouna, S. et al. Patient-derived xenografts and organoids model therapy response in prostate cancer. Nat. Commun. 12, 1117 (2021).
McCulloch, D. R., Opeskin, K., Thompson, E. W. & Williams, E. D. BM18: a novel androgen-dependent human prostate cancer xenograft model derived from a bone metastasis. Prostate 65, 35–43 (2005).
Russell, P. J. et al. Establishing prostate cancer patient derived xenografts: lessons learned from older studies. Prostate 75, 628–636 (2015).
Kohli, M. et al. Mutational landscapes of sequential prostate metastases and matched patient derived xenografts during enzalutamide therapy. PLoS ONE 10, e0145176 (2015).
Yoshikawa, T. et al. An original patient-derived xenograft of prostate cancer with cyst formation. Prostate 76, 994–1003 (2016).
Troyer, D. A. et al. Characterization of PacMetUT1, a recently isolated human prostate cancer cell line. Prostate 68, 883–892 (2008).
Rubin, M. A. et al. Rapid (“warm”) autopsy study for procurement of metastatic prostate cancer. Clin. Cancer Res. 6, 1038–1045 (2000).
Williams, E. S. et al. Generation of prostate cancer patient derived xenograft models from circulating tumor cells. J. Vis. Exp. 105, e53182 (2015).
Kimura, T. et al. A novel androgen-dependent prostate cancer xenograft model derived from skin metastasis of a Japanese patient. Prostate 69, 1660–1667 (2009).
Honda, M. et al. Differential expression of androgen receptor variants in hormone-sensitive prostate cancer xenografts, castration-resistant sublines, and patient specimens according to the treatment sequence. Prostate 79, 1043–1052 (2019).
Einstein, D. J. et al. Metastatic castration-resistant prostate cancer remains dependent on oncogenic drivers found in primary tumors. JCO Precis. Oncol. 5, 1514–1522 (2021).
Patierno, B. M. et al. Characterization of a castrate-resistant prostate cancer xenograft derived from a patient of West African ancestry. Prostate Cancer Prostatic Dis. 25, 513–523 (2021).
Gil, V. et al. HER3 is an actionable target in advanced prostate cancer. Cancer Res. 81, 6207–6218 (2021).
Agemy, L. et al. Irradiation enhances the metastatic potential of prostatic small cell carcinoma xenografts. Prostate 68, 530–539 (2008).
Pinthus, J. H. et al. WISH-PC2: a unique xenograft model of human prostatic small cell carcinoma. Cancer Res. 60, 6563–6567 (2000).
Okasho, K. et al. Establishment and characterization of a novel treatment-related neuroendocrine prostate cancer cell line KUCaP13. Cancer Sci. 112, 2781–2791 (2021).
Lange, T. et al. Development and characterization of a spontaneously metastatic patient-derived xenograft model of human prostate cancer. Sci. Rep. 8, 17535 (2018).
Wetterauer, C. et al. Early development of human lymphomas in a prostate cancer xenograft program using triple knock-out immunocompromised mice. Prostate 75, 585–592 (2015).
Taurozzi, A. J. et al. Spontaneous development of Epstein–Barr virus associated human lymphomas in a prostate cancer xenograft program. PLoS ONE 12, e0188228 (2017).
Alsop, K. et al. A community-based model of rapid autopsy in end-stage cancer patients. Nat. Biotechnol. 34, 1010–1014 (2016).
Ellis, W. J. et al. Characterization of a novel androgen-sensitive, prostate-specific antigen-producing prostatic carcinoma xenograft: LuCaP 23. Clin. Cancer Res. 2, 1039–1048 (1996).
Nguyen, H. M. et al. LuCaP prostate cancer patient‐derived xenografts reflect the molecular heterogeneity of advanced disease an–d serve as models for evaluating cancer therapeutics. Prostate 77, 654–671 (2017).
Lawrence, M. G. et al. Knowing what’s growing: why ductal and intraductal prostate cancer matter. Sci. Transl. Med. 12, eaaz0152 (2020).
Ranasinghe, W. et al. Ductal prostate cancers demonstrate poor outcomes with conventional therapies. Eur. Urol. 79, 298–306 (2021).
Lawrence, M. G. et al. Patient-derived models of abiraterone- and enzalutamide-resistant prostate cancer reveal sensitivity to ribosome-directed therapy. Eur. Urol. 74, 562–572 (2018).
Porter, L. H. et al. Androgen receptor enhancer amplification in matched patient-derived xenografts of primary and castrate-resistant prostate cancer. J. Pathol. 254, 121–134 (2021).
Henzler, C. et al. Truncation and constitutive activation of the androgen receptor by diverse genomic rearrangements in prostate cancer. Nat. Commun. 7, 13668 (2016).
Zhu, Y. et al. Role of androgen receptor splice variant-7 (AR-V7) in prostate cancer resistance to 2nd-generation androgen receptor signaling inhibitors. Oncogene 39, 6935–6949 (2020).
Labrecque, M. P. et al. Molecular profiling stratifies diverse phenotypes of treatment-refractory metastatic castration-resistant prostate cancer. J. Clin. Invest. 129, 4492–4505 (2019).
Li, Z. G. et al. Androgen receptor-negative human prostate cancer cells induce osteogenesis in mice through FGF9-mediated mechanisms. J. Clin. Invest. 118, 2697–2710 (2008).
Tzelepi, V. et al. Modeling a lethal prostate cancer variant with small cell carcinoma features. Clin. Cancer Res. 18, 666–677 (2012).
Shi, M., Wang, Y. & Lin, D. Patient-derived xenograft models of neuroendocrine prostate cancer. Cancer Lett. 525, 160–169 (2022).
Bluemn, E. G. et al. Androgen receptor pathway-independent prostate cancer is sustained through FGF signaling. Cancer Cell 32, 474–489.e476 (2017).
Beltran, H. et al. Whole-exome sequencing of metastatic cancer and biomarkers of treatment response. JAMA Oncol. 1, 466–474 (2015).
Ben-David, U. et al. Patient-derived xenografts undergo mouse-specific tumor evolution. Nat. Genet. 49, 1567–1575 (2017).
Sun, H. et al. Comprehensive characterization of 536 patient-derived xenograft models prioritizes candidatesfor targeted treatment. Nat. Commun. 12, 5086 (2021).
Taylor, B. S. et al. Integrative genomic profiling of human prostate cancer. Cancer Cell 18, 11–22 (2010).
Robinson, D. et al. Integrative clinical genomics of advanced prostate cancer. Cell 161, 1215–1228 (2015).
Armenia, J. et al. The long tail of oncogenic drivers in prostate cancer. Nat. Genet. 50, 645–651 (2018).
The Cancer Genome Atlas Research Network. The molecular taxonomy of primary prostate cancer. Cell 163, 1011–1025 (2015).
Abida, W. et al. Genomic correlates of clinical outcome in advanced prostate cancer. Proc. Natl Acad. Sci. USA 116, 11428–11436 (2019).
Baker, S. C. et al. The external RNA controls consortium: a progress report. Nat. Methods 2, 731–734 (2005).
Yan, Y. et al. The novel BET-CBP/p300 dual inhibitor NEO2734 is active in SPOP mutant and wild-type prostate cancer. EMBO Mol. Med. 11, e10659 (2019).
Brennen, W. N. & Isaacs, J. T. The what, when, and why of human prostate cancer xenografts. Prostate 78, 646–654 (2018).
Wang, Y. et al. Development and characterization of efficient xenograft models for benign and malignant human prostate tissue. Prostate 64, 149–159 (2005).
Michiel Sedelaar, J. P., Dalrymple, S. S. & Isaacs, J. T. Of mice and men — warning: intact versus castrated adult male mice as xenograft hosts are equivalent to hypogonadal versus abiraterone treated aging human males, respectively. Prostate 73, 1316–1325 (2013).
Hassan, S., Blick, T., Wood, J., Thompson, E. W. & Williams, E. D. Circulating tumour cells indicate the presence of residual disease post-castration in prostate cancer patient-derived xenograft models. Front. Cell Dev. Biol. 10, 858013 (2022).
Hassan, S., Blick, T., Thompson, E. W. & Williams, E. D. Diversity of epithelial-mesenchymal phenotypes in circulating tumour cells from prostate cancer patient-derived xenograft models. Cancers 13, 2750 (2021).
De Sarkar, N. et al. Nucleosome patterns in circulating tumor DNA reveal transcriptional regulation of advanced prostate cancer phenotypes. Cancer Discov. https://doi.org/10.1158/2159-8290.CD-22-0692 (2022).
Chatalic, K. L. et al. A novel ¹¹¹In-labeled anti-prostate-specific membrane antigen nanobody for targeted SPECT/CT imaging of prostate cancer. J. Nucl. Med. 56, 1094–1099 (2015).
Asrani, K. et al. Reciprocal YAP1 loss and INSM1 expression in neuroendocrine prostate cancer. J. Pathol. 255, 425–437 (2021).
Baca, S. C. et al. Reprogramming of the FOXA1 cistrome in treatment-emergent neuroendocrine prostate cancer. Nat. Commun. 12, 1979 (2021).
Moll, J. M. et al. Abiraterone switches castration-resistant prostate cancer dependency from adrenal androgens towards androgen receptor variants and glucocorticoid receptor signalling. Prostate 82, 505–516 (2022).
Pomerantz, M. M. et al. Prostate cancer reactivates developmental epigenomic programs during metastatic progression. Nat. Genet. 52, 790–799 (2020).
Xiao, L. et al. Targeting SWI/SNF ATPases in enhancer-addicted prostate cancer. Nature 601, 434–439 (2022).
Lam, H. M. et al. Durable response of enzalutamide-resistant prostate cancer to supraphysiological testosterone is associated with a multifaceted growth suppression and impaired DNA damage response transcriptomic program in patient-derived xenografts. Eur. Urol. 77, 144–155 (2020).
Isaacs, J. T., Brennen, W. N. & Denmeade, S. R. Serial bipolar androgen therapy (sBAT) using cyclic supraphysiologic testosterone (STP) to treat metastatic castration-resistant prostate cancer (mCRPC). Ann. Transl. Med. 7, S311 (2019).
Qiu, X. et al. Response to supraphysiological testosterone is predicted by a distinct androgen receptor cistrome. JCI Insight 7, e157164 (2022).
Linxweiler, J. et al. A novel mouse model of human prostate cancer to study intraprostatic tumor growth and the development of lymph node metastases. Prostate 78, 664–675 (2018).
Migliardi, G. et al. Inhibition of MEK and PI3K/mTOR suppresses tumor growth but does not cause tumor regression in patient-derived xenografts of RAS-mutant colorectal carcinomas. Clin. Cancer Res. 18, 2515–2525 (2012).
Townsend, E. C. et al. The public repository of xenografts (ProXe) enables discovery and randomized phase II-like trials in mice. Cancer Cell 29, 574–586 (2016).
Mer, A. S. et al. Integrative pharmacogenomics analysis of patient-derived xenografts. Cancer Res. 79, 4539–4550 (2019).
Ortmann, J. et al. Assessing therapy response in patient-derived xenografts. Sci. Transl. Med. 13, eabf4969 (2021).
Ci, X. et al. Conditionally reprogrammed cells from patient-derived xenograft to model neuroendocrine prostate cancer development. Cells 9, 1398 (2020).
Beshiri, M. L. et al. A PDX/organoid biobank of advanced prostate cancers captures genomic and phenotypic heterogeneity for disease modeling and therapeutic screening. Clin. Cancer Res. 24, 4332–4345 (2018).
Zhang, W. et al. Ex vivo treatment of prostate tumor tissue recapitulates in vivo therapy response. Prostate 79, 390–402 (2019).
van de Merbel, A. F. et al. An ex vivo tissue culture model for the assessment of individualized drug responses in prostate and bladder cancer. Front. Oncol. 8, 400 (2018).
van de Merbel, A. F. et al. Reovirus mutant jin-3 exhibits lytic and immune-stimulatory effects in preclinical human prostate cancer models. Cancer Gene Ther. 29, 793–802 (2022).
Shokoohmand, A. et al. Microenvironment engineering of osteoblastic bone metastases reveals osteomimicry of patient-derived prostate cancer xenografts. Biomaterials 220, 119402 (2019).
Monterosso, M. E. et al. Using the Microwell-mesh to culture microtissues in vitro and as a carrier to implant microtissues in vivo into mice. Sci. Rep. 11, 5118 (2021).
Choo, N. et al. High-throughput imaging assay for drug screening of 3D prostate cancer organoids. SLAS Discov. 26, 1107–1124 (2021).
Fong, E. L. et al. Hydrogel-based 3D model of patient-derived prostate xenograft tumors suitable for drug screening. Mol. Pharm. 11, 2040–2050 (2014).
Sablatura, L. K. et al. Enhanced viability for ex vivo 3D hydrogel cultures of patient-derived xenografts in a perfused microfluidic platform. J. Vis. Exp. https://doi.org/10.3791/60872 (2020).
Fong, E. L. et al. A 3D in vitro model of patient-derived prostate cancer xenograft for controlled interrogation of in vivo tumor-stromal interactions. Biomaterials 77, 164–172 (2016).
Jansson, K. H. et al. High-throughput screens identify HSP90 inhibitors as potent therapeutics that target inter-related growth and survival pathways in advanced prostate cancer. Sci. Rep. 8, 17239 (2018).
Russell, W. M. S. & Burch, R. L. The Principles Of Humane Experimental Technique (Methuen, 1959).
Van Hemelryk, A. et al. Patient-derived xenografts and organoids recapitulate castration-resistant prostate cancer with sustained androgen receptor signaling. Cells 11, 3632 (2022).
de Morrée, E. S. et al. Loss of SLCO1B3 drives taxane resistance in prostate cancer. Br. J. Cancer 115, 674–681 (2016).
Ewing, C. M. et al. Germline mutations in HOXB13 and prostate-cancer risk. N. Engl. J. Med. 366, 141–149 (2012).
Pritchard, C. C. et al. Inherited DNA-repair gene mutations in men with metastatic prostate cancer. N. Engl. J. Med. 375, 443–453 (2016).
Mo, F. et al. Stromal gene expression is predictive for metastatic primary prostate cancer. Eur. Urol. 73, 524–532 (2018).
Mahal, B. A. et al. Racial differences in genomic profiling of prostate cancer. N. Engl. J. Med. 383, 1083–1085 (2020).
Jaratlerdsiri, W. et al. Whole-genome sequencing reveals elevated tumor mutational burden and initiating driver mutations in African men with treatment-naïve, high-risk prostate cancer. Cancer Res. 78, 6736–6746 (2018).
Rebello, R. J. et al. The dual inhibition of RNA Pol I transcription and PIM Kinase as a new therapeutic approach to treat advanced prostate cancer. Clin. Cancer Res. 22, 5539–5552 (2016).
Lawrence, M. G. et al. CX-5461 sensitizes DNA damage repair-proficient castrate-resistant prostate cancer to PARP inhibition. Mol. Cancer Ther. 20, 2140–2150 (2021).
de Bono, J. et al. Olaparib for metastatic castration-resistant prostate cancer. N. Engl. J. Med. 382, 2091–2102 (2020).
Abida, W. et al. Rucaparib in men with metastatic castration-resistant prostate cancer harboring a BRCA1 or BRCA1 alteration. J. Clin. Oncol. 38, 3763–3772 (2020).
Nombela, P. et al. BRCA2 and other DDR genes in prostate cancer. Cancers 11, 352 (2019).
Evrard, Y. A. et al. Systematic establishment of robustness and standards in patient-derived xenograft experiments and analysis. Cancer Res. 80, 2286–2297 (2020).
Kluin, R. J. C. et al. XenofilteR: computational deconvolution of mouse and human reads in tumor xenograft sequence data. BMC Bioinformatics 19, 366 (2018).
Wakefield, L. M. Xenomapper: mapping reads in a mixed species context. J. Open Source Softw. 1, 18 (2016).
Cerami, E. et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2, 401–404 (2012).
Porter, L. H. et al. Establishing a cryopreservation protocol for patient-derived xenografts of prostate cancer. Prostate 79, 1326–1337 (2019).
Wang, Y. et al. Subrenal capsule grafting technology in human cancer modeling and translational cancer research. Differentiation 91, 15–19 (2016).
El-Hoss, J. et al. A single nucleotide polymorphism genotyping platform for the authentication of patient derived xenografts. Oncotarget 7, 60475–60490 (2016).
Tutty, E., Horsley, P., Forbes Shepherd, R. & Forrest, L. E. The art and science of recruitment to a cancer rapid autopsy programme: a qualitative study exploring patient and clinician experiences. Palliat. Med. 35, 437–446 (2021).
Thompson-Iritani, S. & Schmechel, S. C. in Patient Derived Tumor Xenograft Models (eds Uthamanthil, R. & Tinkey, P.) 93–108 (Academic Press, 2017).
Cassidy, J. W., Caldas, C. & Bruna, A. Maintaining tumor heterogeneity in patient-derived tumor xenografts. Cancer Res. 75, 2963–2968 (2015).
Meraz, I. M. et al. An improved patient-derived xenograft humanized mouse model for evaluation of lung cancer immune responses. Cancer Immunol. Res. 7, 1267–1279 (2019).
Choi, Y. et al. Studying cancer immunotherapy using patient-derived xenografts (PDXs) in humanized mice. Exp. Mol. Med. 50, 1–9 (2018).
Bray, L. J., Hutmacher, D. W. & Bock, N. Addressing patient specificity in the engineering of tumor models. Front. Bioeng. Biotechnol. 7, 217 (2019).
Levesque, C. & Nelson, P. S. Cellular constituents of the prostate stroma: key contributors to prostate cancer progression and therapy resistance. Cold Spring Harb. Perspect. Med. 8, a030510 (2018).
Karkampouna, S. et al. Stroma transcriptomic and proteomic profile of prostate cancer metastasis xenograft models reveals prognostic value of stroma signatures. Cancers 12, 3786 (2020).
Pienta, K. J. et al. The current state of preclinical prostate cancer animal models. Prostate 68, 629–639 (2008).
van Weerden, W. M. et al. Development of seven new human prostate tumor xenograft models and their histopathological characterization. Am. J. Pathol. 149, 1055–1062 (1996).
Hao, J. et al. Patient-derived hormone-naive prostate cancer xenograft models reveal growth factor receptor bound protein 10 as an androgen receptor-repressed gene driving the development of castration-resistant prostate cancer. Eur. Urol. 73, 949–960 (2018).
Acknowledgements
The authors thank the participants of the Virtual Prostate Cancer PDX Symposium series for sharing their views. They acknowledge the patients, clinical co-ordinators, clinicians and scientists who contribute to the establishment and maintenance of each of the collections of prostate cancer PDXs discussed in this Perspective. Authors from Monash University and the Peter MacCallum Cancer Centre thank members of the Melbourne Urological Research Alliance, kConFab, and CASCADE. Authors from the University of Washington/Fred Hutchinson Cancer Research Center would like to thank the patients who generously donated the tissue that made this research possible. The authors also thank J. Conner, M. Dalos, D. Sondheim and the Comparative Medicine Animal Caregivers for assistance with the LuCaP xenograft work. Additionally, they thank P. Lange, R. Vessella, F. Vakar-Lopez, M. Roudier, X. Zhang, B. Nghiem and the rapid autopsy teams and physicians in the Urology and Medical Oncology Departments at the University of Washington. The authors receive funding from the Movember Foundation Global Action Plan 1 PDX Project; US Department of Defense through the Prostate Cancer Research Program (W81XWH1810347, W81XWH1810348 and W81XWH1810349; opinions, interpretations, conclusions, and recommendations are those of the authors and are not necessarily endorsed by the Department of Defense); the Movember Foundation (Global Action Plan 1); the Victorian Cancer Agency (MCRF15023, MCRF18017 and MCRF17005; CAPTIV Program); the National Health and Medical Research Council, Australia (1102752, 1138242, 1140222, 1156570, 1185616, 2011033 and 2011391); the Rotary Club of Manningham; RULE Prostate Cancer and the EJ Whitten Foundation; the Peter and Lyndy White Foundation; TissuPath Pathology; the Peter MacCallum Cancer Foundation; the Pacific Northwest Prostate Cancer SPORE (P50CA97186); the Department of Defense Prostate Cancer Biorepository Network (W81XWH-14-2-0183); CDMRP award W81XWH-21-1-0264; National Institute of Health/National Cancer Institute (P01 CA163227, R01CA234715, U01 CA224044-03, U54 CA233223, SBIR Phase I HHSN26120700015C); the Baylor College of Medicine, Minority PDX Development and Trial Center: Baylor College of Medicine and MD Anderson Cancer Center Collaboration on Mechanistic Studies to Dissect and Combat Health Disparities in Cancer, SBIR Phase II/Mimetas US, Inc); the Prostate Cancer Foundation; the Institute for Prostate Cancer Research; the Richard M. Lucas Foundation; the Craig Watjen Memorial Fund; Fond’Action contre le cancer (Young Investigator Award 2020); the Department of Surgery of the University Hospital Basel; the David H. Koch Center for Applied Research in Genitourinary Cancers at MD Anderson; the Canadian Institutes of Health Research (141635, 144159, 153081 and 173338); the Terry Fox Research Institute (1062); the Mitacs Accelerate Program (IT10125, IT06414, IT12387 and IT14958); and the EU Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant (721746; ‘TRANSPOT’).
Author information
Authors and Affiliations
Contributions
G.P.R., R.A.T. and M.G.L. researched data for the article, made a substantial contribution to discussion of content and wrote the article. G.B.C. wrote the article and made a substantial contribution to discussion of content. E.C., J.T.I. and P.S.N. made a substantial contribution to discussion of content. All authors reviewed and/or edited the manuscript before submission.
Corresponding authors
Ethics declarations
Competing interests
G.P.R., R.A.T. and M.G.L. have been involved in research collaborations with AstraZeneca and Pfizer. W.M.v.W. has been involved in research collaborations with Bayer. E.C. has received research funding under institutional sponsored research agreements from AbbVie, Bayer Pharmaceuticals, Forma Pharmaceutics Foghorn, Gilead, GSK, Janssen Research and Development, KronosBio, MacroGenics and Sanofi. P.S.N. has been a paid consultant to Astellas, Bristol Myers Squibb, Janssen and Pfizer for work unrelated to the present study. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Urology thanks Gabri van der Pluijm, Alastair Lamb and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Jackson Laboratory: https://www.jax.org
Living Tumour Laboratory: http://www.livingtumorlab.com
Patient-Derived Models Repository: https://pdmr.cancer.gov
PDXFinder: https://www.pdxfinder.org
PDXNet: https://www.pdxnetwork.org
Supplementary information
Glossary
- 3D scaffolds
-
Support structures made from natural or synthetic materials used for in vitro cell culture, aiming to mimic the architecture and/or biomechanical features of tissue.
- Conditionally reprogrammed cells
-
In vitro cultures of normal or tumour epithelial cells grown on a feeder layer of irradiated feeder cells (typically mouse 3T3 cells).
- Ex vivo slice cultures
-
Chopped, minced or sliced pieces of tissue submerged in culture media or grown on top of sponges or mesh inserts. Also referred to as explants.
- Hydrogels
-
Synthetic or naturally occurring materials that form 3D matrices to support the growth of cells, including organoids, typically having a more defined composition than Matrigel.
- Matrigel
-
A solution of extracellular matrix proteins secreted by mouse Engelbreth–Holm–Swarm sarcoma cells often used to grow organoid cultures.
- Microwells
-
Arrays of small wells (approximately 150 per cm2) fabricated from silicon or other materials, used to grow separate clusters of cells within a larger tissue culture plate.
- Organoids
-
Clusters of cells grown in suspension or embedded in a matrix, rather than cultured in two dimensions (typically monocultures of tumour cells for prostate cancer).
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lawrence, M.G., Taylor, R.A., Cuffe, G.B. et al. The future of patient-derived xenografts in prostate cancer research. Nat Rev Urol 20, 371–384 (2023). https://doi.org/10.1038/s41585-022-00706-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41585-022-00706-x