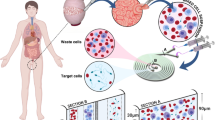
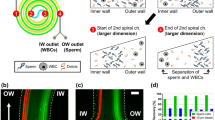
Abstract
Sperm are transcriptionally and translationally quiescent and, therefore, rely on the seminal plasma microenvironment for function, survival and fertilization of the oocyte in the oviduct. The male reproductive system influences sperm function via the binding and fusion of secreted epididymal (epididymosomes) and prostatic (prostasomes) small extracellular vesicles (S-EVs) that facilitate the transfer of proteins, lipids and nucleic acids to sperm. Seminal plasma S-EVs have important roles in sperm maturation, immune and oxidative stress protection, capacitation, fertilization and endometrial implantation and receptivity. Supplementing asthenozoospermic samples with normospermic-derived S-EVs can improve sperm motility and S-EV microRNAs can be used to predict non-obstructive azoospermia. Thus, S-EV influence on sperm physiology might have both therapeutic and diagnostic potential; however, the isolation of pure populations of S-EVs from bodily fluids with current conventional methods presents a substantial hurdle. Many conventional techniques lack accuracy, effectiveness, and practicality; yet microfluidic technology has the potential to simplify and improve S-EV isolation and detection.
Key points
-
Epididymosomes and prostasomes, the two distinct populations of seminal plasma small extracellular vesicles (S-EVs), have substantial roles in sperm function, survival and fertilization of the oocyte.
-
Male reproductive S-EV protein and microRNA cargo might serve as biomarkers for infertility and reproductive dysfunction.
-
Conventional methods of S-EV isolation require often laborious or costly workflows with suboptimal results but have received substantial interest in cancer therapeutics and diagnostics.
-
Microfluidic technology has the potential for miniaturization and simplification of S-EV isolation and analysis for use in point-of-need diagnostics in male infertility.
-
Infertility treatment can use technology developed for cancer diagnostics and therapeutics to approach idiopathic infertility.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Arraud, N. et al. Extracellular vesicles from blood plasma: determination of their morphology, size, phenotype and concentration. J. Thromb. Haemost. 12, 614–627 (2014).
Pisitkun, T., Shen, R.-F. & Knepper, M. A. Identification and proteomic profiling of exosomes in human urine. Proc. Natl Acad. Sci. USA 101, 13368–13373 (2004).
Lässer, C. et al. Human saliva, plasma and breast milk exosomes contain RNA: uptake by macrophages. J. Transl. Med. 9, 1–8 (2011).
Emelyanov, A. et al. Cryo-electron microscopy of extracellular vesicles from cerebrospinal fluid. PLoS ONE 15, e0227949 (2020).
Vallabhaneni, K. C. et al. Extracellular vesicles from bone marrow mesenchymal stem/stromal cells transport tumor regulatory microRNA, proteins, and metabolites. Oncotarget 6, 4953 (2015).
Yáñez-Mó, M. et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 4, 27066 (2015).
Zaborowski, M. P., Balaj, L., Breakefield, X. O. & Lai, C. P. Extracellular vesicles: composition, biological relevance, and methods of study. Bioscience 65, 783–797 (2015).
Deatherage, B. L. & Cookson, B. T. Membrane vesicle release in bacteria, eukaryotes, and archaea: a conserved yet underappreciated aspect of microbial life. Infect. Immun. 80, 1948–1957 (2012).
Doyle, L. M. & Wang, M. Z. Overview of extracellular vesicles, their origin, composition, purpose, and methods for exosome isolation and analysis. Cells 8, 727 (2019).
Colombo, M., Raposo, G. & Théry, C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell Dev. Biol. 30, 255–289 (2014).
Chargaff, E. & West, R. The biological significance of the thromboplastic protein of blood. J. Biol. Chem. 166, 189–197 (1946).
Bonucci, E. Fine structure and histochemistry of “calcifying globules” in epiphyseal cartilage. Z. Zellforsch. Mikroskop. Anat. 103, 192–217 (1970).
Pegtel, D. M. & Gould, S. J. Exosomes. Annu. Rev. Biochem. 88, 487–514 (2019).
Valadi, H. et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 9, 654–659 (2007).
Men, Y. et al. Exosome reporter mice reveal the involvement of exosomes in mediating neuron to astroglia communication in the CNS. Nat. Commun. 10, 4136 (2019).
Raposo, G. & Stoorvogel, W. Extracellular vesicles: exosomes, microvesicles, and friends. J. Cell Biol. 200, 373–383 (2013).
Gurung, S., Perocheau, D., Touramanidou, L. & Baruteau, J. The exosome journey: from biogenesis to uptake and intracellular signalling. Cell Commun. Signal. 19, 47 (2021).
Andaloussi, S. E., Mäger, I., Breakefield, X. O. & Wood, M. J. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat. Rev. Drug Discov. 12, 347–357 (2013).
Ettelaie, C., Collier, M. E., Maraveyas, A. & Ettelaie, R. Characterization of physical properties of tissue factor-containing microvesicles and a comparison of ultracentrifuge-based recovery procedures. J. Extracell. Vesicles 3, 23592 (2014).
Battistelli, M. & Falcieri, E. Apoptotic bodies: particular extracellular vesicles involved in intercellular communication. Biology 9, 21 (2020).
Ihara, T., Yamamoto, T., Sugamata, M., Okumura, H. & Ueno, Y. The process of ultrastructural changes from nuclei to apoptotic body. Virchows Arch. 433, 443–447 (1998).
Hristov, M., Erl, W., Linder, S. & Weber, P. C. Apoptotic bodies from endothelial cells enhance the number and initiate the differentiation of human endothelial progenitor cells in vitro. Blood 104, 2761–2766 (2004).
Elmore, S. Apoptosis: a review of programmed cell death. Toxicol. Pathol. 35, 495–516 (2007).
Ronquist, G., Brody, I., Gottfries, A. & Stegmayr, B. An Mg2+ and Ca2+‐stimulated adenosine triphosphatase in human prostatic fluid — part II. Andrologia 10, 427–433 (1978).
Murdica, V. et al. Seminal plasma of men with severe asthenozoospermia contain exosomes that affect spermatozoa motility and capacitation. Fertil. Steril. 111, 897–908. e892 (2019).
Lin, Y. et al. Proteomic analysis of seminal extracellular vesicle proteins involved in asthenozoospermia by iTRAQ. Mol. Reprod. Dev. 86, 1094–1105 (2019).
Park, K.-H. et al. Ca2+ signaling tools acquired from prostasomes are required for progesterone-induced sperm motility. Sci. Signal. 4, ra31 (2011).
Aalberts, M. et al. Spermatozoa recruit prostasomes in response to capacitation induction. Biochim. Biophys. Acta 1834, 2326–2335 (2013).
Whitesides, G. M. The origins and the future of microfluidics. Nature 442, 368–373 (2006).
Yasui, T. et al. Unveiling massive numbers of cancer-related urinary-microRNA candidates via nanowires. Sci. Adv. 3, e1701133 (2017).
Suwatthanarak, T. et al. Microfluidic-based capture and release of cancer-derived exosomes via peptide–nanowire hybrid interface. Lab Chip 21, 597–607 (2021).
Xu, H., Liao, C., Zuo, P., Liu, Z. & Ye, B. C. Magnetic-based microfluidic device for on-chip isolation and detection of tumor-derived exosomes. Anal. Chem. 90, 13451–13458 (2018).
Zhang, P. et al. Ultrasensitive detection of circulating exosomes with a 3D-nanopatterned microfluidic chip. Nat. Biomed. Eng. 3, 438–451 (2019).
Kanwar, S. S., Dunlay, C. J., Simeone, D. M. & Nagrath, S. Microfluidic device (ExoChip) for on-chip isolation, quantification and characterization of circulating exosomes. Lab Chip 14, 1891–1900 (2014).
Smith, J. T. et al. Integrated nanoscale deterministic lateral displacement arrays for separation of extracellular vesicles from clinically-relevant volumes of biological samples. Lab Chip 18, 3913–3925 (2018).
Wang, Y. et al. Microfluidic Raman biochip detection of exosomes: a promising tool for prostate cancer diagnosis. Lab Chip 20, 4632–4637 (2020).
Machtinger, R., Laurent, L. C. & Baccarelli, A. A. Extracellular vesicles: roles in gamete maturation, fertilization and embryo implantation. Hum. Reprod. Update 22, 182–193 (2016).
Harding, C. V., Heuser, J. E. & Stahl, P. D. Exosomes: looking back three decades and into the future. J. Cell Biol. 200, 367–371 (2013).
Zheng, R. et al. Exosome-transmitted long non-coding RNA PTENP1 suppresses bladder cancer progression. Mol. Cancer 17, 1–13 (2018).
Zamani, P., Fereydouni, N., Butler, A. E., Navashenaq, J. G. & Sahebkar, A. The therapeutic and diagnostic role of exosomes in cardiovascular diseases. Trends Cardiovasc. Med. 29, 313–323 (2019).
Pan, B.-T. & Johnstone, R. M. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell 33, 967–978 (1983).
Cocucci, E., Racchetti, G. & Meldolesi, J. Shedding microvesicles: artefacts no more. Trends Cell Biol. 19, 43–51 (2009).
Denzer, K., Kleijmeer, M. J., Heijnen, H. F. G., Stoorvogel, W. & Geuze, H. J. Exosome: from internal vesicle of the multivesicular body to intercellular signaling device. J. Cell Sci. 113, 3365–3374 (2000).
Zhang, M., Ouyang, H. & Xia, G. The signal pathway of gonadotrophins-induced mammalian oocyte meiotic resumption. Mol. Hum. Reprod. 15, 399–409 (2009).
Momen-Heravi, F. et al. Impact of biofluid viscosity on size and sedimentation efficiency of the isolated microvesicles. Front. Physiol. 3, 162 (2012).
Marleau, A. M., Chen, C.-S., Joyce, J. A. & Tullis, R. H. Exosome removal as a therapeutic adjuvant in cancer. J. Transl. Med. 10, 1–12 (2012).
Sandfeld-Paulsen, B. et al. Exosomal proteins as diagnostic biomarkers in lung cancer. J. Thorac. Oncol. 11, 1701–1710 (2016).
Yuyama, K., Sun, H., Mitsutake, S. & Igarashi, Y. Sphingolipid-modulated exosome secretion promotes clearance of amyloid-β by microglia. J. Biol. Chem. 287, 10977–10989 (2012).
Tomlinson, P. R. et al. Identification of distinct circulating exosomes in Parkinson’s disease. Ann. Clin. Transl. Neurol. 2, 353–361 (2015).
Feng, J., Waqas, A., Zhu, Z. & Chen, L. Exosomes: applications in respiratory infectious diseases and prospects for coronavirus disease 2019 (COVID-19). J. Biomed. Nanotechnol. 16, 399–418 (2020).
Nagashima, S. et al. Hepatitis E virus egress depends on the exosomal pathway, with secretory exosomes derived from multivesicular bodies. J. Gen. Virol. 95, 2166–2175 (2014).
Martin-DeLeon, P. A. Uterosomes: exosomal cargo during the estrus cycle and interaction with sperm. Front. Biosci. 8, 115–122 (2016).
Panner Selvam, M. K., Agarwal, A., Pushparaj, P. N., Baskaran, S. & Bendou, H. Sperm proteome analysis and identification of fertility-associated biomarkers in unexplained male infertility. Genes 10, 522 (2019).
Rao, M. et al. Humanin levels in human seminal plasma and spermatozoa are related to sperm quality. Andrology 7, 859–866 (2019).
Poliakov, A., Spilman, M., Dokland, T., Amling, C. L. & Mobley, J. A. Structural heterogeneity and protein composition of exosome‐like vesicles (prostasomes) in human semen. Prostate 69, 159–167 (2009).
Del Giudice, P. T. et al. Determination of testicular function in adolescents with varicocoele — a proteomics approach. Andrology 4, 447–455 (2016).
Ronquist, G. & Hedström, M. Restoration of detergent-inactivated adenosine triphosphatase activity of human prostatic fluid with concanavalin A. Biochim. Biophys. Acta 483, 483–486 (1977).
Brody, I., Ronquist, G. & Gottfries, A. Ultrastructural localization of the prostasome-an organelle in human seminal plasma. Ups. J. Med. Sci. 88, 63–80 (1983).
Ronquist, G. & Brody, I. The prostasome: its secretion and function in man. Biochim. Biophys. Acta 822, 203–218 (1985).
Ronquist, G. & Nilsson, B. O. The Janus-faced nature of prostasomes: their pluripotency favours the normal reproductive process and malignant prostate growth. Prostate Cancer Prostatic Dis. 7, 21–31 (2004).
Utleg, A. G. et al. Proteomic analysis of human prostasomes. Prostate 56, 150–161 (2003).
Stegmayr, B. & Ronquist, G. Promotive effect on human sperm progressive motility by prostasomes. Urol. Res. 10, 253–257 (1982).
Thimon, V., Frenette, G., Saez, F., Thabet, M. & Sullivan, R. Protein composition of human epididymosomes collected during surgical vasectomy reversal: a proteomic and genomic approach. Hum. Reprod. 23, 1698–1707 (2008).
Sahlén, G. et al. Secretions from seminal vesicles lack characteristic markers for prostasomes. Ups. J. Med. Sci. 115, 107–112 (2010).
Le Tortorec, A. et al. From ancient to emerging infections: the odyssey of viruses in the male genital tract. Physiol. Rev. 100, 1349–1414 (2020).
Agrawal, Y. & Vanha-Perttula, T. Effect of secretory particles in bovine seminal vesicle secretion on sperm motility and acrosome reaction. Reproduction 79, 409–419 (1987).
Zhang, X., Vos, H. R., Tao, W. & Stoorvogel, W. Proteomic profiling of two distinct populations of extracellular vesicles isolated from human seminal plasma. Int. J. Mol. Sci. 21, 7957 (2020).
Tauber, P., Zaneveld, L., Propping, D. & Schumacher, G. Components of human split ejaculates. Reproduction 43, 249–267 (1975).
Taylor, P. & Kelly, R. 19-HydroxyIated E prostaglandins as the major prostaglandins of human semen. Nature 250, 665–667 (1974).
Robert, M. & Gagnon, C. Semenogelin I: a coagulum forming, multifunctional seminal vesicle protein. Cell. Mol. Life Sci. 55, 944–960 (1999).
Belleannée, C., Calvo, É., Caballero, J. & Sullivan, R. Epididymosomes convey different repertoires of microRNAs throughout the bovine epididymis. Biol. Reprod. 89, 30 (2013).
Conine, C. C., Sun, F., Song, L., Rivera-Pérez, J. A. & Rando, O. J. Small RNAs gained during epididymal transit of sperm are essential for embryonic development in mice. Dev. Cell 46, 470–480. e473 (2018).
Grunewald, S., Paasch, U., Glander, H. J. & Anderegg, U. Mature human spermatozoa do not transcribe novel RNA. Andrologia 37, 69–71 (2005).
Goodrich, R. J., Anton, E. & Krawetz, S. A. in Spermatogenesis (eds Carrell, D. T. & Aston, K.I.) 385–396 (Springer, 2013).
Rodriguez-Caro, H. et al. In vitro decidualisation of human endometrial stromal cells is enhanced by seminal fluid extracellular vesicles. J. Extracell. Vesicles 8, 1565262 (2019).
Bai, R. et al. Induction of immune-related gene expression by seminal exosomes in the porcine endometrium. Biochem. Biophys. Res. Commun. 495, 1094–1101 (2018).
Wang, D. et al. Seminal plasma and seminal plasma exosomes of aged male mice affect early embryo implantation via immunomodulation. Front. Immunol. 12, 723409 (2021).
Yanagimachi, R., Kamiguchi, Y., Mikamo, K., Suzuki, F. & Yanagimachi, H. Maturation of spermatozoa in the epididymis of the Chinese hamster. Am. J. Anat. 172, 317–330 (1985).
Thimon, V., Koukoui, O., Calvo, E. & Sullivan, R. Region-specific gene expression profiling along the human epididymis. Mol. Hum. Reprod. 13, 691–704 (2007).
Frenette, G. & Sullivan, R. Prostasome‐like particles are involved in the transfer of P25b from the bovine epididymal fluid to the sperm surface. Mol. Reprod. Dev. 59, 115–121 (2001).
Frenette, G., Lessard, C. & Sullivan, R. Selected proteins of “prostasome-like particles” from epididymal cauda fluid are transferred to epididymal caput spermatozoa in bull. Biol. Reprod. 67, 308–313 (2002).
Rejraji, H. et al. Lipid remodeling of murine epididymosomes and spermatozoa during epididymal maturation. Biol. Reprod. 74, 1104–1113 (2006).
Griffiths, G. S., Galileo, D. S., Reese, K. & Martin‐DeLeon, P. A. Investigating the role of murine epididymosomes and uterosomes in GPI‐linked protein transfer to sperm using SPAM1 as a model. Mol. Reprod. Dev. 75, 1627–1636 (2008).
Ecroyd, H., Sarradin, P., Dacheux, J.-L. & Gatti, J.-L. Compartmentalization of prion isoforms within the reproductive tract of the ram. Biol. Reprod. 71, 993–1001 (2004).
Gatti, J.-L. et al. Post-testicular sperm environment and fertility. Anim. Reprod. Sci. 82, 321–339 (2004).
Fornes, M., Barbieri, A. & Cavicchia, J. Morphological and enzymatic study of membrane‐bound vesicles from the lumen of the rat epididymis. Andrologia 27, 1–5 (1995).
Grimalt, P., Bertini, F. & Fornes, M. High-affinity sites for β-D-galactosidase on membrane-bound vesicles isolated from rat epididymal fluid. Arch. Androl. 44, 85–91 (2000).
Candenas, L. & Chianese, R. Exosome composition and seminal plasma proteome: a promising source of biomarkers of male infertility. Int. J. Mol. Sci. 21 (2020).
Johnston, D. S. et al. The mouse epididymal transcriptome: transcriptional profiling of segmental gene expression in the epididymis. Biol. Reprod. 73, 404–413 (2005).
Zhou, W., De Iuliis, G. N., Dun, M. D. & Nixon, B. Characteristics of the epididymal luminal environment responsible for sperm maturation and storage. Front. Endocrinol. 9, 59 (2018).
Nixon, B. et al. The identification of mouse sperm-surface-associated proteins and characterization of their ability to act as decapacitation factors. Biol. Reprod. 74, 275–287 (2006).
Dacheux, J. & Voglmayr, J. Sequence of sperm cell surface differentiation and its relationship to exogenous fluid proteins in the ram epididymis. Biol. Reprod. 29, 1033–1046 (1983).
Dacheux, J.-L. et al. Mammalian epididymal proteome. Mol. Cell. Endocrinol. 306, 45–50 (2009).
Belleannée, C., Thimon, V. & Sullivan, R. Region-specific gene expression in the epididymis. Cell Tissue Res. 349, 717–731 (2012).
Ecroyd, H., Belghazi, M., Dacheux, J.-L. & Gatti, J.-L. The epididymal soluble prion protein forms a high-molecular-mass complex in association with hydrophobic proteins. Biochem. J. 392, 211–219 (2005).
Girouard, J., Frenette, G. & Sullivan, R. Comparative proteome and lipid profiles of bovine epididymosomes collected in the intraluminal compartment of the caput and cauda epididymidis. Int. J. Androl. 34, e475–e486 (2011).
Nixon, B. et al. Proteomic profiling of mouse epididymosomes reveals their contributions to post-testicular sperm maturation. Mol. Cell. Proteom. 18, S91–S108 (2019).
Johnson, A. L. & Howards, S. S. Intratubular hydrostatic pressure in testis and epididymis before and after long-term vasectomy in the guinea pig. Biol. Reprod. 14, 371–376 (1976).
Turner, T., Gleavy, J. & Harris, J. Fluid movement in the lumen of the rat epididymis: effect of vasectomy and subsequent vasovasostomy. J. Androl. 11, 422–428 (1990).
Dacheux, J.-L. et al. The contribution of proteomics to understanding epididymal maturation of mammalian spermatozoa. Syst. Biol. Reprod. Med. 58, 197–210 (2012).
Girouard, J., Frenette, G. & Sullivan, R. Compartmentalization of proteins in epididymosomes coordinates the association of epididymal proteins with the different functional structures of bovine spermatozoa. Biol. Reprod. 80, 965–972 (2009).
Zhou, W. et al. Mechanisms of tethering and cargo transfer during epididymosome-sperm interactions. BMC Biol. 17, 35 (2019).
Edidin, M. The state of lipid rafts: from model membranes to cells. Annu. Rev. Biophys. Biomol. Struct. 32, 257–283 (2003).
Sengupta, P., Baird, B. & Holowka, D. Lipid rafts, fluid/fluid phase separation, and their relevance to plasma membrane structure and function. Semin. Cell Dev. Biol. 18, 583–590 (2007).
Aitken, R. J. & De Iuliis, G. N. Origins and consequences of DNA damage in male germ cells. Reprod. Biomed. Online 14, 727–733 (2007).
Rooney, I. A., Heuser, J. E. & Atkinson, J. P. GPI-anchored complement regulatory proteins in seminal plasma. An analysis of their physical condition and the mechanisms of their binding to exogenous cells. J. Clin. Invest. 97, 1675–1686 (1996).
Sloand, E. M. et al. Correction of the PNH defect by GPI-anchored protein transfer. Blood 92, 4439–4445 (1998).
Samanta, L., Parida, R., Dias, T. R. & Agarwal, A. The enigmatic seminal plasma: a proteomics insight from ejaculation to fertilization. Reprod. Biol. Endocrinol. 16, 41 (2018).
Caballero, J. N., Frenette, G., Belleannée, C. & Sullivan, R. CD9-positive microvesicles mediate the transfer of molecules to bovine spermatozoa during epididymal maturation. PLoS ONE 8, e65364 (2013).
Sullivan, R., Frenette, G. & Girouard, J. Epididymosomes are involved in the acquisition of new sperm proteins during epididymal transit. Asian J. Androl. 9, 483–491 (2007).
Sullivan, R. Epididymosomes: a heterogeneous population of microvesicles with multiple functions in sperm maturation and storage. Asian J. Androl. 17, 726 (2015).
D’Amours, O. et al. Evidences of biological functions of biliverdin reductase A in the bovine epididymis. J. Cell. Physiol. 231, 1077–1089 (2016).
Hu, J. et al. Epididymal cysteine-rich secretory proteins are required for epididymal sperm maturation and optimal sperm function. Mol. Hum. Reprod. 24, 111–122 (2018).
Martin-DeLeon, P. A. Epididymal SPAM1 and its impact on sperm function. Mol. Cell. Endocrinol. 250, 114–121 (2006).
Eickhoff, R. et al. Influence of macrophage migration inhibitory factor (MIF) on the zinc content and redox state of protein-bound sulphydryl groups in rat sperm: indications for a new role of MIF in sperm maturation. Mol. Hum. Reprod. 10, 605–611 (2004).
Saez, F., Frenette, G. & Sullivan, R. Epididymosomes and prostasomes: their roles in posttesticular maturation of the sperm cells. J. Androl. 24, 149–154 (2003).
Patel, R. et al. Plasma membrane Ca2+-ATPase 4 in murine epididymis: secretion of splice variants in the luminal fluid and a role in sperm maturation. Biol. Reprod. 89, 1–11 (2013).
Sullivan, R. Epididymosomes: role of extracellular microvesicles in sperm maturation. Front. Biosci. 8, 106–114 (2016).
Simon, C. et al. Extracellular vesicles in human reproduction in health and disease. Endocr. Rev. 39, 292–332 (2018).
Ishijima, S., Okuno, M. & Mohri, H. Zeta potential of human X‐ and Y‐bearing sperm. Int. J. Androl. 14, 340–347 (1991).
Cuasnicú, P. S. et al. in The Epididymis: From Molecules to Clinical Practice (eds Robaire, B. & Hinton, B. T.) 389–403 (Springer, 2002).
Kirchhoff, C. & Hale, G. Cell-to-cell transfer of glycosylphosphatidylinositol-anchored membrane proteins during sperm maturation. Mol. Hum. Reprod. 2, 177–184 (1996).
Cooper, T. G. & Yeung, C.-H. in Sperm Cell: Production, Maturation, Fertilization, Regeneration (eds De Jonge, C. & Barratt, C.) 72–107 (Cambridge Univ. Press, 2006).
Miller, D., Brinkworth, M. & Iles, D. Paternal DNA packaging in spermatozoa: more than the sum of its parts? DNA, histones, protamines and epigenetics. Reproduction 139, 287–301 (2010).
Cooper, T. in Tissue Renin-Angiotensin Systems (eds Mukhopadhyay, A. K. & Raizada, M. K.) 87–101 (Springer, 1995).
Jones, R. Membrane remodelling during sperm maturation in the epididymis. Oxf. Rev. Reprod. Biol. 11, 285–337 (1989).
Vickram, A. et al. Seminal exosomes — an important biological marker for various disorders and syndrome in human reproduction. Saudi J. Biol. Sci. 28, 3607–3615 (2021).
Llorente, A., de Marco, M. C. & Alonso, M. A. Caveolin-1 and MAL are located on prostasomes secreted by the prostate cancer PC-3 cell line. J. Cell Sci. 117, 5343–5351 (2004).
Llorente, A., van Deurs, B. & Sandvig, K. Cholesterol regulates prostasome release from secretory lysosomes in PC-3 human prostate cancer cells. Eur. J. Cell Biol. 86, 405–415 (2007).
Aalberts, M., Stout, T. & Stoorvogel, W. Prostasomes: extracellular vesicles from the prostate. Reproduction 147, R1–R14 (2014).
Sullivan, R. & Saez, F. Epididymosomes, prostasomes, and liposomes: their roles in mammalian male reproductive physiology. Reproduction 146, R21–R35 (2013).
Brouwers, J. F. et al. Distinct lipid compositions of two types of human prostasomes. Proteomics 13, 1660–1666 (2013).
Arienti, G., Carlini, E. & Palmerini, C. Fusion of human sperm to prostasomes at acidic pH. J. Membr. Biol. 155, 89–94 (1997).
Aalberts, M. et al. Identification of distinct populations of prostasomes that differentially express prostate stem cell antigen, annexin A1, and GLIPR2 in humans. Biol. Reprod. 86, 82–82 (2012).
Publicover, S., Harper, C. V. & Barratt, C. [Ca2+]i signalling in sperm — making the most of what you’ve got. Nat. Cell Biol. 9, 235–242 (2007).
Bailey, J. L. Factors regulating sperm capacitation. Syst. Biol. Reprod. Med. 56, 334–348 (2010).
Fraser, L. R. The “switching on” of mammalian spermatozoa: molecular events involved in promotion and regulation of capacitation. Mol. Reprod. Dev. 77, 197–208 (2010).
Harrison, R. & Miller, N. cAMP‐dependent protein kinase control of plasma membrane lipid architecture in boar sperm. Mol. Reprod. Dev. 55, 220–228 (2000).
Pons-Rejraji, H. et al. Prostasomes: inhibitors of capacitation and modulators of cellular signalling in human sperm. Int. J. Androl. 34, 568–580 (2011).
García-Rodríguez, A., Gosálvez, J., Agarwal, A., Roy, R. & Johnston, S. DNA damage and repair in human reproductive cells. Int. J. Mol. Sci. 20, 31 (2019).
Clark, G. F. & Schust, D. J. Manifestations of immune tolerance in the human female reproductive tract. Front. Immunol. 4, 26 (2013).
Vojtech, L. et al. Exosomes in human semen carry a distinctive repertoire of small non-coding RNAs with potential regulatory functions. Nucleic Acids Res. 42, 7290–7304 (2014).
Kelly, R. et al. Extracellular organelles (prostasomes) are immunosuppressive components of human semen. Clin. Exp. Immunol. 86, 550–556 (1991).
Johansson, M., Bromfield, J. J., Jasper, M. J. & Robertson, S. A. Semen activates the female immune response during early pregnancy in mice. Immunology 112, 290–300 (2004).
Robertson, S. A., Guerin, L. R., Moldenhauer, L. M. & Hayball, J. D. Activating T regulatory cells for tolerance in early pregnancy — the contribution of seminal fluid. J. Reprod. Immunol. 83, 109–116 (2009).
Malla, B., Zaugg, K., Vassella, E., Aebersold, D. M. & Dal Pra, A. Exosomes and exosomal microRNAs in prostate cancer radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 98, 982–995 (2017).
Yang, C. et al. Comprehensive proteomics analysis of exosomes derived from human seminal plasma. Andrology 5, 1007–1015 (2017).
Taylor, D. D. & Gercel-Taylor, C. MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol. Oncol. 110, 13–21 (2008).
Milardi, D. et al. Proteomic approach in the identification of fertility pattern in seminal plasma of fertile men. Fertil. Steril. 97, 67–73.e61 (2012).
De Lazari, F. L. et al. Seminal plasma proteins and their relationship with sperm motility and morphology in boars. Andrologia 51, e13222 (2019).
Gilany, K., Minai-Tehrani, A., Savadi-Shiraz, E., Rezadoost, H. & Lakpour, N. Exploring the human seminal plasma proteome: an unexplored gold mine of biomarker for male infertility and male reproduction disorder. J. Reprod. Infertil. 16, 61 (2015).
Vernet, P., Aitken, R. & Drevet, J. Antioxidant strategies in the epididymis. Mol. Cell. Endocrinol. 216, 31–39 (2004).
Chabory, E. et al. Epididymis seleno-independent glutathione peroxidase 5 maintains sperm DNA integrity in mice. J. Clin. Invest. 119, 2074–2085 (2009).
Buschow, S. I. et al. MHC II in dendritic cells is targeted to lysosomes or T cell-induced exosomes via distinct multivesicular body pathways. Traffic 10, 1528–1542 (2009).
Gibbs, G. M. et al. Glioma pathogenesis-related 1-like 1 is testis enriched, dynamically modified, and redistributed during male germ cell maturation and has a potential role in sperm-oocyte binding. Endocrinology 151, 2331–2342 (2010).
Ronquist, G. The Male Role in Pregnancy Loss and Embryo Implantation Failure (ed. Bronson, R.) 191–209 (Springer, 2015).
Tarazona, R. et al. Human prostasomes express CD48 and interfere with NK cell function. Immunobiology 216, 41–46 (2011).
Burden, H., Holmes, C., Persad, R. & Whittington, K. Prostasomes — their effects on human male reproduction and fertility. Hum. Reprod. Update 12, 283–292 (2006).
Garcia-Rodriguez, A., de la Casa, M., Peinado, H., Gosalvez, J. & Roy, R. Human prostasomes from normozoospermic and non-normozoospermic men show a differential protein expression pattern. Andrology 6, 585–596 (2018).
Ernesto, J. I. et al. CRISP1 as a novel CatSper regulator that modulates sperm motility and orientation during fertilization. J. Cell Biol. 210, 1213–1224 (2015).
Roberts, K. P. et al. Epididymal secreted protein Crisp-1 and sperm function. Mol. Cell. Endocrinol. 250, 122–127 (2006).
Roberts, K. P., Wamstad, J. A., Ensrud, K. M. & Hamilton, D. W. Inhibition of capacitation-associated tyrosine phosphorylation signaling in rat sperm by epididymal protein Crisp-1. Biol. Reprod. 69, 572–581 (2003).
Weigel Muñoz, M. et al. Influence of the genetic background on the reproductive phenotype of mice lacking Cysteine-Rich Secretory Protein 1 (CRISP1). Biol. Reprod. 99, 373–383 (2018).
Maldera, J. A. et al. Human fertilization: epididymal hCRISP1 mediates sperm–zona pellucida binding through its interaction with ZP3. Mol. Hum. Reprod. 20, 341–349 (2014).
Da Ros, V. G. et al. Impaired sperm fertilizing ability in mice lacking Cysteine-RIch Secretory Protein 1 (CRISP1). Dev. Biol. 320, 12–18 (2008).
Miki, K. Energy metabolism and sperm function. Soc. Reprod. Fertil. Suppl. 65, 309–325 (2007).
Odet, F. et al. Lactate dehydrogenase C and energy metabolism in mouse sperm. Biol. Reprod. 85, 556–564 (2011).
Rolland, A. D. et al. Identification of genital tract markers in the human seminal plasma using an integrative genomics approach. Hum. Reprod. 28, 199–209 (2013).
Li, S. S.-L. et al. Differential activity and synthesis of lactate dehydrogenase isozymes A (muscle), B (heart), and C (testis) in mouse spermatogenic cells. Biol. Reprod. 40, 173–180 (1989).
O’Flaherty, C., Beorlegui, N. & Beconi, M. Lactate dehydrogenase‐C4 is involved in heparin‐and NADH‐dependent bovine sperm capacitation. Andrologia 34, 91–97 (2002).
Duan, C. & Goldberg, E. Inhibition of lactate dehydrogenase C4 (LDH-C4) blocks capacitation of mouse sperm in vitro. Cytogenet. Genome Res. 103, 352–359 (2003).
Odet, F. et al. Expression of the gene for mouse lactate dehydrogenase C (Ldhc) is required for male fertility. Biol. Reprod. 79, 26–34 (2008).
Oddo, M., Calandra, T., Bucala, R. & Meylan, P. R. Macrophage migration inhibitory factor reduces the growth of virulent Mycobacterium tuberculosis in human macrophages. Infect. Immun. 73, 3783–3786 (2005).
Meinhardt, A. et al. Macrophage migration inhibitory factor production by Leydig cells: evidence for a role in the regulation of testicular function. Endocrinology 137, 5090–5095 (1996).
Frenette, G., Lessard, C., Madore, E., Fortier, M. A. & Sullivan, R. Aldose reductase and macrophage migration inhibitory factor are associated with epididymosomes and spermatozoa in the bovine epididymis. Biol. Reprod. 69, 1586–1592 (2003).
Huleihel, M. et al. Production of macrophage inhibitory factor (MIF) by primary Sertoli cells; its possible involvement in migration of spermatogonial cells. J. Cell. Physiol. 232, 2869–2877 (2017).
Henkel, R., Bittner, J., Weber, R., Hüther, F. & Miska, W. Relevance of zinc in human sperm flagella and its relation to motility. Fertil. Steril. 71, 1138–1143 (1999).
Frenette, G., Légaré, C., Saez, F. & Sullivan, R. Macrophage migration inhibitory factor in the human epididymis and semen. Mol. Hum. Reprod. 11, 575–582 (2005).
Aljabari, B. et al. Imbalance in seminal fluid MIF indicates male infertility. Mol. Med. 13, 199–202 (2007).
Ebert, B., Kisiela, M. & Maser, E. Human DCXR — another ‘moonlighting protein’ involved in sugar metabolism, carbonyl detoxification, cell adhesion and male fertility? Biol. Rev. 90, 254–278 (2015).
Légaré, C., Gaudreault, C., St-Jacques, S. & Sullivan, R. P34H sperm protein is preferentially expressed by the human corpus epididymidis. Endocrinology 140, 3318–3327 (1999).
Sullivan, R., Saez, F., Girouard, J. & Frenette, G. Role of exosomes in sperm maturation during the transit along the male reproductive tract. Blood Cells Mol. Dis. 35, 1–10 (2005).
Parent, S., Lefievre, L., Brindle, Y. & Sullivan, R. Bull subfertility is associated with low levels of a sperm membrane antigen. Mol. Reprod. Dev. 52, 57–65 (1999).
Boué, F. & Sullivan, R. Cases of human infertility are associated with the absence of P34H, an epididymal sperm antigen. Biol. Reprod. 54, 1018–1024 (1996).
Frapsauce, C. et al. Proteomic identification of target proteins in normal but nonfertilizing sperm. Fertil. Steril. 102, 372–380 (2014).
Sullivan, R., Légaré, C., Villeneuve, M., Foliguet, B. & Bissonnette, F. Levels of P34H, a sperm protein of epididymal origin, as a predictor of conventional in vitro fertilization outcome. Fertil. Steril. 85, 1557–1559 (2006).
Moskovtsev, S. I., Jarvi, K., Légaré, C., Sullivan, R. & Mullen, J. B. M. Epididymal P34H protein deficiency in men evaluated for infertility. Fertil. Steril. 88, 1455–1457 (2007).
Oh, J. S., Han, C. & Cho, C. ADAM7 is associated with epididymosomes and integrated into sperm plasma membrane. Mol. Cell 28, 441–446 (2009).
Han, C. et al. Identification of heat shock protein 5, calnexin and integral membrane protein 2B as Adam7‐interacting membrane proteins in mouse sperm. J. Cell. Physiol. 226, 1186–1195 (2011).
Légaré, C., Thabet, M., Gatti, J.-L. & Sullivan, R. HE1/NPC2 status in human reproductive tract and ejaculated spermatozoa: consequence of vasectomy. Mol. Hum. Reprod. 12, 461–468 (2006).
Busso, D. et al. Spermatozoa from mice deficient in Niemann-Pick disease type C2 (NPC2) protein have defective cholesterol content and reduced in vitro fertilising ability. Reprod. Fertil. Dev. 26, 609–621 (2014).
Okamura, N. et al. Molecular cloning and characterization of the epididymis-specific glutathione peroxidase-like protein secreted in the porcine epididymal fluid. Biochim. Biophys. Acta 1336, 99–109 (1997).
Légaré, C., Thabet, M., Picard, S. & Sullivan, R. Effect of vasectomy on P34H messenger ribonucleic acid expression along the human excurrent duct: a reflection on the function of the human epididymis. Biol. Reprod. 64, 720–727 (2001).
Giacomini, E. et al. Comparative analysis of the seminal plasma proteomes of oligoasthenozoospermic and normozoospermic men. Reprod. Biomed. Online 30, 522–531 (2015).
Taylor, A. et al. Epididymal specific, selenium-independent GPX5 protects cells from oxidative stress-induced lipid peroxidation and DNA mutation. Hum. Reprod. 28, 2332–2342 (2013).
Noblanc, A. et al. Glutathione peroxidases at work on epididymal spermatozoa: an example of the dual effect of reactive oxygen species on mammalian male fertilizing ability. J. Androl. 32, 641–650 (2011).
Rejraji, H., Vernet, P. & Drevet, J. L. R. GPX5 is present in the mouse caput and cauda epididymidis lumen at three different locations. Mol. Reprod. Dev. 63, 96–103 (2002).
Barranco, I. et al. Glutathione peroxidase 5 is expressed by the entire pig male genital tract and once in the seminal plasma contributes to sperm survival and in vivo fertility. PLoS ONE 11, e0162958 (2016).
Kim, E. et al. Sperm penetration through cumulus mass and zona pellucida. Int. J. Dev. Biol. 52, 677–682 (2004).
Baba, D. et al. Mouse sperm lacking cell surface hyaluronidase PH-20 can pass through the layer of cumulus cells and fertilize the egg. J. Biol. Chem. 277, 30310–30314 (2002).
Myles, D. G., Hyatt, H. & Primakoff, P. Binding of both acrosome-intact and acrosome-reacted guinea pig sperm to the zona pellucida during in vitro fertilization. Dev. Biol. 121, 559–567 (1987).
Primakoff, P., Hyatt, H. & Myles, D. G. A role for the migrating sperm surface antigen PH-20 in guinea pig sperm binding to the egg zona pellucida. J. Cell Biol. 101, 2239–2244 (1985).
Reese, K. L. et al. Acidic hyaluronidase activity is present in mouse sperm and is reduced in the absence of SPAM1: evidence for a role for hyaluronidase 3 in mouse and human sperm. Mol. Reprod. Dev. 77, 759–772 (2010).
Kimura, M. et al. Functional roles of mouse sperm hyaluronidases, HYAL5 and SPAM1, in fertilization. Biol. Reprod. 81, 939–947 (2009).
Honbou, K. et al. The crystal structure of DJ-1, a protein related to male fertility and Parkinson’s disease. J. Biol. Chem. 278, 31380–31384 (2003).
Junn, E., Jang, W. H., Zhao, X., Jeong, B. S. & Mouradian, M. M. Mitochondrial localization of DJ-1 leads to enhanced neuroprotection. J. Neurosci. Res. 87, 123–129 (2009).
An, C.-N. et al. Down-regulation of DJ-1 protein in the ejaculated spermatozoa from Chinese asthenozoospermia patients. Fertil. Steril. 96, 19–23.e12 (2011).
Yoshida, K. et al. Immunocytochemical localization of DJ-1 in human male reproductive tissue. Mol. Reprod. Dev. 66, 391–397 (2003).
Whyard, T. C., Cheung, W., Sheynkin, Y., Waltzer, W. C. & Hod, Y. Identification of RS as a flagellar and head sperm protein. Mol. Reprod. Dev. 55, 189–196 (2000).
Wang, J. et al. Proteomic analysis of seminal plasma from asthenozoospermia patients reveals proteins that affect oxidative stress responses and semen quality. Asian J. Androl. 11, 484–491 (2009).
Nishinaga, H. et al. Expression profiles of genes in DJ-1-knockdown and L166P DJ-1 mutant cells. Neurosci. Lett. 390, 54–59 (2005).
Pegtel, D. M. et al. Functional delivery of viral miRNAs via exosomes. Proc. Natl Acad. Sci. USA 107, 6328–6333 (2010).
Hergenreider, E. et al. Atheroprotective communication between endothelial cells and smooth muscle cells through miRNAs. Nat. Cell Biol. 14, 249–256 (2012).
Kalluri, R. & LeBleu, V. S. The biology, function, and biomedical applications of exosomes. Science 367 (2020).
Ma, J. et al. Testosterone-dependent miR-26a-5p and let-7g-5p act as signaling mediators to regulate sperm apoptosis via targeting PTEN and PMAIP1. Int. J. Mol. Sci. 19, 1233 (2018).
Barceló, M., Castells, M., Bassas, L., Vigués, F. & Larriba, S. Semen miRNAs contained in exosomes as non-invasive biomarkers for prostate cancer diagnosis. Sci. Rep. 9, 1–16 (2019).
Twenter, H. et al. Transfer of microRNAs from epididymal epithelium to equine spermatozoa. J. Equine Vet. Sci. 87, 102841 (2020).
Reilly, J. N. et al. Characterisation of mouse epididymosomes reveals a complex profile of microRNAs and a potential mechanism for modification of the sperm epigenome. Sci. Rep. 6, 31794 (2016).
Alshanbayeva, A., Tanwar, D. K., Roszkowski, M., Manuella, F. & Mansuy, I. M. Early life stress affects the miRNA cargo of epididymal extracellular vesicles in mouse. Biol. Reprod. 105, 593–602 (2021).
Barcelo, M., Mata, A., Bassas, L. & Larriba, S. Exosomal microRNAs in seminal plasma are markers of the origin of azoospermia and can predict the presence of sperm in testicular tissue. Hum. Reprod. 33, 1087–1098 (2018).
Wang, C. et al. Altered profile of seminal plasma microRNAs in the molecular diagnosis of male infertility. Clin. Chem. 57, 1722–1731 (2011).
Wu, W. et al. Seminal plasma microRNAs: potential biomarkers for spermatogenesis status. Mol. Hum. Reprod. 18, 489–497 (2012).
Abu-Halima, M. et al. Altered micro-ribonucleic acid expression profiles of extracellular microvesicles in the seminal plasma of patients with oligoasthenozoospermia. Fertil. Steril. 106, 1061–1069.e3 (2016).
World Health Organization. Laboratory Manual for the Examination and Processing of Human Semen 6th edn (WHO, 2021).
Santi, D., Spaggiari, G. & Simoni, M. Sperm DNA fragmentation index as a promising predictive tool for male infertility diagnosis and treatment management–meta-analyses. Reprod. Biomed. Online 37, 315–326 (2018).
Ferlin, A. et al. Male infertility: role of genetic background. Reprod. Biomed. Online 14, 734–745 (2007).
Vashisht, A. & Gahlay, G. K. Using miRNAs as diagnostic biomarkers for male infertility: opportunities and challenges. Mol. Hum. Reprod. 26, 199–214 (2020).
Murdica, V. et al. Proteomic analysis reveals the negative modulator of sperm function glycodelin as over-represented in semen exosomes isolated from asthenozoospermic patients. Hum. Reprod. 34, 1416–1427 (2019).
Madison, M. N., Roller, R. J. & Okeoma, C. M. Human semen contains exosomes with potent anti-HIV-1 activity. Retrovirology 11, 1–16 (2014).
Hiemstra, T. F. et al. Human urinary exosomes as innate immune effectors. J. Am. Soc. Nephrol. 25, 2017–2027 (2014).
György, B. et al. Detection and isolation of cell-derived microparticles are compromised by protein complexes resulting from shared biophysical parameters. Blood J. Am. Soc. Hematol. 117, e39–e48 (2011).
He, M., Crow, J., Roth, M., Zeng, Y. & Godwin, A. K. Integrated immunoisolation and protein analysis of circulating exosomes using microfluidic technology. Lab Chip 14, 3773–3780 (2014).
Muller, L., Hong, C.-S., Stolz, D. B., Watkins, S. C. & Whiteside, T. L. Isolation of biologically-active exosomes from human plasma. J. Immunol. Methods 411, 55–65 (2014).
Das, C. K. et al. Exosome as a novel shuttle for delivery of therapeutics across biological barriers. Mol. Pharm. 16, 24–40 (2018).
Yamashita, T., Takahashi, Y. & Takakura, Y. Possibility of exosome-based therapeutics and challenges in production of exosomes eligible for therapeutic application. Biol. Pharm. Bull. 41, 835–842 (2018).
Théry, C. et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 7, 1535750 (2018).
Fedder, J. Nonsperm cells in human semen: with special reference to seminal leukocytes and their possible influence on fertility. Arch. Androl. 36, 41–65 (1996).
Soares Martins, T., Catita, J., Martins Rosa, I., O, A. B. D. C. E. S. & Henriques, A. G. Exosome isolation from distinct biofluids using precipitation and column-based approaches. PLoS ONE 13, e0198820 (2018).
Lane, R. E., Korbie, D., Anderson, W., Vaidyanathan, R. & Trau, M. Analysis of exosome purification methods using a model liposome system and tunable-resistive pulse sensing. Sci. Rep. 5, 1–7 (2015).
Lobb, R. J. et al. Optimized exosome isolation protocol for cell culture supernatant and human plasma. J. Extracell. Vesicles 4, 27031 (2015).
Coughlan, C. et al. Exosome isolation by ultracentrifugation and precipitation and techniques for downstream analyses. Curr. Protoc. Cell Biol. 88, e110 (2020).
Cao, F. et al. Proteomics comparison of exosomes from serum and plasma between ultracentrifugation and polymer‐based precipitation kit methods. Electrophoresis 40, 3092–3098 (2019).
Madison, M. N., Welch, J. L. & Okeoma, C. M. Isolation of exosomes from semen for in vitro uptake and HIV-1 infection assays. Bio Protoc. 7, e2216 (2017).
Kaddour, H. et al. Proteomics profiling of autologous blood and semen exosomes from HIV-infected and uninfected individuals reveals compositional and functional variabilities. Mol. Cell. Proteom. 19, 78–100 (2020).
Welch, J. L., Kaufman, T. M., Stapleton, J. T. & Okeoma, C. M. Semen exosomes inhibit HIV infection and HIV‐induced proinflammatory cytokine production independent of the activation state of primary lymphocytes. FEBS Lett. 594, 695–709 (2020).
Welch, J. L., Kaddour, H., Schlievert, P. M., Stapleton, J. T. & Okeoma, C. M. Semen exosomes promote transcriptional silencing of HIV-1 by disrupting NF-κB/Sp1/Tat circuitry. J. Virol. 92, e00731–18 (2018).
Chang, X. et al. Exosomes from women with preeclampsia induced vascular dysfunction by delivering sFlt (soluble Fms-like tyrosine kinase)-1 and sEng (soluble endoglin) to endothelial cells. Hypertension 72, 1381–1390 (2018).
Gemoll, T. et al. Protein profiling of serum extracellular vesicles reveals qualitative and quantitative differences after differential ultracentrifugation and ExoQuick™ isolation. J. Clin. Med. 9, 1429 (2020).
Wang, X. in Extracellular Vesicles (eds Kuo, W. P. & Jia, S.) 351–353 (Springer, 2017).
Yamada, T., Inoshima, Y., Matsuda, T. & Ishiguro, N. Comparison of methods for isolating exosomes from bovine milk. J. Vet. Med. Sci., 12-0032 (2012).
Lin, S. et al. Progress in microfluidics-based exosome separation and detection technologies for diagnostic applications. Small 16, e1903916 (2020).
Théry, C., Amigorena, S., Raposo, G. & Clayton, A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr. Protoc. Cell Biol. 30, 3.22. 21–23.22. 29 (2006).
Contreras-Naranjo, J. C., Wu, H. J. & Ugaz, V. M. Microfluidics for exosome isolation and analysis: enabling liquid biopsy for personalized medicine. Lab Chip 17, 3558–3577 (2017).
Webber, J. & Clayton, A. How pure are your vesicles? J. Extracell. Vesicles 2, 19861 (2013).
Vlassov, A. V., Magdaleno, S., Setterquist, R. & Conrad, R. Exosomes: current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim. Biophys. Acta 1820, 940–948 (2012).
Liga, A., Vliegenthart, A. D., Oosthuyzen, W., Dear, J. W. & Kersaudy-Kerhoas, M. Exosome isolation: a microfluidic road-map. Lab Chip 15, 2388–2394 (2015).
Momen-Heravi, F. et al. Current methods for the isolation of extracellular vesicles. Biol. Chem. 394, 1253–1262 (2013).
Faruqu, F. N., Xu, L. & Al-Jamal, K. T. Preparation of exosomes for siRNA delivery to cancer cells. J. Vis. Exp. 142, e58814 (2018).
Greening, D. W., Xu, R., Ji, H., Tauro, B. J. & Simpson, R. J. in Proteomic Profiling (ed. Posch, A.) 179–209 (Springer, 2015).
Yang, D. et al. Progress, opportunity, and perspective on exosome isolation-efforts for efficient exosome-based theranostics. Theranostics 10, 3684–3707 (2020).
Baranyai, T. et al. Isolation of exosomes from blood plasma: qualitative and quantitative comparison of ultracentrifugation and size exclusion chromatography methods. PLoS ONE 10, e0145686 (2015).
Guan, S. et al. Characterization of urinary exosomes purified with size exclusion chromatography and ultracentrifugation. J. Proteome Res. 19, 2217–2225 (2020).
Stranska, R. et al. Comparison of membrane affinity-based method with size-exclusion chromatography for isolation of exosome-like vesicles from human plasma. J. Transl. Med. 16, 1–9 (2018).
Al Ali, J. et al. TAF1 transcripts and neurofilament light chain as biomarkers for x‐linked dystonia‐parkinsonism. Mov. Disord. 36, 206–215 (2021).
Vandendriessche, C. et al. Importance of extracellular vesicle secretion at the blood–cerebrospinal fluid interface in the pathogenesis of Alzheimer’s disease. Acta Neuropathol. Commun. 9, 1–25 (2021).
Salarpour, S. et al. Paclitaxel incorporated exosomes derived from glioblastoma cells: comparative study of two loading techniques. Daru 27, 533–539 (2019).
Macías, M. et al. Comparison of six commercial serum exosome isolation methods suitable for clinical laboratories. Effect in cytokine analysis. Clin. Chem. Lab. Med. 57, 1539–1545 (2019).
Keller, S., Sanderson, M. P., Stoeck, A. & Altevogt, P. Exosomes: from biogenesis and secretion to biological function. Immunol. Lett. 107, 102–108 (2006).
Kastelowitz, N. & Yin, H. Exosomes and microvesicles: identification and targeting by particle size and lipid chemical probes. Chembiochem Eur. J. Chem. Biol. 15, 923 (2014).
Song, Z. et al. Development of a CD63 aptamer for efficient cancer immunochemistry and immunoaffinity-based exosome isolation. Molecules 25, 5585 (2020).
Zarovni, N. et al. Integrated isolation and quantitative analysis of exosome shuttled proteins and nucleic acids using immunocapture approaches. Methods 87, 46–58 (2015).
Alvarez, M. L., Khosroheidari, M., Ravi, R. K. & DiStefano, J. K. Comparison of protein, microRNA, and mRNA yields using different methods of urinary exosome isolation for the discovery of kidney disease biomarkers. Kidney Int. 82, 1024–1032 (2012).
Li, P., Kaslan, M., Lee, S. H., Yao, J. & Gao, Z. Progress in exosome isolation techniques. Theranostics 7, 789 (2017).
Phan, T. H. et al. New multiscale characterization methodology for effective determination of isolation–structure–function relationship of extracellular vesicles. Front. Bioeng. Biotechnol. 9, 358 (2021).
Heinemann, M. L. et al. Benchtop isolation and characterization of functional exosomes by sequential filtration. J. Chromatogr. A 1371, 125–135 (2014).
Marsh, S. R., Pridham, K. J., Jourdan, J. & Gourdie, R. G. Novel protocols for scalable production of high quality purified small extracellular vesicles from bovine milk. Nanotheranostics 5, 488–498 (2021).
Manz, A., Graber, N. & Widmer, H. Á. Miniaturized total chemical analysis systems: a novel concept for chemical sensing. Sens. Actuators B Chem. 1, 244–248 (1990).
Chen, Y.-S., Ma, Y.-D., Chen, C., Shiesh, S.-C. & Lee, G.-B. An integrated microfluidic system for on-chip enrichment and quantification of circulating extracellular vesicles from whole blood. Lab Chip 19, 3305–3315 (2019).
Panesar, S. & Neethirajan, S. Microfluidics: rapid diagnosis for breast cancer. Nanomicro Lett. 8, 204–220 (2016).
Nosrati, R. et al. Rapid selection of sperm with high DNA integrity. Lab Chip 14, 1142–1150 (2014).
Quinn, M. M. et al. Microfluidic sorting selects sperm for clinical use with reduced DNA damage compared to density gradient centrifugation with swim-up in split semen samples. Hum. Reprod. 33, 1388–1393 (2018).
Tung, C.-K. et al. Fluid viscoelasticity promotes collective swimming of sperm. Sci. Rep. 7, 1–9 (2017).
Vasilescu, S. A. et al. A microfluidic approach to rapid sperm recovery from heterogeneous cell suspensions. Sci. Rep. 11, 7917 (2021).
Son, J. et al. Non-motile sperm cell separation using a spiral channel. Anal. Methods 7, 8041–8047 (2015).
Son, J., Samuel, R., Gale, B. K., Carrell, D. T. & Hotaling, J. M. Separation of sperm cells from samples containing high concentrations of white blood cells using a spiral channel. Biomicrofluidics 11, 054106 (2017).
Roy, T. K. et al. Embryo vitrification using a novel semi-automated closed system yields in vitro outcomes equivalent to the manual Cryotop method. Hum. Reprod. 29, 2431–2438 (2014).
Smith, D., Gaffney, E., Blake, J. & Kirkman-Brown, J. Human sperm accumulation near surfaces: a simulation study. J. Fluid Mech. 621, 289–320 (2009).
Ramadan, S. et al. Carbon-dot-enhanced graphene field-effect transistors for ultrasensitive detection of exosomes. ACS Appl. Mater. Interfaces 13, 7854–7864 (2021).
Zhand, S. et al. Improving capture efficiency of human cancer cell derived exosomes with nanostructured metal organic framework functionalized beads. Appl. Mater. Today 23, 100994 (2021).
Sayyadi, N., Zhand, S., Razavi Bazaz, S. & Warkiani, M. E. Affibody functionalized beads for the highly sensitive detection of cancer cell-derived exosomes. Int. J. Mol. Sci. 22, 12014 (2021).
Dorayappan, K. D. P. et al. A microfluidic chip enables isolation of exosomes and establishment of their protein profiles and associated signaling pathways in ovarian cancer. Cancer Res. 79, 3503–3513 (2019).
Sancho-Albero, M. et al. Isolation of exosomes from whole blood by a new microfluidic device: proof of concept application in the diagnosis and monitoring of pancreatic cancer. J. Nanobiotechnol. 18, 1–15 (2020).
Chen, C. et al. Microfluidic isolation and transcriptome analysis of serum microvesicles. Lab Chip 10, 505–511 (2010).
Fang, S. et al. Clinical application of a microfluidic chip for immunocapture and quantification of circulating exosomes to assist breast cancer diagnosis and molecular classification. PLoS ONE 12, e0175050 (2017).
Ashcroft, B. A. et al. Determination of the size distribution of blood microparticles directly in plasma using atomic force microscopy and microfluidics. Biomed. Microdevices 14, 641–649 (2012).
Zhang, P., He, M. & Zeng, Y. Ultrasensitive microfluidic analysis of circulating exosomes using a nanostructured graphene oxide/polydopamine coating. Lab Chip 16, 3033–3042 (2016).
Shao, H. et al. Chip-based analysis of exosomal mRNA mediating drug resistance in glioblastoma. Nat. Commun. 6, 6999 (2015).
Im, H. et al. Label-free detection and molecular profiling of exosomes with a nano-plasmonic sensor. Nat. Biotechnol. 32, 490–495 (2014).
Vaidyanathan, R. et al. Detecting exosomes specifically: a multiplexed device based on alternating current electrohydrodynamic induced nanoshearing. Anal. Chem. 86, 11125–11132 (2014).
Zhao, Z., Yang, Y., Zeng, Y. & He, M. A microfluidic ExoSearch chip for multiplexed exosome detection towards blood-based ovarian cancer diagnosis. Lab Chip 16, 489–496 (2016).
Sina, A. A. I. et al. Real time and label free profiling of clinically relevant exosomes. Sci. Rep. 6, 1–9 (2016).
Ko, J. et al. Smartphone-enabled optofluidic exosome diagnostic for concussion recovery. Sci. Rep. 6, 1–12 (2016).
Hisey, C. L., Dorayappan, K. D. P., Cohn, D. E., Selvendiran, K. & Hansford, D. J. Microfluidic affinity separation chip for selective capture and release of label-free ovarian cancer exosomes. Lab Chip 18, 3144–3153 (2018).
Kang, Y. T. et al. Dual‐isolation and profiling of circulating tumor cells and cancer exosomes from blood samples with melanoma using immunoaffinity‐based microfluidic interfaces. Adv. Sci. 7, 2001581 (2020).
Tauro, B. J. et al. Two distinct populations of exosomes are released from LIM1863 colon carcinoma cell-derived organoids. Mol. Cell. Proteom. 12, 587–598 (2013).
Crescitelli, R. et al. Distinct RNA profiles in subpopulations of extracellular vesicles: apoptotic bodies, microvesicles and exosomes. J. Extracell. Vesicles 2, 20677 (2013).
Oliveira-Rodríguez, M. et al. Development of a rapid lateral flow immunoassay test for detection of exosomes previously enriched from cell culture medium and body fluids. J. Extracell. Vesicles 5, 31803 (2016).
Moyano, A. et al. Magnetic lateral flow immunoassay for small extracellular vesicles quantification: application to colorectal cancer biomarker detection. Sensors 21, 3756 (2021).
Liu, F. et al. The exosome total isolation chip. ACS Nano 11, 10712–10723 (2017).
Chen, Z., Yang, Y., Yamaguchi, H., Hung, M.-C. & Kameoka, J. Isolation of cancer-derived extracellular vesicle subpopulations by a size-selective microfluidic platform. Biomicrofluidics 14, 034113 (2020).
Wu, M. et al. Acoustofluidic separation of cells and particles. Microsyst. Nanoeng. 5, 1–18 (2019).
Wu, M. et al. Isolation of exosomes from whole blood by integrating acoustics and microfluidics. Proc. Natl Acad. Sci. USA 114, 10584–10589 (2017).
Jubery, T. Z., Srivastava, S. K. & Dutta, P. Dielectrophoretic separation of bioparticles in microdevices: a review. Electrophoresis 35, 691–713 (2014).
Yun, H., Kim, K. & Lee, W. G. Cell manipulation in microfluidics. Biofabrication 5, 022001 (2013).
Zhu, H., Lin, X., Su, Y., Dong, H. & Wu, J. Screen-printed microfluidic dielectrophoresis chip for cell separation. Biosens. Bioelectron. 63, 371–378 (2015).
Zheng, L., Brody, J. P. & Burke, P. J. Electronic manipulation of DNA, proteins, and nanoparticles for potential circuit assembly. Biosens. Bioelectron. 20, 606–619 (2004).
Ibsen, S. D. et al. Rapid isolation and detection of exosomes and associated biomarkers from plasma. ACS Nano 11, 6641–6651 (2017).
Zhao, W. et al. Microsphere mediated exosome isolation and ultra-sensitive detection on a dielectrophoresis integrated microfluidic device. Analyst 146, 5962–5972 (2021).
Zeming, K. K., Thakor, N. V., Zhang, Y. & Chen, C.-H. Real-time modulated nanoparticle separation with an ultra-large dynamic range. Lab Chip 16, 75–85 (2016).
Santana, S. M., Antonyak, M. A., Cerione, R. A. & Kirby, B. J. Microfluidic isolation of cancer-cell-derived microvesicles from hetergeneous extracellular shed vesicle populations. Biomed. Microdevices 16, 869–877 (2014).
Tottori, N., Muramoto, Y., Sakai, H. & Nisisako, T. Nanoparticle separation through deterministic lateral displacement arrays in poly (dimethylsiloxane). J. Chem. Eng. Jpn. 53, 414–421 (2020).
Calero, V., Garcia-Sanchez, P., Ramos, A. & Morgan, H. Combining DC and AC electric fields with deterministic lateral displacement for micro- and nano-particle separation. Biomicrofluidics 13, 054110 (2019).
Woo, H.-K. et al. Exodisc for rapid, size-selective, and efficient isolation and analysis of nanoscale extracellular vesicles from biological samples. Acs Nano 11, 1360–1370 (2017).
Liu, C. et al. Field-free isolation of exosomes from extracellular vesicles by microfluidic viscoelastic flows. ACS Nano 11, 6968–6976 (2017).
Kang, D., Oh, S., Ahn, S.-M., Lee, B.-H. & Moon, M. H. Proteomic analysis of exosomes from human neural stem cells by flow field-flow fractionation and nanoflow liquid chromatography−tandem mass spectrometry. J. Proteome Res. 7, 3475–3480 (2008).
Wang, Z. et al. Ciliated micropillars for the microfluidic-based isolation of nanoscale lipid vesicles. Lab Chip 13, 2879–2882 (2013).
Qi, R. et al. Microfluidic device for the analysis of MDR cancerous cell-derived exosomes’ response to nanotherapy. Biomed. Microdevices 21, 1–9 (2019).
Willis, G. R., Kourembanas, S. & Mitsialis, S. A. Toward exosome-based therapeutics: isolation, heterogeneity, and fit-for-purpose potency. Front. Cardiovasc. Med. 4, 63 (2017).
Luo, J. et al. Immunogenicity study of plasmid DNA encoding mouse cysteine‐rich secretory protein‐1 (mCRISP 1) as a contraceptive vaccine. Am. J. Reprod. Immunol. 68, 47–55 (2012).
Batruch, I. et al. Analysis of seminal plasma from patients with non-obstructive azoospermia and identification of candidate biomarkers of male infertility. J. Proteome Res. 11, 1503–1511 (2012).
Batruch, I. et al. Proteomic analysis of seminal plasma from normal volunteers and post-vasectomy patients identifies over 2000 proteins and candidate biomarkers of the urogenital system. J. Proteome Res. 10, 941–953 (2011).
Tang, H., Duan, C., Bleher, R. & Goldberg, E. Human lactate dehydrogenase A (LDHA) rescues mouse Ldhc-null sperm function. Biol. Reprod. 88, 91–96 (2013).
Anahara, R. et al. Deletion of macrophage migration inhibitory factor gene induces down regulation of sex hormones and ultrastructural abnormalities in mouse testes. Reprod. Toxicol. 21, 167–170 (2006).
Grzmil, P. et al. Human cyritestin genes (CYRN1 and CYRN2) are non-functional. Biochem. J. 357, 551–556 (2001).
Choi, H. et al. Reduced fertility and altered epididymal and sperm integrity in mice lacking ADAM7. Biol. Reprod. 93, 70 (2015).
Kim, T. et al. Expression and relationship of male reproductive ADAMs in mouse. Biol. Reprod. 74, 744–750 (2006).
Ronquist, G. K. et al. Biochemical characterization of stallion prostasomes and comparison to their human counterparts. Syst. Biol. Reprod. Med. 59, 297–303 (2013).
Chotwiwatthanakun, C. et al. Expression of Penaeus monodon ortholog of Niemann–Pick type C‐2 in the spermatic tract, and its role in sperm cholesterol removal. Mol. Reprod. Dev. 83, 259–270 (2016).
Vilagran, I., Castillo-Martín, M., Prieto-Martínez, N., Bonet, S. & Yeste, M. Triosephosphate isomerase (TPI) and epididymal secretory glutathione peroxidase (GPX5) are markers for boar sperm quality. Anim. Reprod. Sci. 100, 22–30 (2016).
Zhou, C., Kang, W. & Baba, T. Functional characterization of double-knockout mouse sperm lacking SPAM1 and ACR or SPAM1 and PRSS21 in fertilization. J. Reprod. Dev. 58, 330–337 (2012).
Lin, Y., Mahan, K., Lathrop, W. F., Myles, D. G. & Primakoff, P. A hyaluronidase activity of the sperm plasma membrane protein PH-20 enables sperm to penetrate the cumulus cell layer surrounding the egg. J. Cell Biol. 125, 1157–1163 (1994).
Primakoff, P. & Myles, D. G. Penetration, adhesion, and fusion in mammalian sperm-egg interaction. Science 296, 2183–2185 (2002).
Sun, Y. et al. DJ-1 deficiency causes metabolic abnormality in ornidazole-induced asthenozoospermia. Reproduction 160, 931–941 (2020).
Klinefelter, G., Laskey, J., Ferrell, J., Suarez, J. & Roberts, N. Discriminant analysis indicates a single sperm protein (SP22) is predictive of fertility following exposure to epididymal toxicants. J. Androl. 18, 139–150 (1997).
Lyu, Y. et al. Human immunodeficiency virus (HIV) infection and use of illicit substances promote secretion of semen exosomes that enhance monocyte adhesion and induce actin reorganization and chemotactic migration. Cells 8, 1027 (2019).
Fabiani, R., Johansson, L., Lundkvist, Ö. & Ronquist, G. Enhanced recruitment of motile spermatozoa by prostasome inclusion in swim-up medium. Hum. Reprod. 9, 1485–1489 (1994).
Minelli, A., Moroni, M., Martinez, E., Mezzasoma, I. & Ronquist, G. Occurrence of prostasome-like membrane vesicles in equine seminal plasma. Reproduction 114, 237–243 (1998).
Carlsson, L. et al. Characteristics of human prostasomes isolated from three different sources. Prostate 54, 322–330 (2003).
Ronquist, K. G., Ronquist, G., Larsson, A. & Carlsson, L. Proteomic analysis of prostate cancer metastasis-derived prostasomes. Anticancer Res. 30, 285–290 (2010).
Carlsson, L. et al. Association of cystatin C with prostasomes in human seminal plasma. Int. J. Androl. 34, 363–368 (2011).
Chevillet, J. R. et al. Quantitative and stoichiometric analysis of the microRNA content of exosomes. Proc. Natl Acad. Sci. USA 111, 14888–14893 (2014).
Du, J. et al. Boar seminal plasma exosomes maintain sperm function by infiltrating into the sperm membrane. Oncotarget 7, 58832 (2016).
Milutinović, B., Goč, S., Mitić, N., Kosanović, M. & Janković, M. Surface glycans contribute to differences between seminal prostasomes from normozoospermic and oligozoospermic men. Ups. J. Med. Sci. 124, 111–118 (2019).
Lee, K., Shao, H., Weissleder, R. & Lee, H. Acoustic purification of extracellular microvesicles. ACS Nano 9, 2321–2327 (2015).
Ku, A. et al. Acoustic enrichment of extracellular vesicles from biological fluids. Anal. Chem. 90, 8011–8019 (2018).
Wu, M. et al. Separating extracellular vesicles and lipoproteins via acoustofluidics. Lab Chip 19, 1174–1182 (2019).
Shi, L. et al. Rapid and label-free isolation of small extracellular vesicles from biofluids utilizing a novel insulator based dielectrophoretic device. Lab Chip 19, 3726–3734 (2019).
Wunsch, B. H. et al. Nanoscale lateral displacement arrays for the separation of exosomes and colloids down to 20 nm. Nat. Nanotechnol. 11, 936–940 (2016).
Bai, Y. et al. Rapid isolation and multiplexed detection of exosome tumor markers via queued beads combined with quantum dots in a microarray. Nanomicro Lett. 11, 59 (2019).
Davies, R. T. et al. Microfluidic filtration system to isolate extracellular vesicles from blood. Lab Chip 12, 5202–5210 (2012).
Cho, S. et al. Isolation of extracellular vesicle from blood plasma using electrophoretic migration through porous membrane. Sens. Actuators B Chem. 233, 289–297 (2016).
Liang, L.-G. et al. An integrated double-filtration microfluidic device for isolation, enrichment and quantification of urinary extracellular vesicles for detection of bladder cancer. Sci. Rep. 7, 1–10 (2017).
Dong, X. et al. Efficient isolation and sensitive quantification of extracellular vesicles based on an integrated ExoID-Chip using photonic crystals. Lab Chip 19, 2897–2904 (2019).
Casadei, L. et al. Cross‐flow microfiltration for isolation, selective capture and release of liposarcoma extracellular vesicles. J. Extracell. Vesicles 10, e12062 (2021).
Han, Z. et al. Highly efficient exosome purification from human plasma by tangential flow filtration based microfluidic chip. Sens. Actuators B Chem. 333, 129563 (2021).
Han, B. H. et al. Isolation of extracellular vesicles from small volumes of plasma using a microfluidic aqueous two-phase system. Lab Chip 20, 3552–3559 (2020).
Dudani, J. S. et al. Rapid inertial solution exchange for enrichment and flow cytometric detection of microvesicles. Biomicrofluidics 9, 014112 (2015).
Yeo, J. C. et al. Label-free extraction of extracellular vesicles using centrifugal microfluidics. Biomicrofluidics 12, 024103 (2018).
Zhou, Y., Ma, Z., Tayebi, M. & Ai, Y. Submicron particle focusing and exosome sorting by wavy microchannel structures within viscoelastic fluids. Anal. Chem. 91, 4577–4584 (2019).
Teoh, B. Y. et al. Isolation of exosome from the culture medium of nasopharyngeal cancer (NPC) C666-1 cells using inertial based microfluidic channel. Biomed. Microdevices 24, 12 (2022).
Linxweiler, J. & Junker, K. Extracellular vesicles in urological malignancies: an update. Nat. Rev. Urol. 17, 11–27 (2020).
Author information
Authors and Affiliations
Contributions
D.M.G. researched data for the article. All authors contributed substantially to discussion of the content. D.M.G. wrote the article. D.M.G., S.A.V., D.K.G. and M.E.W. reviewed and/or edited the manuscript before submission.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Urology thanks the anonymous reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Goss, D.M., Vasilescu, S.A., Sacks, G. et al. Microfluidics facilitating the use of small extracellular vesicles in innovative approaches to male infertility. Nat Rev Urol 20, 66–95 (2023). https://doi.org/10.1038/s41585-022-00660-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41585-022-00660-8
This article is cited by
-
Unlocking the mystery associated with infertility and prostate cancer: an update
Medical Oncology (2023)