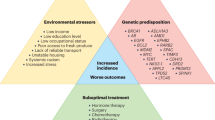
Abstract
In the past 20 years, new insights into the genomic pathogenesis of prostate cancer have been provided. Large-scale integrative genomics approaches enabled researchers to characterize the genetic and epigenetic landscape of prostate cancer and to define different molecular subclasses based on the combination of genetic alterations, gene expression patterns and methylation profiles. Several molecular drivers of prostate cancer have been identified, some of which are different in men of different races. However, the extent to which genomics can explain racial disparities in prostate cancer outcomes is unclear. Future collaborative genomic studies overcoming the underrepresentation of non-white patients and other minority populations are essential.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Siegel, R. L., Miller, K. D., Fuchs, H. E. & Jemal, A. Cancer statistics, 2021. CA Cancer J. Clin. 71, 7–33 (2021).
Abeshouse, A. et al. The molecular taxonomy of primary prostate cancer. Cell 163, 1011–1025 (2015).
Grasso, C. S. et al. The mutational landscape of lethal castration-resistant prostate cancer. Nature 487, 239–243 (2012).
Taylor, B. S. et al. Integrative genomic profiling of human prostate cancer. Cancer Cell 18, 11–22 (2010).
Rencsok, E. M. et al. Diversity of enrollment in prostate cancer clinical trials: current status and future directions. Cancer Epidemiol. Biomark. Prev. 29, 1374–1380 (2020).
Hooker, S. E. et al. Genetic ancestry analysis reveals misclassification of commonly used cancer cell lines. Cancer Epidemiol. Biomark. Prev. 28, 1003–1009 (2019).
Mersha, T. B. & Abebe, T. Self-reported race/ethnicity in the age of genomic research: its potential impact on understanding health disparities. Hum. Genomics 9, 1 (2015).
Banda, Y. et al. Characterizing race/ethnicity and genetic ancestry for 100,000 subjects in the genetic epidemiology research on adult health and aging (GERA) cohort. Genetics 200, 1285–1295 (2015).
Stopsack, K. H. et al. Differences in prostate cancer genomes by self-reported race: contributions of genetic ancestry, modifiable cancer risk factors, and clinical factors. Clin. Cancer Res. https://doi.org/10.1158/1078-0432.CCR-21-2577 (2021).
Sivakumar, S. et al. Ancestral characterization of the genomic landscape, comprehensive genomic profiling utilization, and treatment patterns may inform disparities in advanced prostate cancer: a large-scale analysis. J. Clin. Oncol. 39, 5003–5003 (2021).
Li, J. et al. A genomic and epigenomic atlas of prostate cancer in Asian populations. Nature 580, 93–99 (2020).
Koga, Y. et al. Genomic profiling of prostate cancers from men with African and European ancestry. Clin. Cancer Res. 26, 4651–4660 (2020).
Shoag, J. & Barbieri, C. Clinical variability and molecular heterogeneity in prostate cancer. Asian J. Androl. 18, 543 (2016).
Tomlins, S. A. et al. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science 310, 644–648 (2005).
Brenner, J. C., Chinnaiyan, A. M. & Tomlins, S. A. ETS Fusion Genes in Prostate Cancer. Prostate Cancer: Biochemistry, Molecular Biology and Genetics (Springer New York, 2013).
Tomlins, S. A. et al. ETS gene fusions in prostate cancer: from discovery to daily clinical practice. Eur. Urol. 56, 275–286 (2009).
Mehra, R. et al. Characterization of TMPRSS2-ETS gene aberrations in androgen-independent metastatic prostate cancer. Cancer Res. 68, 3584–3590 (2008).
Attard, G. et al. Characterization of ERG, AR and PTEN gene status in circulating tumor cells from patients with castration-resistant prostate cancer. Cancer Res. 69, 2912–2918 (2009).
Perner, S. et al. TMPRSS2:ERG fusion-associated deletions provide insight into the heterogeneity of prostate cancer. Cancer Res. 66, 8337–8341 (2006).
Pettersson, A. et al. The TMPRSS2:ERG rearrangement, ERG expression, and prostate cancer outcomes: a cohort study and meta-analysis. Cancer Epidemiol. Biomark. Prev. 21, 1497–1509 (2012).
Tomlins, S. A. et al. TMPRSS2:ETV4 gene fusions define a third molecular subtype of prostate cancer. Cancer Res. 66, 3396–3400 (2006).
Helgeson, B. E. et al. Characterization of TMPRSS2:ETV5 and SLC45A3:ETV5 gene fusions in prostate cancer. Cancer Res. 68, 73–80 (2008).
Paulo, P. et al. FLI1 is a novel ETS transcription factor involved in gene fusions in prostate cancer. Genes Chromosomes Cancer 51, 2430–249 (2012).
Magi-Galluzzi, C. et al. TMPRSS2-ERG gene fusion prevalence and class are significantly different in prostate cancer of Caucasian, African-American and Japanese patients. Prostate 71, 489–497 (2011).
Minner, S. et al. ERG status is unrelated to PSA recurrence in radically operated prostate cancer in the absence of antihormonal therapy. Clin. Cancer Res. 17, 5878–5888 (2011).
Park, K. et al. Antibody-based detection of ERG rearrangement-positive prostate cancer. Neoplasia 12, 590–IN21 (2010).
Schumacher, F. R. et al. Race and genetic alterations in prostate cancer. JCO Precis. Oncol. https://doi.org/10.1200/PO.21.00324 (2021).
Park, K. et al. TMPRSS2:ERG gene fusion predicts subsequent detection of prostate cancer in patients with high-grade prostatic intraepithelial neoplasia. J. Clin. Oncol. 32, 206–211 (2014).
Taneja, S. S. et al. Prostate cancer diagnosis among men with isolated high-grade intraepithelial neoplasia enrolled onto a 3-year prospective phase III clinical trial of oral toremifene. J. Clin. Oncol. 31, 523–529 (2013).
Demichelis, F. et al. TMPRSS2:ERG gene fusion associated with lethal prostate cancer in a watchful waiting cohort. Oncogene 26, 4596–4599 (2007).
Attard, G. et al. Duplication of the fusion of TMPRSS2 to ERG sequences identifies fatal human prostate cancer. Oncogene 27, 253–263 (2008).
Gopalan, A. et al. TMPRSS2-ERG gene fusion is not associated with outcome in patients treated by prostatectomy. Cancer Res. 69, 1400–1406 (2009).
Swanson, T. A. et al. TMPRSS2/ERG fusion gene expression alters chemo- and radio-responsiveness in cell culture models of androgen independent prostate cancer. Prostate 71, 1548–1558 (2011).
Mani, R.-S. et al. Induced chromosomal proximity and gene fusions in prostate cancer. Science 326, 1230–1230 (2009).
Dal Pra, A. et al. TMPRSS2-ERG status is not prognostic following prostate cancer radiotherapy: implications for fusion status and DSB repair. Clin. Cancer Res. 19, 5202–5209 (2013).
Song, C. & Chen, H. Predictive significance of TMRPSS2-ERG fusion in prostate cancer: a meta-analysis. Cancer Cell Int. 18, 177 (2018).
Khani, F. et al. Evidence for molecular differences in prostate cancer between African American and Caucasian men. Clin. Cancer Res. 20, 4925–4934 (2014).
Mahal, B. A. et al. Racial differences in genomic profiling of prostate cancer. N. Engl. J. Med. 383, 1083–1085 (2020).
Petrovics, G. et al. Frequent overexpression of ETS-related gene-1 (ERG1) in prostate cancer transcriptome. Oncogene 24, 3847–3852 (2005).
Rosen, P. et al. Differences in frequency of ERG oncoprotein expression between index tumors of Caucasian and African American patients with prostate cancer. Urology 80, 749–753 (2012).
Farrell, J. et al. Predominance of ERG-negative high-grade prostate cancers in African American men. Mol. Clin. Oncol. 2, 982–986 (2014).
Faisal, F. A. et al. Racial variations in prostate cancer molecular subtypes and androgen receptor signaling reflect anatomic tumor location. Eur. Urol. 70, 14–17 (2016).
Sedarsky, J., Degon, M., Srivastava, S. & Dobi, A. Ethnicity and ERG frequency in prostate cancer. Nat. Rev. Urol. 15, 125–131 (2018).
Zhu, Y. et al. Epidemiology and genomics of prostate cancer in Asian men. Nat. Rev. Urol. https://doi.org/10.1038/s41585-021-00442-8 (2021).
Xu, C. et al. Detection of TMPRSS2-ERG and TMPRSS2-EGR1 gene fusion in prostate cancer from a Chinese population. Egypt. J. Med. Hum. Genet. 21, 53 (2020).
Miyagi, Y. et al. ETS family-associated gene fusions in Japanese prostate cancer: analysis of 194 radical prostatectomy samples. Mod. Pathol. 23, 1492–1498 (2010).
Tomlins, S. A. et al. The role of SPINK1 in ETS rearrangement-negative prostate cancers. Cancer Cell 13, 519–528 (2008).
Tiwari, R. et al. Androgen deprivation upregulates SPINK1 expression and potentiates cellular plasticity in prostate cancer. Nat. Commun. 11, 384 (2020).
Zhang, X. et al. The association between SPINK1 and clinical outcomes in patients with prostate cancer: a systematic review and meta-analysis. OncoTargets Ther. 10, 3123–3130 (2017).
Pan, X. et al. The expression profile and prognostic value of SPINK1 in initially diagnosed bone metastatic prostate cancer. Prostate 76, 823–833 (2016).
Wang, C. et al. Serine protease inhibitor Kazal type 1 promotes epithelial mesenchymal transition through EGFR signaling pathway in prostate cancer. Prostate 74, 689–701 (2014).
Koide, H. et al. Comparison of ERG and SPINK1 expression among incidental and metastatic prostate cancer in Japanese men. Prostate 79, 3–8 (2019).
Barbieri, C. E. et al. Exome sequencing identifies recurrent SPOP, FOXA1 and MED12 mutations in prostate cancer. Nat. Genet. 44, 685–689 (2012).
Blattner, M. et al. SPOP mutations in prostate cancer across demographically diverse patient cohorts. Neoplasia 16, 14–W10 (2014).
Boysen, G. et al. SPOP mutation leads to genomic instability in prostate cancer. Elife 4, 1–18 (2015).
Geng, C. et al. Prostate cancer-associated mutations in speckle-type POZ protein (SPOP) regulate steroid receptor coactivator 3 protein turnover. Proc. Natl Acad. Sci. USA 110, 6997–7002 (2013).
Zhang, P. et al. Destruction of DDIT3/CHOP protein by wild-type SPOP but not prostate cancer-associated mutants. Hum. Mutat. 35, 1142–1151 (2014).
Zhang, D. et al. Speckle-type POZ protein, SPOP, is involved in the DNA damage response. Carcinogenesis 35, 1691–1697 (2014).
Shoag, J. et al. SPOP mutation drives prostate neoplasia without stabilizing oncogenic transcription factor ERG. J. Clin. Investig. 128, 381–386 (2017).
Gan, W. et al. SPOP promotes ubiquitination and degradation of the ERG oncoprotein to suppress prostate cancer progression. Mol. Cell 59, 917–930 (2015).
An, J. et al. Truncated ERG oncoproteins from TMPRSS2-ERG fusions are resistant to SPOP-mediated proteasome degradation. Mol. Cell 59, 904–916 (2015).
Duan, S. & Pagano, M. SPOP mutations or ERG rearrangements result in enhanced levels of ERG to promote cell invasion in prostate cancer. Mol. Cell 59, 883–884 (2015).
Bernasocchi, T. et al. Dual functions of SPOP and ERG dictate androgen therapy responses in prostate cancer. Nat. Commun. 12, 734 (2021).
Liu, D. et al. Impact of the SPOP mutant subtype on the interpretation of clinical parameters in prostate cancer. JCO Precis. Oncol. https://doi.org/10.1200/PO.18.00036 (2018).
Tewari, A. K. et al. Molecular features of exceptional response to neoadjuvant anti-androgen therapy in high-risk localized prostate cancer. Cell Rep. 36, 109665 (2021).
Shoag, J. et al. Prognostic value of the SPOP mutant genomic subclass in prostate cancer. Urol. Oncol. Semin. Orig. Investig. 38, 418–422 (2020).
Parolia, A. et al. Distinct structural classes of activating FOXA1 alterations in advanced prostate cancer. Nature 571, 413–418 (2019).
Fraser, M. et al. Genomic hallmarks of localized, non-indolent prostate cancer. Nature 541, 359–364 (2017).
Adams, E. J. et al. FOXA1 mutations alter pioneering activity, differentiation and prostate cancer phenotypes. Nature 571, 408–412 (2019).
Sahu, B. et al. Dual role of FoxA1 in androgen receptor binding to chromatin, androgen signalling and prostate cancer. EMBO J. 30, 3962–3976 (2011).
Lindquist, K. J. et al. Mutational landscape of aggressive prostate tumors in African American men. Cancer Res. 76, 1860–1868 (2016).
Yuan, J. et al. Integrative comparison of the genomic and transcriptomic landscape between prostate cancer patients of predominantly African or European genetic ancestry. PLoS Genet. 16, e1008641 (2020).
Qian, J., Jenkins, R. B. & Bostwick, D. G. Detection of chromosomal anomalies and c-myc gene amplification in the cribriform pattern of prostatic intraepithelial neoplasia and carcinoma by fluorescence in situ hybridization. Mod. Pathol. 10, 1113–1119 (1997).
Sun, J. et al. DNA copy number alterations in prostate cancers: a combined analysis of published CGH studies. Prostate 67, 692–700 (2007).
Dominguez-Sola, D. & Gautier, J. MYC and the control of DNA replication. Cold Spring Harb. Perspect. Med. 4, a014423–a014423 (2014).
Gil, J. et al. Immortalization of primary human prostate epithelial cells by c-Myc. Cancer Res. 65, 2179–2185 (2005).
Qiu, X. et al. MYC drives aggressive prostate cancer by disrupting transcriptional pause release at androgen receptor targets. Nat. Commun. 13, 2559 (2022).
Hawksworth, D. et al. Overexpression of C-MYC oncogene in prostate cancer predicts biochemical recurrence. Prostate Cancer Prostatic Dis. 13, 311–315 (2010).
Sato, K. et al. Clinical significance of alterations of chromosome 8 in high-grade, advanced, nonmetastatic prostate carcinoma. J. Natl Cancer Inst. 91, 1574–1580 (1999).
Bai, S. et al. A positive role of c-Myc in regulating androgen receptor and its splice variants in prostate cancer. Oncogene 38, 4977–4989 (2019).
Rebello, R., Pearson, R., Hannan, R. & Furic, L. Therapeutic approaches targeting MYC-driven prostate cancer. Genes 8, 71 (2017).
Leinonen, K. A. et al. Loss of PTEN is associated with aggressive behavior in ERG-positive prostate cancer. Cancer Epidemiol. Biomark. Prev. 22, 2333–2344 (2013).
Jamaspishvili, T. et al. Clinical implications of PTEN loss in prostate cancer. Nat. Rev. Urol. 15, 222–234 (2018).
Yoshimoto, M. et al. PTEN genomic deletions that characterize aggressive prostate cancer originate close to segmental duplications. Genes Chromosomes Cancer 51, 149–160 (2012).
Papa & Pandolfi The PTEN–PI3K axis in cancer. Biomolecules 9, 153 (2019).
Krohn, A. et al. Genomic deletion of PTEN is associated with tumor progression and early PSA recurrence in ERG fusion-positive and fusion-negative prostate cancer. Am. J. Pathol. 181, 401–412 (2012).
Ahearn, T. U. et al. A prospective investigation of PTEN loss and ERG expression in lethal prostate cancer. J. Natl Cancer Inst. 108, djv346 (2015).
Tosoian, J. J. et al. Prevalence and prognostic significance of PTEN loss in African-American and European-American men undergoing radical prostatectomy. Eur. Urol. 71, 697–700 (2017).
Huang, F. W. et al. Exome sequencing of African-American prostate cancer reveals loss-of-function ERF mutations. Cancer Discov. 7, 973–983 (2017).
Hoffman, R. M. et al. Racial and ethnic differences in advanced-stage prostate cancer: the Prostate Cancer Outcomes Study. J. Natl Cancer Inst. 93, 388–395 (2001).
Dess, R. T. et al. Association of Black race with prostate cancer-specific and other-cause mortality. JAMA Oncol. 5, 975 (2019).
Sundi, D. et al. Pathological examination of radical prostatectomy specimens in men with very low risk disease at biopsy reveals distinct zonal distribution of cancer in Black American men. J. Urol. 191, 60–67 (2014).
Awasthi, S. et al. Comparative genomics reveals distinct immune-oncologic pathways in African American men with prostate cancer. Clin. Cancer Res. 27, 320–329 (2021).
Abida, W. et al. Prospective genomic profiling of prostate cancer across disease states reveals germline and somatic alterations that may affect clinical decision making. JCO Precis. Oncol. https://doi.org/10.1200/PO.17.00029 (2017).
Bose, R. et al. ERF mutations reveal a balance of ETS factors controlling prostate oncogenesis. Nature 546, 671–675 (2017).
Troutman, S. M. et al. Racial disparities in the association between variants on 8q24 and prostate cancer: a systematic review and meta-analysis. Oncologist 17, 312–320 (2012).
Cairns, P. et al. Frequent inactivation of PTEN/MMAC1 in primary prostate cancer. Cancer Res. 57, 4997–5000 (1997).
Choucair, K. et al. PTEN genomic deletion predicts prostate cancer recurrence and is associated with low AR expression and transcriptional activity. BMC Cancer 12, 543 (2012).
Petrovics, G. et al. A novel genomic alteration of LSAMP associates with aggressive prostate cancer in African American men. EBioMedicine 2, 1957–1964 (2015).
Carracedo, A. & Pandolfi, P. P. The PTEN–PI3K pathway: of feedbacks and cross-talks. Oncogene 27, 5527–5541 (2008).
de Bono, J. et al. IPATential150: phase III study of ipatasertib (ipat) plus abiraterone (abi) vs placebo (pbo) plus abi in metastatic castration-resistant prostate cancer (mCRPC). Ann. Oncol. 31, S1153–S1154 (2020).
Rotimi, S. O., Rotimi, O. A. & Salhia, B. A review of cancer genetics and genomics studies in Africa. Front. Oncol. 10, 606400 (2021).
Cook, M. B. et al. A genome-wide association study of prostate cancer in West African men. Hum. Genet. 133, 509–521 (2014).
Petersen, D. C. et al. African KhoeSan ancestry linked to high-risk prostate cancer. BMC Med. Genomics 12, 82 (2019).
Mallick, S., Blanchet, P. & Multigner, L. Prostate cancer incidence in Guadeloupe, a French Caribbean archipelago. Eur. Urol. 47, 769–772 (2005).
Tonon, L. et al. Mutational profile of aggressive, localised prostate cancer from African Caribbean men versus European Ancestry men. Eur. Urol. 75, 11–15 (2019).
Jaratlerdsiri, W. et al. Whole-genome sequencing reveals elevated tumor mutational burden and initiating driver mutations in African men with treatment-naïve, high-risk prostate cancer. Cancer Res. 78, 6736–6746 (2018).
Tabei, T. et al. Does screening for prostate cancer improve cancer-specific mortality in Asian men? Real-world data in Yokosuka City 15 years after introducing PSA-based population screening. Prostate 80, 824–830 (2020).
Kimura, T. & Egawa, S. Epidemiology of prostate cancer in Asian countries. Int. J. Urol. 25, 524–531 (2018).
Zhang, J., Dhakal, I. B., Zhao, Z. & Li, L. Trends in mortality from cancers of the breast, colon, prostate, esophagus, and stomach in East Asia. Eur. J. Cancer Prev. 21, 480–489 (2012).
Shi, Z. et al. Biomarker analysis of the phase III IPATential150 trial of first-line ipatasertib (Ipat) plus abiraterone (Abi) in metastatic castration-resistant prostate cancer (mCRPC). J. Clin. Oncol. 38, 182–182 (2020).
Ren, S. et al. Whole-genome and transcriptome sequencing of prostate cancer identify new genetic alterations driving disease progression. Eur. Urol. 73, 322–339 (2018).
American Cancer Society. Cancer Facts & Figures for Hispanics/Latinos 2021–2023. Atlanta: American Cancer Society, Inc. https://www.cancer.org/research/cancer-facts-statistics/hispanics-latinos-facts-figures.html (2018).
Aldaco-Sarvide, F. et al. Mortalidad por Cáncer en México: actualización 2015. Gac. Mex. de. Oncol. https://doi.org/10.24875/j.gamo.M18000105 (2019).
Bravo, L. E. & Muñoz, N. Epidemiology of cancer in Colombia. Colomb. Médica 49, 9–12 (2018).
Lora, D. et al. Tendencia de la mortalidad por cáncer en Argentina, Cuba y Uruguay en un período de. 15 años. Rev. Cub. Salud Pública 36, 115–125 (2010).
González Burchard, E. et al. Latino populations: a unique opportunity for the study of race, genetics, and social environment in epidemiological research. Am. J. Public. Health 95, 2161–2168 (2005).
Pinheiro, P. S. et al. Cancer mortality in Hispanic ethnic groups. Cancer Epidemiol. Biomark. Prev. 26, 376–382 (2017).
Spratt, D. E. et al. Racial/ethnic disparities in genomic sequencing. JAMA Oncol. 2, 1070 (2016).
Du, Z. et al. A genome-wide association study of prostate cancer in Latinos. Int. J. Cancer 146, 1819–1826 (2020).
Sugiura, M. et al. Epigenetic modifications in prostate cancer. Int. J. Urol. 28, 140–149 (2021).
Kron, K. J. et al. TMPRSS2–ERG fusion co-opts master transcription factors and activates NOTCH signaling in primary prostate cancer. Nat. Genet. 49, 1336–1345 (2017).
Grbesa, I. et al. Reshaping of the androgen-driven chromatin landscape in normal prostate cells by early cancer drivers and effect on therapeutic sensitivity. Cell Rep. 36, 109625 (2021).
Wang, R. & Liu, X. Epigenetic regulation of prostate cancer. Genes Dis. 7, 606–613 (2020).
Rickman, D. S., Beltran, H., Demichelis, F. & Rubin, M. A. Biology and evolution of poorly differentiated neuroendocrine tumors. Nat. Med. 23, 664–673 (2017).
Nelson, W. G., de Marzo, A. M. & Yegnasubramanian, S. Minireview: epigenetic alterations in human prostate cancers. Endocrinology 150, 3991–4002 (2009).
Santric, V. et al. GSTP1 rs1138272 polymorphism affects prostate cancer risk. Medicina 56, 128 (2020).
Parray, A. et al. ROBO1, a tumor suppressor and critical molecular barrier for localized tumor cells to acquire invasive phenotype: study in African-American and Caucasian prostate cancer models. Int. J. Cancer 135, 2493–2506 (2014).
Kwabi-Addo, B. et al. Identification of differentially methylated genes in normal prostate tissues from African American and Caucasian men. Clin. Cancer Res. 16, 3539–3547 (2010).
Yegnasubramanian, S. et al. DNA hypomethylation arises later in prostate cancer progression than CpG island hypermethylation and contributes to metastatic tumor heterogeneity. Cancer Res. 68, 8954–8967 (2008).
Zhao, S. G. et al. The DNA methylation landscape of advanced prostate cancer. Nat. Genet. 52, 778–789 (2020).
Edwards, S. M. et al. Prostate cancer in BRCA2 germline mutation carriers is associated with poorer prognosis. Br. J. Cancer 103, 918–924 (2010).
Taylor, R. A. et al. Germline BRCA2 mutations drive prostate cancers with distinct evolutionary trajectories. Nat. Commun. 8, 13671 (2017).
Edwards, S. M. et al. Two percent of men with early-onset prostate cancer harbor germline mutations in the BRCA2 gene. Am. J. Hum. Genet. 72, 1–12 (2003).
Devaney, J. et al. Genome-wide differentially methylated genes in prostate cancer tissues from African-American and Caucasian men. Epigenetics 10, 319–328 (2015).
Sharda, D. R. et al. Regulation of macrophage arginase expression and tumor growth by the Ron receptor tyrosine kinase. J. Immunol. 187, 2181–2192 (2011).
Barrow, T. M. et al. Aberrant methylation of imprinted genes is associated with negative hormone receptor status in invasive breast cancer. Int. J. Cancer 137, 537–547 (2015).
Bedford, M. T. & van Helden, P. D. Hypomethylation of DNA in pathological conditions of the human prostate1. Cancer Res. 47, 5274–5276 (1987).
Schulz, W. A. et al. Genomewide DNA hypomethylation is associated with alterations on chromosome 8 in prostate carcinoma. Genes Chromosomes Cancer 35, 58–65 (2002).
Santourlidis, S., Florl, A., Ackermann, R., Wirtz, H.-C. & Schulz, W. A. High frequency of alterations in DNA methylation in adenocarcinoma of the prostate. Prostate 39, 166–174 (1999).
Desai, M. M. et al. Trends in incidence of metastatic prostate cancer in the US. JAMA Netw. Open 5, e222246 (2022).
Grossman, D. C. et al. Screening for prostate cancer. JAMA 319, 1901 (2018).
Carter, H. B. et al. Early detection of prostate cancer: AUA guideline. J. Urol. 190, 419–426 (2013).
Mottet, N. et al. EAU-ESTRO-SIOG guidelines on prostate cancer. Part 1: screening, diagnosis, and local treatment with curative intent. Eur. Urol. 71, 618–629 (2017).
Carroll, P. R. et al. NCCN Guidelines for Prostate Cancer Early Detection V.1.2021. https://www.nccn.org/professionals/physician_gls/pdf/prostate_detection.pdf (2020).
Basourakos, S. P. et al. Harm-to-benefit of three decades of prostate cancer screening in Black men. NEJM Evid. https://doi.org/10.1056/evidoa2200031 (2022).
Shoag, J. E., Nyame, Y. A., Gulati, R., Etzioni, R. & Hu, J. C. Reconsidering the trade-offs of prostate cancer screening. N. Engl. J. Med. 382, 2465–2468 (2020).
Black, M. H. et al. Validation of a prostate cancer polygenic risk score. Prostate 80, 1314–1321 (2020).
Sipeky, C. et al. Prostate cancer risk prediction using a polygenic risk score. Sci. Rep. 10, 17075 (2020).
Plym, A. et al. Evaluation of a multiethnic polygenic risk score model for prostate cancer. J. Natl Cancer Inst. https://doi.org/10.1093/jnci/djab058 (2021).
Huynh-Le, M.-P. et al. Polygenic hazard score is associated with prostate cancer in multi-ethnic populations. Nat. Commun. 12, 1236 (2021).
Song, S. H. & Byun, S.-S. Polygenic risk score for genetic evaluation of prostate cancer risk in Asian populations: a narrative review. Investig. Clin. Urol. 62, 256–266 (2021).
Seibert, T. M. et al. Polygenic hazard score to guide screening for aggressive prostate cancer: development and validation in large scale cohorts. BMJ https://doi.org/10.1136/bmj.j5757 (2018).
NIH. National Institutes of Health Policy and Guidelines on the Inclusion of Women and Minorities as Subjects in Clinical Research. https://grants.nih.gov/policy/inclusion/women-and-minorities/guidelines.htm (2017).
Witham, M. D. et al. Developing a roadmap to improve trial delivery for under-served groups: results from a UK multi-stakeholder process. Trials 21, 694 (2020).
Polite, B. N. et al. Charting the future of cancer health disparities research: a position statement from the American Association for Cancer Research, the American Cancer Society, the American Society of Clinical Oncology, and the National Cancer Institute. Cancer Res. 77, 4548–4555 (2017).
Mohler, J. L. & Antonarakis, E. S. NCCN guidelines updates: management of prostate cancer. J. Natl Compr. Canc Netw. 17, 583–586 (2019).
Bancroft, E. K. et al. A prospective prostate cancer screening programme for men with pathogenic variants in mismatch repair genes (IMPACT): initial results from an international prospective study. Lancet Oncol. 22, 1618–1631 (2021).
Castro, E. et al. Germline BRCA mutations are associated with higher risk of nodal involvement, distant metastasis, and poor survival outcomes in prostate cancer. J. Clin. Oncol. 31, 1748–1757 (2013).
Cheng, H. H., Pritchard, C. C., Montgomery, B., Lin, D. W. & Nelson, P. S. Prostate cancer screening in a new era of genetics. Clin. Genitourin. Cancer 15, 625–628 (2017).
Das, S. et al. Bringing prostate cancer germline genetics into clinical practice. J. Urol. 202, 223–230 (2019).
Giri, V. N. et al. Role of genetic testing for inherited prostate cancer risk: Philadelphia Prostate Cancer Consensus Conference 2017. J. Clin. Oncol. 36, 414–424 (2018).
Leongamornlert, D. et al. Frequent germline deleterious mutations in DNA repair genes in familial prostate cancer cases are associated with advanced disease. Br. J. Cancer 110, 1663–1672 (2014).
Maia, S. et al. The role of germline mutations in the BRCA1/2 and mismatch repair genes in men ascertained for early-onset and/or familial prostate cancer. Fam. Cancer 15, 111–121 (2016).
McNevin, C. S. et al. Pathogenic BRCA variants as biomarkers for risk in prostate cancer. Cancers 13, 5697 (2021).
Nicolosi, P. et al. Prevalence of germline variants in prostate cancer and implications for current genetic testing guidelines. JAMA Oncol. 5, 523 (2019).
Pomerantz, M. M. et al. The association between germline BRCA2 variants and sensitivity to platinum-based chemotherapy among men with metastatic prostate cancer. Cancer 123, 3532–3539 (2017).
Pritchard, C. C. et al. Inherited DNA-repair gene mutations in men with metastatic prostate cancer. N. Engl. J. Med. 375, 443–453 (2016).
Shah, S. et al. BRCA mutations in prostate cancer: assessment, implications and treatment considerations. Int. J. Mol. Sci. 22, 12628 (2021).
Telvizian, T. & Mukherji, D. Germline mutations and prostate cancer: is it time to change treatment algorithms? Chin. Clin. Oncol. 9, 65–65 (2020).
Zhen, J. T. et al. Genetic testing for hereditary prostate cancer: current status and limitations. Cancer 124, 3105–3117 (2018).
Robinson, D. et al. Integrative clinical genomics of advanced prostate cancer. Cell 161, 1215–1228 (2015).
Stenson, P. D. et al. The human gene mutation database: towards a comprehensive repository of inherited mutation data for medical research, genetic diagnosis and next-generation sequencing studies. Hum. Genet. 136, 665–677 (2017).
Tate, J. G. et al. COSMIC: the catalogue of somatic mutations in cancer. Nucleic Acids Res. 47, D941–D947 (2019).
Fullerton, S. M., Knerr, S. & Burke, W. Finding a place for genomics in health disparities research. Public Health Genomics 15, 156–163 (2012).
Telfah, M., Holzbeierlein, J. M., Shen, X., Wulff-Burchfield, E. M. & Parikh, R. A. Abiraterone acetate in comparison to enzalutamide in African American patients with metastatic castrate-resistant prostate cancer: a single-center retrospective study. J. Clin. Oncol. 37(suppl_15), e16547 (2019).
George, D. J. et al. Survival by race in men with chemotherapy-naive enzalutamide- or abiraterone-treated metastatic castration-resistant prostate cancer. Prostate Cancer Prostatic Dis. https://doi.org/10.1038/s41391-021-00463-9 (2021).
George, D. J. et al. A prospective trial of abiraterone acetate plus prednisone in Black and white men with metastatic castrate-resistant prostate cancer. Cancer 127, 2954–2965 (2021).
Bernard, B. et al. Impact of ethnicity on the outcome of men with metastatic, hormone-sensitive prostate cancer. Cancer 123, 1536–1544 (2017).
Oni-Orisan, A., Mavura, Y., Banda, Y., Thornton, T. A. & Sebro, R. Embracing genetic diversity to improve Black health. N. Engl. J. Med. https://doi.org/10.1056/NEJMms2031080 (2021).
Bergström, A. et al. Insights into human genetic variation and population history from 929 diverse genomes. Science 367, eaay5012 (2020).
Minas, T. Z., Kiely, M., Ajao, A. & Ambs, S. An overview of cancer health disparities: new approaches and insights and why they matter. Carcinogenesis https://doi.org/10.1093/carcin/bgaa121 (2020).
Na, R. et al. Germline mutations in ATM and BRCA1/2 distinguish risk for lethal and indolent prostate cancer and are associated with early age at death. Eur. Urol. 71, 740–747 (2017).
Author information
Authors and Affiliations
Contributions
C.A.G., J.O., I.W., P.L., S.P.B., B.A.H.A.A., F.R.S., D.E.S., C.B. and J.E.S. researched data for the article. All authors contributed substantially to discussion of the content. All authors wrote the article. All authors reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
D.E.S. reports personal fees from Boston Scientific, AstraZeneca, Janssen, and Blue Earth. J.E.S. is supported by the Frederick J. and Theresa Dow Foundation of the New York Community Trust, Vinney Scholars Award and a Damon Runyon Cancer Research Foundation Physician-Scientist Training Award. F.R.S. is supported by NCI CA2333216, CA043703, CA241956 and CA254566. The other authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Urology thanks S. Chanock and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Flatiron Health: https://flatiron.com/
Foundation Medicine, Inc: https://www.foundationmedicine.com/
Rights and permissions
About this article
Cite this article
Arenas-Gallo, C., Owiredu, J., Weinstein, I. et al. Race and prostate cancer: genomic landscape. Nat Rev Urol 19, 547–561 (2022). https://doi.org/10.1038/s41585-022-00622-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41585-022-00622-0
This article is cited by
-
Association between patient ethnicity and prostate cancer diagnosis following a prostate-specific antigen test: a cohort study of 730,000 men in primary care in the UK
BMC Medicine (2024)
-
Genetic and biological drivers of prostate cancer disparities in Black men
Nature Reviews Urology (2023)
-
Preclinical models of prostate cancer — modelling androgen dependency and castration resistance in vitro, ex vivo and in vivo
Nature Reviews Urology (2023)