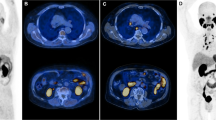
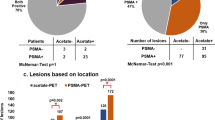
Abstract
Radiolabelled prostate-specific membrane antigen (PSMA)-based PET–CT has been shown in numerous studies to be superior to conventional imaging in the detection of nodal or distant metastatic lesions. 68Ga-PSMA PET–CT is now recommended by many guidelines for the detection of biochemically relapsed disease after radical local therapy. PSMA radioligands can also function as radiotheranostics, and Lu-PSMA has been shown to be a potential new line of treatment for metastatic castration-resistant prostate cancer. Whole-body (WB) MRI has been shown to have a high diagnostic performance in the detection and monitoring of metastatic bone disease. Prospective, randomized, multicentre studies comparing 68Ga-PSMA PET–CT and WB MRI for pelvic nodal and metastatic disease detection are yet to be performed. Challenges for interpretation of PSMA include tracer trapping in non-target tissues and also urinary excretion of tracers, which confounds image interpretation at the vesicoureteral junction. Additionally, studies have shown how long-term androgen deprivation therapy (ADT) affects PSMA expression and could, therefore, reduce tracer uptake and visibility of PSMA+ lesions. Furthermore, ADT of short duration might increase PSMA expression, leading to the PSMA flare phenomenon, which makes the accurate monitoring of treatment response to ADT with PSMA PET challenging. Scan duration, detection of incidentalomas and presence of metallic implants are some of the major challenges with WB MRI. Emerging data support the wider adoption of PSMA PET and WB MRI for diagnosis, staging, disease burden evaluation and response monitoring, although their relative roles in the standard-of-care management of patients are yet to be fully defined.
Key points
-
Next-generation imaging techniques have been found to affect prostate cancer disease state classifications as their increased sensitivity can result in stage migration.
-
Prostate-specific membrane antigen (PSMA) PET has been shown to have higher sensitivity and specificity in detecting nodal and metastatic lesions than conventional imaging. PSMA-derived tumour volume and total lesion PSMA are experimental quantitative volumetric measures for whole-body (WB) tumour burden with good prognostic value for progression-free survival and can be used in treatment response assessment.
-
177Lu-labelled PSMA is a potential new line of therapy in patients with metastatic castration-resistant prostate cancer who have progressed on at least one line of chemotherapy.
-
WB MRI is showing increasing promise as an ‘all-in-one’ modality for cancer diagnosis and staging without the need for radiation exposure. WB MRI-derived markers include apparent diffusion coefficient (ADC), signal fat fraction (sFF) and proton density fat fraction (PDFF). ADC values are especially useful for assessing bone metastases; PDFF and sFF are emerging quantitative imaging biomarkers that might be useful in assessing nodal and bone marrow metastases.
-
Limitations of PSMA PET include tracer trapping in non-target tissue, PSMA flare phenomenon, limited availability and radiation exposure related to radiotracers. Limitations of WB MRI include long acquisition time, metal-related and motion-related artefacts, fat–water swapping, incidentalomas, differential diagnoses of findings and limited availability.
-
Well-designed, powered, randomized multicentre studies are needed to assess the value of PSMA PET, WB MRI and standard imaging for disease detection, disease burden evaluation and survival across different prostate cancer disease states.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Ferlay, J. et al. Cancer Today (International Agency for Research on Cancer, 2018).
D’Amico, A. V. et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA 280, 969–974 (1998).
van Leeuwen, F. W. B. & van der Poel, H. G. Oligometastases: the art of providing metastases-directed therapy in prostate cancer. Nat. Rev. Urol. 19, 259–260 (2022).
Schatzl, G. et al. High-grade prostate cancer is associated with low serum testosterone levels. Prostate 47, 52–58 (2001).
Carceles-Cordon, M. et al. Cellular rewiring in lethal prostate cancer: the architect of drug resistance. Nat. Rev. Urol. 17, 292–307 (2020).
Artibani, W., Porcaro, A. B., De Marco, V., Cerruto, M. A. & Siracusano, S. Management of biochemical recurrence after primary curative treatment for prostate cancer: a review. Urol. Int. 100, 251–262 (2018).
Conteduca, V. et al. Flare phenomenon in prostate cancer: recent evidence on new drugs and next generation imaging. Ther. Adv. Med. Oncol. https://doi.org/10.1177/1758835920987654 (2021).
Taylor, S. A. et al. Diagnostic accuracy of whole-body MRI versus standard imaging pathways for metastatic disease in newly diagnosed non-small-cell lung cancer: the prospective Streamline L trial. Lancet Respir. Med. 7, 523–532 (2019).
Taylor, S. A. et al. Diagnostic accuracy of whole-body MRI versus standard imaging pathways for metastatic disease in newly diagnosed colorectal cancer: the prospective Streamline C trial. Lancet Gastroenterol. Hepatol. 4, 529–537 (2019).
Messiou, C. et al. Prospective evaluation of whole-body MRI versus FDG PET/CT for lesion detection in participants with myeloma. Radiol. Imaging Cancer 3, e210048 (2021).
Tikkinen, K. A. O. et al. Prostate cancer screening with prostate-specific antigen (PSA) test: a clinical practice guideline. BMJ 362, k3581 (2018).
Kohestani, K., Chilov, M. & Carlsson, S. V. Prostate cancer screening-when to start and how to screen? Transl. Androl. Urol. 7, 34–45 (2018).
O’Sullivan, J. M., Norman, A. R., Cook, G. J., Fisher, C. & Dearnaley, D. P. Broadening the criteria for avoiding staging bone scans in prostate cancer: a retrospective study of patients at the Royal Marsden Hospital. BJU Int. 92, 685–689 (2003).
Trabulsi, E. J. et al. Optimum imaging strategies for advanced prostate cancer: ASCO guideline. J. Clin. Oncol. 38, 1963–1996 (2020).
Hofman, M. S. et al. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): a prospective, randomised, multicentre study. Lancet 395, 1208–1216 (2020).
Ceci, F. et al. (68)Ga-PSMA PET/CT for restaging recurrent prostate cancer: which factors are associated with PET/CT detection rate? Eur. J. Nucl. Med. Mol. Imaging 42, 1284–1294 (2015).
Peng, L. et al. Can 68Ga-prostate specific membrane antigen positron emission tomography/computerized tomography provide an accurate lymph node staging for patients with medium/high risk prostate cancer? A diagnostic meta-analysis. Radiat. Oncol. 15, 227 (2020).
Petersen, L. J. & Zacho, H. D. PSMA PET for primary lymph node staging of intermediate and high-risk prostate cancer: an expedited systematic review. Cancer Imaging 20, 10 (2020).
Rahman, L. A. et al. High negative predictive value of 68Ga PSMA PET-CT for local lymph node metastases in high risk primary prostate cancer with histopathological correlation. Cancer Imaging 19, 86 (2019).
Johnston, E. W. et al. Multiparametric whole-body 3.0-T MRI in newly diagnosed intermediate- and high-risk prostate cancer: diagnostic accuracy and interobserver agreement for nodal and metastatic staging. Eur. Radiol. 29, 3159–3169 (2019).
Lecouvet, F. E. et al. Can whole-body magnetic resonance imaging with diffusion-weighted imaging replace Tc 99m bone scanning and computed tomography for single-step detection of metastases in patients with high-risk prostate cancer? Eur. Urol. 62, 68–75 (2012).
Kesch, C., Kratochwil, C., Mier, W., Kopka, K. & Giesel, F. L. 68Ga or 18F for prostate cancer imaging? J. Nucl. Med. 58, 687–688 (2017).
Raveenthiran, S. et al. The use of 68Ga-PET/CT PSMA to determine patterns of disease for biochemically recurrent prostate cancer following primary radiotherapy. Prostate Cancer Prostatic Dis. 22, 385–390 (2019).
Bluemel, C. et al. 68Ga-PSMA-PET/CT in patients with biochemical prostate cancer recurrence and negative 18F-choline-PET/CT. Clin. Nucl. Med. 41, 515–521 (2016).
Sawicki, L. M. et al. Prospective comparison of whole-body MRI and 68Ga-PSMA PET/CT for the detection of biochemical recurrence of prostate cancer after radical prostatectomy. Eur. J. Nucl. Med. Mol. Imaging 46, 1542–1550 (2019).
Adeleke, S. et al. Localising occult prostate cancer metastasis with advanced imaging techniques (LOCATE trial): a prospective cohort, observational diagnostic accuracy trial investigating whole-body magnetic resonance imaging in radio-recurrent prostate cancer. BMC Med. Imaging 19, 90 (2019).
Palma, D. A. et al. Stereotactic ablative radiotherapy for the comprehensive treatment of oligometastatic cancers: long-term results of the SABR-COMET phase II randomized trial. J. Clin. Oncol. 38, 2830–2838 (2020).
Ost, P. et al. Surveillance or metastasis-directed therapy for oligometastatic prostate cancer recurrence: a prospective, randomized, multicenter phase II trial. J. Clin. Oncol. 36, 446–453 (2018).
Chalkidou, A. et al. Stereotactic ablative body radiotherapy in patients with oligometastatic cancers: a prospective, registry-based, single-arm, observational, evaluation study. Lancet Oncol. 22, 98–106 (2021).
Phillips, R. et al. Outcomes of observation vs stereotactic ablative radiation for oligometastatic prostate cancer: the ORIOLE phase 2 randomized clinical trial. JAMA Oncol. 6, 650–659 (2020).
Guckenberger, M. et al. Characterisation and classification of oligometastatic disease: a European Society for Radiotherapy and Oncology and European Organisation for Research and Treatment of Cancer consensus recommendation. Lancet Oncol. 21, e18–e28 (2020).
Lievens, Y. et al. Defining oligometastatic disease from a radiation oncology perspective: an ESTRO-ASTRO consensus document. Radiother. Oncol. 148, 157–166 (2020).
Foster, C. C., Pitroda, S. P. & Weichselbaum, R. R. Definition, biology, and history of oligometastatic and oligoprogressive disease. Cancer J. 26, 96–99 (2020).
Sweeney, C. J. et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N. Engl. J. Med. 373, 737–746 (2015).
Lecouvet, F. E. et al. Use of modern imaging methods to facilitate trials of metastasis-directed therapy for oligometastatic disease in prostate cancer: a consensus recommendation from the EORTC Imaging Group. Lancet Oncol. 19, e534–e545 (2018).
Gillessen, S. et al. Management of patients with advanced prostate cancer: report of the Advanced Prostate Cancer Consensus Conference 2019. Eur. Urol. 77, 508–547 (2020).
Aggarwal, R. et al. Clinical and genomic characterization of low PSA secretors: a unique subset of metastatic castration resistant prostate cancer. Prostate Cancer Prostatic Dis. 24, 81–87 (2021).
Eisenhauer, E. A. et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur. J. Cancer 45, 228–247 (2009).
Scher, H. I. et al. Trial design and objectives for castration-resistant prostate cancer: updated recommendations from the Prostate Cancer Clinical Trials Working Group 3. J. Clin. Oncol. 34, 1402–1418 (2016).
Seitz, A. K. et al. Preliminary results on response assessment using 68Ga-HBED-CC-PSMA PET/CT in patients with metastatic prostate cancer undergoing docetaxel chemotherapy. Eur. J. Nucl. Med. Mol. Imaging 45, 602–612 (2018).
Blackledge, M. D. et al. Assessment of treatment response by total tumor volume and global apparent diffusion coefficient using diffusion-weighted MRI in patients with metastatic bone disease: a feasibility study. PLoS ONE 9, e91779 (2014).
Turpin, A. et al. Imaging for metastasis in prostate cancer: a review of the literature. Front. Oncol. 10, 55 (2020).
Messiou, C. et al. Guidelines for acquisition, interpretation, and reporting of whole-body MRI in myeloma: Myeloma Response Assessment and Diagnosis System (MY-RADS). Radiology 291, 5–13 (2019).
Maurer, T., Eiber, M., Schwaiger, M. & Gschwend, J. E. Current use of PSMA-PET in prostate cancer management. Nat. Rev. Urol. 13, 226–235 (2016).
van der Sar, E. C. A., van Kalmthout, L. M. & Lam, M. G. E. H. PSMA PET/CT in primary prostate cancer diagnostics: an overview of the literature. Tijdschr. Urol. 10, 101–108 (2020).
Eder, M. et al. Novel preclinical and radiopharmaceutical aspects of [68Ga]Ga-PSMA-HBED-CC: a new PET tracer for imaging of prostate cancer. Pharmaceuticals 7, 779–796 (2014).
Afshar-Oromieh, A. et al. PET imaging with a [68Ga]gallium-labelled PSMA ligand for the diagnosis of prostate cancer: biodistribution in humans and first evaluation of tumour lesions. Eur. J. Nucl. Med. Mol. Imaging 40, 486–495 (2013); erratum 40, 797–798 (2013).
Andaglia, G. et al. Distribution of metastatic sites in patients with prostate cancer: a population-based analysis. Prostate 74, 210–216 (2014).
Kamaleshwaran, K. K. et al. Predictive value of serum prostate specific antigen in detecting bone metastasis in prostate cancer patients using bone scintigraphy. Indian. J. Nucl. Med. 27, 81–84 (2012).
Guo, Y., Wang, L., Hu, J., Feng, D. & Xu, L. Diagnostic performance of choline PET/CT for the detection of bone metastasis in prostate cancer: a systematic review and meta-analysis. PLoS ONE 13, e0203400 (2018).
Lengana, T. et al. 68Ga-PSMA PET/CT replacing bone scan in the initial staging of skeletal metastasis in prostate cancer: a fait accompli? Clin. Genitourin. Cancer 16, 392–401 (2018).
von Eyben, F. E., Picchio, M., von Eyben, R., Rhee, H. & Bauman, G. 68Ga-labeled prostate-specific membrane antigen ligand positron emission tomography/computed tomography for prostate cancer: a systematic review and meta-analysis. Eur. Urol. Focus 4, 686–693 (2018).
Perera, M. et al. Gallium-68 prostate-specific membrane antigen positron emission tomography in advanced prostate cancer-updated diagnostic utility, sensitivity, specificity, and distribution of prostate-specific membrane antigen-avid lesions: a systematic review and meta-analysis. Eur. Urol. 77, 403–417 (2020).
Afshar-Oromieh, A. et al. Comparison of PET imaging with a (68)Ga-labelled PSMA ligand and (18)F-choline-based PET/CT for the diagnosis of recurrent prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 41, 11–20 (2014).
Morigi, J. J. et al. Prospective comparison of 18F-fluoromethylcholine versus 68Ga-PSMA PET/CT in prostate cancer patients who have rising PSA after curative treatment and are being considered for targeted therapy. J. Nucl. Med. 56, 1185–1190 (2015).
Glicksman, R. M. et al. [18F]DCFPyL PET-MRI/CT for unveiling a molecularly defined oligorecurrent prostate cancer state amenable for curative-intent ablative therapy: study protocol for a phase II trial. BMJ Open 10, e035959 (2020).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/record/NCT04742361?view=record (2021).
Aksu, A. et al. Evaluation of 68Ga-PSMA PET/CT with volumetric parameters for staging of prostate cancer patients. Nucl. Med. Commun. 42, 503–509 (2021).
Zou, Q. et al. Semi-automatic evaluation of baseline whole-body tumor burden as an imaging biomarker of 68Ga-PSMA-11 PET/CT in newly diagnosed prostate cancer. Abdom. Radiol. 45, 4202–4213 (2020).
Karyagar, S. S., Karyagar, S. & Guven, O. Correlations of the 68Ga-PSMA PET/CT derived primary prostate tumor PSMA expression parameters and metastatic patterns in patients with Gleason score >7 prostate cancer. Hell. J. Nucl. Med. 23, 120–124 (2020).
Yildirim, Ö. A. et al. Correlations between whole body volumetric parameters of 68Ga-PSMA PET/CT and biochemical-histopathological parameters in castration-naive and resistant prostate cancer patients. Ann. Nucl. Med 35, 540–548 (2021).
Acar, E. et al. The use of molecular volumetric parameters for the evaluation of Lu-177 PSMA I&T therapy response and survival. Ann. Nucl. Med. 33, 681–688 (2019).
Schmidkonz, C. et al. 68Ga-PSMA-11 PET/CT-derived metabolic parameters for determination of whole-body tumor burden and treatment response in prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 45, 1862–1872 (2018).
Michalski, K., Mix, M., Meyer, P. T. & Ruf, J. Determination of whole-body tumour burden on [68Ga]PSMA-11 PET/CT for response assessment of [177Lu]PSMA-617 radioligand therapy: a retrospective analysis of serum PSA level and imaging derived parameters before and after two cycles of therapy. Nuklearmedizin 58, 443–450 (2019).
Has Simsek, D. et al. Can PSMA-based tumor burden predict response to docetaxel treatment in metastatic castration-resistant prostate cancer? Ann. Nucl. Med. 35, 680–690 (2021).
Wahl, R. L., Jacene, H., Kasamon, Y. & Lodge, M. A. From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J. Nucl. Med. 50 (Suppl. 1), 122S–150S (2009).
Gupta, M., Choudhury, P. S., Rawal, S., Goel, H. C. & Rao, S. A. Evaluation of RECIST, PERCIST, EORTC, and MDA criteria for assessing treatment response with Ga68-PSMA PET-CT in metastatic prostate cancer patient with biochemical progression: a comparative study. Nucl. Med. Mol. Imaging 52, 420–429 (2018); erratum 54, 267 (2020).
Lawhn-Heath, C. et al. Prostate-specific membrane antigen PET in prostate cancer. Radiology 299, 248–260 (2021).
Ceci, F. et al. E-PSMA: the EANM standardized reporting guidelines v1.0 for PSMA-PET. Eur. J. Nucl. Med. Mol. Imaging 48, 1626–1638 (2021).
Vierasu, I. et al. Clinical experience with 18F-JK-PSMA-7 when using a digital PET/CT. Eur. J. Hybrid Imaging 6, 6 (2022).
Bodar, Y. J. L. et al. Prospective analysis of clinically significant prostate cancer detection with [18F]DCFPyL PET/MRI compared to multiparametric MRI: a comparison with the histopathology in the radical prostatectomy specimen, the ProStaPET study. Eur. J. Nucl. Med. Mol. Imaging 49, 1731–1742 (2022).
Krohn, T. et al. [(68)Ga]PSMA-HBED uptake mimicking lymph node metastasis in coeliac ganglia: an important pitfall in clinical practice. Eur. J. Nucl. Med. Mol. Imaging 42, 210–214 (2015).
Bialek, E. J. & Malkowski, B. Celiac ganglia: can they be misinterpreted on multimodal 68Ga-PSMA-11 PET/MR? Nucl. Med. Commun. 40, 175–184 (2019).
de Galiza Barbosa, F. et al. Nonprostatic diseases on PSMA PET imaging: a spectrum of benign and malignant findings. Cancer Imaging 20, 23 (2020).
Gykiere, P., Goethals, L. & Everaert, H. Healing sacral fracture masquerading as metastatic bone disease on a 68Ga-PSMA PET/CT. Clin. Nucl. Med. 41, e346–e347 (2016).
Kanthan, G. L. et al. Follicular thyroid adenoma showing avid uptake on 68Ga PSMA-HBED-CC PET/CT. Clin. Nucl. Med. 41, 331–332 (2016).
Radzina, M. et al. Accuracy of 68Ga-PSMA-11 PET/CT and multiparametric MRI for the detection of local tumor and lymph node metastases in early biochemical recurrence of prostate cancer. Am. J. Nucl. Med. Mol. Imaging 10, 106–118 (2020).
Iravani, A. et al. 68Ga PSMA-11 PET with CT urography protocol in the initial staging and biochemical relapse of prostate cancer. Cancer Imaging 17, 31 (2017).
Kroenke, M. et al. Matched-pair comparison of 68Ga-PSMA-11 and 18F-rhPSMA-7 PET/CT in patients with primary and biochemical recurrence of prostate cancer: frequency of non-tumor-related uptake and tumor positivity. J. Nucl. Med. 62, 1082–1088 (2021).
Dietlein, F. et al. Intraindividual comparison of 18F-PSMA-1007 with renally excreted PSMA ligands for PSMA PET imaging in patients with relapsed prostate cancer. J. Nucl. Med. 61, 729–734 (2020).
Ghadanfer, L., Usmani, S., Marafi, F., Al-Kandari, F. & Rasheed, R. Incremental value of post diuretic 68Ga-PSMA-11 PET-CT in characterization of indeterminate lesions in prostate cancer. Asian Pac. J. Cancer Prev. 21, 3719–3723 (2020).
Morawitz, J. et al. Is there a diagnostic benefit of late-phase abdomino-pelvic PET/CT after urination as part of whole-body 68Ga-PSMA-11 PET/CT for restaging patients with biochemical recurrence of prostate cancer after radical prostatectomy? EJNMMI Res. 12, 12 (2022).
Afshar-Oromieh, A. et al. The clinical impact of additional late PET/CT imaging with 68Ga-PSMA-11 (HBED-CC) in the diagnosis of prostate cancer. J. Nucl. Med. 58, 750–755 (2017).
Hoffmann, M. A. et al. Dual-time point [68Ga]Ga-PSMA-11 PET/CT hybrid imaging for staging and restaging of prostate cancer. Cancers 12, 2788 (2020).
Perveen, G. et al. Role of early dynamic positron emission tomography/computed tomography with 68Ga-prostate-specific membrane antigen-HBED-CC in patients with adenocarcinoma prostate: initial results. Indian. J. Nucl. Med. 33, 112–117 (2018).
Uprimny, C. et al. Early dynamic imaging in 68Ga-PSMA-11 PET/CT allows discrimination of urinary bladder activity and prostate cancer lesions. Eur. J. Nucl. Med. Mol. Imaging 44, 765–775 (2017).
Freitag, M. T. et al. Local recurrence of prostate cancer after radical prostatectomy is at risk to be missed in 68Ga-PSMA-11-PET of PET/CT and PET/MRI: comparison with mpMRI integrated in simultaneous PET/MRI. Eur. J. Nucl. Med. Mol. Imaging 44, 776–787 (2017).
Burger, I. A. et al. 68Ga-PSMA-11 PET/MR detects local recurrence occult on mpMRI in prostate cancer patients after HIFU. J. Nucl. Med. 60, 1118–1123 (2019).
Wright, G. L. Jr et al. Upregulation of prostate-specific membrane antigen after androgen-deprivation therapy. Urology 48, 326–334 (1996).
Evans, M. J. et al. Noninvasive measurement of androgen receptor signaling with a positron-emitting radiopharmaceutical that targets prostate-specific membrane antigen. Proc. Natl Acad. Sci. USA 108, 9578–9582 (2011).
Hope, T. A. et al. 68Ga-PSMA-11 PET imaging of response to androgen receptor inhibition: first human experience. J. Nucl. Med. 58, 81–84 (2017).
Murga, J. D. et al. Synergistic co-targeting of prostate-specific membrane antigen and androgen receptor in prostate cancer. Prostate 75, 242–254 (2015).
Afshar-Oromieh, A. et al. Impact of long-term androgen deprivation therapy on PSMA ligand PET/CT in patients with castration-sensitive prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 45, 2045–2054 (2018).
Afshar-Oromieh, A. et al. Diagnostic performance of (68)Ga-PSMA-11 (HBED-CC) PET/CT in patients with recurrent prostate cancer: evaluation in 1007 patients. Eur. J. Nucl. Med. Mol. Imaging 44, 1258–1268 (2017).
Emmett, L. et al. Rapid modulation of PSMA expression by androgen deprivation: serial 68Ga-PSMA-11 PET in men with hormone-sensitive and castrate-resistant prostate cancer commencing androgen blockade. J. Nucl. Med. 60, 950–954 (2019).
Plouznikoff, N. et al. Evaluation of PSMA expression changes on PET/CT before and after initiation of novel antiandrogen drugs (enzalutamide or abiraterone) in metastatic castration-resistant prostate cancer patients. Ann. Nucl. Med. 33, 945–954 (2019).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03876912 (2021).
Hofman, M. S., Hicks, R. J., Maurer, T. & Eiber, M. Prostate-specific membrane antigen PET: clinical utility in prostate cancer, normal patterns, pearls, and pitfalls. Radiographics 38, 200–217 (2018).
Rischpler, C. et al. 68Ga-PSMA-HBED-CC uptake in cervical, celiac, and sacral ganglia as an important pitfall in prostate cancer PET imaging. J. Nucl. Med. 59, 1406–1411 (2018).
UK Health Security Agency. Radiation Protection Services. UKHSA https://www.phe-protectionservices.org.uk/radiationandyou/ (2022).
Siva, S. et al. Expanding the role of small-molecule PSMA ligands beyond PET staging of prostate cancer. Nat. Rev. Urol. 17, 107–118 (2020).
Hofman, M. S. et al. TheraP trial investigators and the Australian and New Zealand Urogenital and Prostate Cancer Trials Group. [177Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): a randomised, open-label, phase 2 trial. Lancet 397, 797–804 (2021).
Sartor, O. et al. Lutetium-177-PSMA-617 for metastatic castration-resistant prostate cancer. N. Engl. J. Med. https://doi.org/10.1056/NEJMoa2107322 (2021).
O’ Connor, M. Transformative prostate cancer therapy ‘should not be accepted’ without PET imaging. HealthImaging https://www.healthimaging.com/topics/medical-imaging/molecular-imaging/prostate-cancer-therapy-not-be-accepted-without-pet (2021).
Ormond Filho, A. G. et al. Whole-body imaging of multiple myeloma: diagnostic criteria. Radiographics 39, 1077–1097 (2019).
Pasoglou, V., Michoux, N., Tombal, B. & Lecouvet, F. Optimising TNM staging of patients with prostate cancer using WB-MRI. J. Belg. Soc. Radiol. 100, 101 (2016).
Van Damme, J. et al. Comparison of 68Ga-prostate specific membrane antigen (PSMA) positron emission tomography computed tomography (PET-CT) and whole-body magnetic resonance imaging (WB-MRI) with diffusion sequences (DWI) in the staging of advanced prostate cancer. Cancers 13, 5286 (2021).
Lecouvet, F. E. et al. Shortening the acquisition time of whole-body MRI: 3D T1 gradient echo Dixon vs fast spin echo for metastatic screening in prostate cancer. Eur. Radiol. 30, 3083–3093 (2020).
Bitar, R. et al. MR pulse sequences: what every radiologist wants to know but is afraid to ask. Radiographics 26, 513–537 (2006).
Padhani, A. R. et al. METastasis Reporting and Sata System for Prostate Cancer: practical guidelines for acquisition, interpretation, and reporting of whole-body magnetic resonance imaging-based evaluations of multiorgan involvement in advanced prostate cancer. Eur. Urol. 71, 81–92 (2017).
Pricolo, P. et al. Whole-body magnetic resonance imaging (WB-MRI) reporting with the METastasis Reporting and Data System for Prostate Cancer (MET-RADS-P): inter-observer agreement between readers of different expertise levels. Cancer Imaging 20, 77 (2020).
Harisinghani, M. G. et al. Ferumoxtran-10-enhanced MR lymphangiography: does contrast-enhanced imaging alone suffice for accurate lymph node characterization? AJR Am. J. Roentgenol. 186, 144–148 (2006).
Heesakkers, R. A. et al. MRI with a lymph-node-specific contrast agent as an alternative to CT scan and lymph-node dissection in patients with prostate cancer: a prospective multicohort study. Lancet Oncol. 9, 850–856 (2008).
Thoeny, H. C. et al. Combined ultrasmall superparamagnetic particles of iron oxide-enhanced and diffusion-weighted magnetic resonance imaging reliably detect pelvic lymph node metastases in normal-sized nodes of bladder and prostate cancer patients. Eur. Urol. 55, 761–769 (2009).
Heesakkers, R. A. et al. Prostate cancer: detection of lymph node metastases outside the routine surgical area with ferumoxtran-10-enhanced MR imaging. Radiology 251, 408–414 (2009).
Meijer et al. High occurrence of aberrant lymph node spread on magnetic resonance lymphography in prostate cancer patients with a biochemical recurrence after radical prostatectomy. Int. J. Radiat. Oncol. Biol. Phys. 82, 1405–1410 (2012).
Fortuin, A. S. et al. Value of PET/CT and MR lymphography in treatment of prostate cancer patients with lymph node metastases. Int. J. Radiat. Oncol. Biol. Phys. 84, 712–718 (2012).
Schilham, M. G. M. et al. Head-to-head comparison of 68Ga-prostate-specific membrane antigen PET/CT and ferumoxtran-10-enhanced MRI for the diagnosis of lymph node metastases in prostate cancer patients. J. Nucl. Med. 62, 1258–1263 (2021).
Daldrup-Link, H. E. Ten things you might not know about iron oxide nanoparticles. Radiology 284, 616–629 (2017).
Li, C. S. et al. Enhancement characteristics of ultrasmall superparamagnetic iron oxide particle within the prostate gland in patients with primary prostate cancer. J. Comput. Assist. Tomogr. 32, 523–528 (2008).
Fukuda, Y. et al. Superparamagnetic iron oxide (SPIO) MRI contrast agent for bone marrow imaging: differentiating bone metastasis and osteomyelitis. Magn. Reson. Med. Sci. 5, 191–196 (2006).
Padhani, A. R. et al. Rationale for modernising imaging in advanced prostate cancer. Eur. Urol. Focus 3, 223–239 (2017).
Patterson, D. M., Padhani, A. R. & Collins, D. J. Technology insight: water diffusion MRI–a potential new biomarker of response to cancer therapy. Nat. Clin. Pract. Oncol. 5, 220–233 (2008).
Padhani, A. R. et al. Therapy monitoring of skeletal metastases with whole-body diffusion MRI. J. Magn. Reson. Imaging 39, 1049–1078 (2014).
Perez-Lopez, R. et al. Diffusion-weighted imaging as a treatment response biomarker for evaluating bone metastases in prostate cancer: a pilot study. Radiology 283, 168–177 (2017).
Reischauer, C. et al. Bone metastases from prostate cancer: assessing treatment response by using diffusion-weighted imaging and functional diffusion maps–initial observations. Radiology 257, 523–531 (2010).
Jacobs, M. A. et al. Multiparametric whole-body MRI with diffusion-weighted imaging and ADC mapping for the identification of visceral and osseous metastases from solid tumors. Acad. Radiol. 25, 1405–1414 (2018).
Dong, H. et al. Prediction of early treatment response in multiple myeloma using MY-RADS total burden score, ADC, and fat fraction from whole-body MRI: impact of anemia on predictive performance. AJR Am. J. Roentgenol. 218, 310–319 (2022).
Gross, B. H., Glazer, G. M., Orringer, M. B., Spizarny, D. L. & Flint, A. Bronchogenic carcinoma metastatic to normal-sized lymph nodes: frequency and significance. Radiology 166, 71–74 (1988).
Ganeshalingam, S. & Koh, D. M. Nodal staging. Cancer Imaging 9, 104–111 (2009).
Adeleke S. et al. Fat-fraction provides classification and treatment response assessment of metastatic lymph nodes for patients with radio-recurrent prostate cancer [abstract]. UCL https://discovery.ucl.ac.uk/id/eprint/10078201/1/Fat%20fraction%20abstract-Montreal%202019.pdf (2019).
Appayya, M. B. et al. Quantitative mDixon fat fraction can differentiate metastatic nodes from benign nodes in prostate cancer patients. Proc. Int. Soc. Mag. Reson. Med. 26, 0721 (2018).
Cho, S. Y. et al. Biodistribution, tumor detection, and radiation dosimetry of 18F-DCFBC, a low-molecular-weight inhibitor of prostate-specific membrane antigen, in patients with metastatic prostate cancer. J. Nucl. Med. 53, 1883–1891 (2012).
Kwack, K. S., Lee, H. D., Jeon, S. W., Lee, H. Y. & Park, S. Comparison of proton density fat fraction, simultaneous R2*, and apparent diffusion coefficient for assessment of focal vertebral bone marrow lesions. Clin. Radiol. 75, 123–130 (2020).
Food and Drug Administration. Understanding MRI safety labeling. FDA https://www.fda.gov/media/101221/download (2020).
Evans, R. E. C. et al. Patient deprivation and perceived scan burden negatively impact the quality of whole-body MRI. Clin. Radiol. 75, 308–315 (2020).
Cancer Research UK & UCL Cancer Trials Centre. Whole-body MRI can save money and stress, according to CTC study. CRUK https://www.ctc.ucl.ac.uk/ViewNews.aspx?Item=73 (2019).
Cieszanowski, A. et al. Non-contrast-enhanced whole-body magnetic resonance imaging in the general population: the incidence of abnormal findings in patients 50 years old and younger compared to older subjects. PLoS ONE 9, e107840 (2014).
Tarnoki, D. L. et al. Clinical value of whole-body magnetic resonance imaging in health screening of general adult population. Radiol. Oncol. 49, 10–16 (2015).
Hegenscheid, K. et al. Potentially relevant incidental findings on research whole-body MRI in the general adult population: frequencies and management. Eur. Radiol. 23, 816–826 (2013).
Poustchi-Amin, M., Mirowitz, S. A., Brown, J. J., McKinstry, R. C. & Li, T. Principles and applications of echo-planar imaging: a review for the general radiologist. Radiographics 21, 767–779 (2001).
Schallmo, M. P., Weldon, K. B., Burton, P. C., Sponheim, S. R. & Olman, C. A. Assessing methods for geometric distortion compensation in 7T gradient echo functional MRI data. Hum. Brain Mapp. 42, 4205–4223 (2021).
Donato, F. Jr et al. Geometric distortion in diffusion-weighted MR imaging of the prostate–contributing factors and strategies for improvement. Acad. Radiol. 21, 817–823 (2014).
Dixon, W. T. Simple proton spectroscopic imaging. Radiology 153, 189–194 (1984).
Glocker, B. et al. in Medical Image Computing and Computer-Assisted Intervention - MICCAI 2016. Lecture Notes in Computer Science Vol. 9902 (eds Ourselin, S., Joskowicz, L., Sabuncu,M., Unal, G. & Wells, W.) 536–543 (Springer, 2016).
Kirchgesner, T. et al. Two-point Dixon fat-water swapping artifact: lesion mimicker at musculoskeletal T2-weighted MRI. Skelet. Radiol. 49, 2081–2086 (2020).
Ladefoged, C. N. et al. Impact of incorrect tissue classification in Dixon-based MR-AC: fat-water tissue inversion. EJNMMI Phys. 1, 101 (2014).
Bray, T. J. P., Chouhan, M. D., Punwani, S., Bainbridge, A. & Hall-Craggs, M. A. Fat fraction mapping using magnetic resonance imaging: insight into pathophysiology. Br. J. Radiol. 90, 20170344 (2017).
Sciarra, A. et al. Magnetic resonance spectroscopic imaging (1H-MRSI) and dynamic contrast-enhanced magnetic resonance (DCE-MRI): pattern changes from inflammation to prostate cancer. Cancer Invest. 28, 424–432 (2010).
Cheng, Y., Zhang, X., Ji, Q. & Shen, W. Xanthogranulomatous prostatitis: multiparametric MRI appearances. Clin. Imaging 38, 755–757 (2014).
Suditu, N. & Negru, D. Bacillus Calmette-Guérin therapy-associated granulomatous prostatitis mimicking prostate cancer on MRI: a case report and literature review. Mol. Clin. Oncol. 3, 249–251 (2015).
Lecouvet, F. E. et al. Monitoring the response of bone metastases to treatment with magnetic resonance imaging and nuclear medicine techniques: a review and position statement by the European Organisation for Research and Treatment of Cancer imaging group. Eur. J. Cancer 50, 2519–2531 (2014).
Małkiewicz, A. & Dziedzic, M. Bone marrow reconversion–imaging of physiological changes in bone marrow. Pol. J. Radiol. 77, 45–50 (2012).
Yu, Y. S. et al. False-positive diagnosis of disease progression by magnetic resonance imaging for response assessment in prostate cancer with bone metastases: a case report and review of the pitfalls of images in the literature. Oncol. Lett. 10, 3585–3590 (2015).
Tanaka, T. et al. A case of focal bone marrow reconversion mimicking bone metastasis: the value of 111indium chloride. Acta Med. Okayama 70, 285–289 (2016).
Mottet, N. et al. EAU–EANM–ESTRO–ESUR–ISUP–SIOG guidelines on prostate cancer 2022. EAU https://uroweb.org/guidelines/prostate-cancer (2022).
Sundahl, N., Gillessen, S., Sweeney, C. & Ost, P. When what you see is not always what you get: raising the bar of evidence for new diagnostic imaging modalities. Eur. Urol. 79, 565–567 (2021).
Rodnick, M. E. et al. Cyclotron-based production of 68Ga, [68Ga]GaCl3, and [68Ga]Ga-PSMA-11 from a liquid target. EJNMMI Radiopharm. Chem. 5, 25 (2020).
Block Imaging. PET/CT price guide. Block Imaging https://info.blockimaging.com/bid/68875/how-much-does-a-pet-ct-scanner-cost (2021).
LBN Medical. How much does an MRI scanner cost: a complete overview. LBN Medical https://lbnmedical.com/how-much-does-an-mri-machine-cost/ (2021).
Qin, C. et al. Sustainable low-field cardiovascular magnetic resonance in changing healthcare systems. Eur. Heart J. Cardiovasc. Imaging https://doi.org/10.1093/ehjci/jeab286 (2022).
NHS. National cost collection for the NHS. NHS https://www.england.nhs.uk/national-cost-collection/ (2020).
de Feria Cardet, R. E. et al. Is prostate-specific membrane antigen positron emission tomography/computed tomography imaging cost-effective in prostate cancer: an analysis informed by the proPSMA trial. Eur. Urol. 79, 413–418 (2021).
Farolfi, A. et al. Positron emission tomography and whole-body magnetic resonance imaging for metastasis-directed therapy in hormone-sensitive oligometastatic prostate cancer after primary radical treatment: a systematic review. Eur. Urol. Oncol. 4, 714–730 (2021).
Dyrberg, E. et al. 68Ga-PSMA-PET/CT in comparison with 18F-fluoride-PET/CT and whole-body MRI for the detection of bone metastases in patients with prostate cancer: a prospective diagnostic accuracy study. Eur. Radiol. 29, 1221–1230 (2019).
Stecco, A. et al. Whole-body MRI with diffusion-weighted imaging in bone metastases: a narrative review. Diagnostics 8, 45 (2018).
Giesel, F. L. et al. PSMA PET/CT with glu-urea-Lys-(Ahx)-[68Ga(HBED-CC)] versus 3D CT volumetric lymph node assessment in recurrent prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 42, 1794–1800 (2015).
Pernthaler, B. et al. A prospective head-to-head comparison of 18F-fluciclovine with 68Ga-PSMA-11 in biochemical recurrence of prostate cancer in PET/CT. Clin. Nucl. Med. 44, e566–e573 (2019).
Emmett, L. et al. Prospective, multisite, international comparison of 18F-fluoromethylcholine PET/CT, multiparametric MRI, and 68Ga-HBED-CC PSMA-11 PET/CT in men with high-risk features and biochemical failure after radical prostatectomy: clinical performance and patient outcomes. J. Nucl. Med. 60, 794–800 (2019).
Calais, J. et al. 18F-fluciclovine PET-CT and 68Ga-PSMA-11 PET-CT in patients with early biochemical recurrence after prostatectomy: a prospective, single-centre, single-arm, comparative imaging trial. Lancet Oncol. 20, 1286–1294 (2019).
Schwenck, J. et al. Comparison of 68Ga-labelled PSMA-11 and 11C-choline in the detection of prostate cancer metastases by PET/CT. Eur. J. Nucl. Med. Mol. Imaging 44, 92–101 (2017).
Dietlein, M. et al. Comparison of [(18)F]DCFPyL and [(68)Ga]Ga-PSMA-HBED-CC for PSMA-PET imaging in patients with relapsed prostate cancer. Mol. Imaging Biol. 17, 575–584 (2015).
Szabo, Z. et al. Initial evaluation of [(18)F]DCFPyL for prostate-specific membrane antigen (PSMA)-targeted PET imaging of prostate cancer. Mol. Imaging Biol. 17, 565–574 (2015).
Pienta, K. J. et al. A phase 2/3 prospective multicenter study of the diagnostic accuracy of prostate specific membrane antigen PET/CT with 18F-DCFPyL in prostate cancer patients (OSPREY). J. Urol. 206, 52–61 (2021).
Morris, M. J. et al. Diagnostic performance of 18F-DCFPyL-PET/CT in men with biochemically recurrent prostate cancer: results from the CONDOR phase III, multicenter study. Clin. Cancer Res. 27, 3674–3682 (2021).
Behr, S. C. et al. Phase I study of CTT1057, an 18F-labeled imaging agent with phosphoramidate core targeting prostate-specific membrane antigen in prostate cancer. J. Nucl. Med. 60, 910–916 (2019).
Turkbey, B. et al. 18F-DCFBC prostate-specific membrane antigen-targeted PET/CT imaging in localized prostate cancer: correlation with multiparametric MRI and histopathology. Clin. Nucl. Med. 42, 735–740 (2017).
Harmon, S. A. et al. A prospective comparison of 18F-sodium fluoride PET/CT and PSMA-targeted 18F-DCFBC PET/CT in metastatic prostate cancer. J. Nucl. Med. 59, 1665–1671 (2018).
Mena, E. et al. Clinical impact of PSMA-based 18F-DCFBC PET/CT imaging in patients with biochemically recurrent prostate cancer after primary local therapy. Eur. J. Nucl. Med. Mol. Imaging 45, 4–11 (2018).
Rowe, S. P. et al. 18F-DCFBC PET/CT for PSMA-based detection and characterization of primary prostate cancer. J. Nucl. Med. 56, 1003–1010 (2015).
Hoberück, S. et al. Dual-time-point 64Cu-PSMA-617-PET/CT in patients suffering from prostate cancer. J. Labelled Comp. Radiopharm. 62, 523–532 (2019).
Cantiello, F. et al. Comparison between 64Cu-PSMA-617 PET/CT and 18F-choline PET/CT imaging in early diagnosis of prostate cancer biochemical recurrence. Clin. Genitourin. Cancer 16, 385–391 (2018).
Giesel, F. L. et al. Detection efficacy of 18F-PSMA-1007 PET/CT in 251 patients with biochemical recurrence of prostate cancer after radical prostatectomy. J. Nucl. Med. 60, 362–368 (2019).
Malaspina, S. et al. Prospective comparison of 18F-PSMA-1007 PET/CT, whole-body MRI and CT in primary nodal staging of unfavourable intermediate- and high-risk prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 49, 2670–2671 (2021).
Alberts, I. et al. Comparing the clinical performance and cost efficacy of [68Ga]Ga-PSMA-11 and [18F]PSMA-1007 in the diagnosis of recurrent prostate cancer: a Markov chain decision analysis. Eur. J. Nucl. Med. Mol. Imaging https://doi.org/10.1007/s00259-021-05620-9 (2021).
Witkowska-Patena, E., Giżewska, A., Miśko, J. & Dziuk, M. 18F-prostate-specific membrane antigen 1007 and 18F-FCH PET/CT in local recurrence of prostate cancer. Clin. Nucl. Med. 44, e401–e403 (2019).
Derlin, T. et al. PSA-stratified detection rates for [68Ga]THP-PSMA, a novel probe for rapid kit-based 68Ga-labeling and PET imaging, in patients with biochemical recurrence after primary therapy for prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 45, 913–922 (2018).
Afaq, A. et al. A Phase II, open-label study to assess safety and management change using 68Ga-THP PSMA PET/CT in patients with high risk primary prostate cancer or biochemical recurrence after radical treatment: the PRONOUNCED study. J. Nucl. Med. 62, 1727–1734 (2021).
Kulkarni, M. et al. The management impact of 68gallium-tris(hydroxypyridinone) prostate-specific membrane antigen (68Ga-THP-PSMA) PET-CT imaging for high-risk and biochemically recurrent prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 47, 674–686 (2020).
Schmuck et al. Multiple time-point 68Ga-PSMA I&T PET/CT for characterization of primary prostate cancer. Clin. Nucl. Med. 42, e286–e293 (2017).
Asokendaran, M. E., Meyrick, D. P., Skelly, L. A., Lenzo, N. P. & Henderson, A. Gallium-68 prostate-specific membrane antigen positron emission tomography/computed tomography compared with diagnostic computed tomography in relapsed prostate cancer. World J. Nucl. Med. 18, 232–237 (2019).
Oh, S. W. et al. Quantitative and qualitative analyses of biodistribution and PET image quality of a novel radiohybrid PSMA, 18F-rhPSMA-7, in patients with prostate cancer. J. Nucl. Med. 61, 702–709 (2020).
Hohberg, M. et al. Biodistribution and radiation dosimetry of [18F]-JK-PSMA-7 as a novel prostate-specific membrane antigen-specific ligand for PET/CT imaging of prostate cancer. EJNMMI Res. 9, 66 (2019).
Dietlein, F. et al. [18F]-JK-PSMA-7 PET/CT under androgen deprivation therapy in advanced prostate cancer. Mol. Imaging Biol. 23, 277–286 (2021).
Piron, S. et al. Radiation dosimetry and biodistribution of 18F-PSMA-11 for PET imaging of prostate cancer. J. Nucl. Med. 60, 1736–1742 (2019).
Piron, S. et al. Optimization of PET protocol and interrater reliability of 18F-PSMA-11 imaging of prostate cancer. EJNMMI Res. 10, 14 (2020).
Schottelius, M. et al. Synthesis and preclinical characterization of the PSMA-targeted hybrid tracer PSMA-I&F for nuclear and fluorescence imaging of prostate cancer. J. Nucl. Med. 60, 71–78 (2019).
Depardon, E. et al. FDG PET/CT for prognostic stratification of patients with metastatic breast cancer treated with first line systemic therapy: comparison of EORTC criteria and PERCIST. PLoS ONE 13, e0199529 (2018).
Author information
Authors and Affiliations
Contributions
Y.W., J.R.G., S.W. and S.A. researched data for the article. All authors contributed substantially to discussion of the content. Y.W., J.R.G., S.A. and V.K. wrote the article. Y.W., J.R.G., A.H., S.W., H.P., S.A. and V.K. reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Urology thanks the anonymous reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Echo-planar imaging
-
(EPI). An MRI pulse sequence in which data for the entire image is collected following a single radiofrequency excitation. It has the advantage of rapid image acquisition but with poor resolution.
- B0
-
The B0 in MRI refers to the main static magnetic field (scanner magnetic field) used to polarize spins and is measured in teslas. The majority of MRI systems in clinical use are 1.5 T or 3 T.
Rights and permissions
About this article
Cite this article
Wang, Y., Galante, J.R., Haroon, A. et al. The future of PSMA PET and WB MRI as next-generation imaging tools in prostate cancer. Nat Rev Urol 19, 475–493 (2022). https://doi.org/10.1038/s41585-022-00618-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41585-022-00618-w
This article is cited by
-
Ontwikkelingen in de behandeling van gemetastaseerd hormoongevoelig prostaatcarcinoom
Tijdschrift voor Urologie (2024)
-
MRI fat fraction imaging of nodal and bone metastases in prostate cancer
European Radiology (2023)