Abstract
Penile cancer is a rare genitourinary malignancy that is associated with poor outcomes and severely limited therapeutic options that are generally non-curative when used to treat localized disease with high-risk features or advanced disease. To address the unmet need for treatment modalities with increased effectiveness, immune-based therapies such as immune-checkpoint blockade, human papilloma virus (HPV)-directed vaccines and adoptive T cell therapies have emerged as potential treatment options for advanced penile cancer. A diverse array of immune cells such as cytotoxic T lymphocytes (CTLs), tumour-associated macrophages and myeloid-derived suppressor cells have been shown to infiltrate penile cancer tumours, with distinct immune landscapes being demonstrated in HPV-positive compared with HPV-negative tumours. Study results have also demonstrated the prognostic value of immune cells such as tumour-associated macrophages, immune markers such as programmed death ligand-1, and HPV-status in penile cancer. Taken together, these findings underscore the clinical relevance of the tumour immune microenvironment as a source of both prognostic indicators and potential therapeutic targets for immune-based therapies. Current evidence regarding the safety and efficacy of immune-based therapies is limited in penile cancer, but a number of clinical and preclinical studies are ongoing to evaluate these therapies in this disease based on promising results from studies in other malignancies, including other squamous cell carcinomas. In addition, an opportunity exists to combine immune-based therapies with existing lines of systemic therapy to offer the most benefit to patients with advanced penile cancer. Future work should focus on expansion of preclinical models for immune-based drug discovery.
Key points
-
The immune landscape of penile cancer is defined by unique patterns of immune cell infiltration that also serve as prognostic indicators of metastasis and survival.
-
Human papilloma virus (HPV) infection status can be used to stratify patients into two groups with differing tumour immune microenvironments (TIMEs) based on key markers such as programmed death-ligand 1.
-
Immune-based therapies including immune-checkpoint blockade, adoptive T cell therapies, and HPV-targeting therapeutic vaccines are each promising candidate therapies, although these treatments are largely unexplored in penile cancer; however, they are currently being evaluated prospectively.
-
The optimal management of locally advanced penile cancer might involve a multimodal approach that combines immune-based therapies with chemotherapeutic and/or targeted agents early in the disease course followed by surgery.
-
Preclinical models that will improve understanding of the TIME and the mechanisms underlying responses to immune-based therapies are needed.
-
In this rare disease context, future preclinical and clinical work on immune-based therapies will benefit from the centralization of care and the pooling of collaborative scientific knowledge and resources.
Similar content being viewed by others
Introduction
Penile cancer is a rare disease that disproportionately affects men in specific parts of the world, including regions in South Africa, South-Central Asia and most of South America1,2. Human papilloma virus (HPV) infection is a major risk factor for developing penile cancer, and results of a meta-analysis published in 2019 demonstrated that 50.8% of patients with penile cancer are positive for HPV;3,4 other risk factors include lack of circumcision, phimosis, smoking and socioeconomic disadvantages5,6,7,8,9,10.
Surgery alone can be effective in treating localized disease, but is usually non-curative in patients with considerable inguinal or pelvic lymph node involvement11. Depending on the location, the laterality, number and presence of the extranodal extension (ENE) of positive lymph nodes, survival outcomes substantially vary and can be very poor in patients with advanced disease12,13,14,15. In fact, 5-year overall survival (OS) among patients with pelvic or bilateral inguinal positive nodes is only 10–20%16. Thus, based on the node size, number and whether the nodes are fixed or mobile, patients with positive lymph nodes are often recommended to receive neoadjuvant chemotherapy (NAC) followed by lymph node dissection per National Comprehensive Cancer Network (NCCN)17 and European Association of Urology (EAU) guidelines18. In this regard, a bilateral approach yielding an increased number of nodes leads to improved outcomes. For example, a retrospective analysis of 364 patients with penile cancer who underwent lymph node dissection demonstrated improved 5-year overall survival among those who had >15 versus ≤15 nodes removed (67% versus 49%, P = 0.008)19,20,21. Notably, owing to the rarity of penile cancer, guidelines on disease management are largely based on low-level evidence from limited studies within this disease22; thus, higher-level evidence from prospective studies in other squamous cell carcinomas (SCCs) is sometimes used to support practice guidelines for penile cancer. For example, data on the use of chemoradiotherapy in penile cancer are scarce; therefore, data from phase II/III studies in vulvar and anal SCCs are cited by NCCN guidelines as evidence for its potential use in the treatment of locally advanced or metastatic disease in certain instances17.
Current NCCN guidelines17 recommending four cycles of neoadjuvant paclitaxel, ifosfamide and cisplatin (TIP) for locally advanced disease mainly rely on the results of a single-arm, prospective phase II study of the TIP regimen for penile cancer in the neoadjuvant setting for evidence23. However, results of a subsequent pooled meta-analysis of ten studies on taxane-based NAC for penile cancer demonstrated an objective response rate of 53%24, suggesting an unmet need to both predict non-responders to NAC and develop effective neoadjuvant therapy combinations and subsequent lines of systemic treatment for patients with chemo-refractory disease, as well as those who are ineligible for cisplatin-based chemotherapy25. Similarly, adjuvant chemotherapy is recommended for certain patients with considerable residual disease or ENE at the time of inguinal lymph node dissection (ILND), but this recommendation is based on results of a small number of retrospective studies26,27,28. Patients with disease with high-risk features such as ENE, bilateral positive inguinal nodes, positive pelvic nodes, and lymph node tumours measuring >4 cm can be offered adjuvant chemoradiotherapy, which has shown some efficacy in vulvar SCC29, as an alternative to TIP. For patients with relapsed or metastatic disease in whom outcomes are dismal30, results of prospective studies of chemotherapy show only moderate efficacy and/or high toxic effects with median overall survival of 5 months31,32,33,34,35. Thus, an unmet need remains for effective systemic therapies across the full spectrum of advanced penile cancer36.
In an effort to address some of these challenges and provide higher-level evidence for treatment guidelines for locally advanced disease than is currently available, the International Penile Advanced Cancer Trial (InPACT; NCT02305654) was launched in 2017 to evaluate chemotherapy and chemoradiotherapy in both the neoadjuvant and adjuvant settings37. Specifically, this study will enrol and randomize 200 patients to one of three arms: ILND alone; NAC plus ILND; or neoadjuvant chemoradiotherapy followed by ILND. If patients who have received previous neoadjuvant chemoradiotherapy have disease with high-risk features following surgery, they will either receive a pelvic lymph node dissection or will receive active surveillance imaging as per the NCCN guidelines. If patients who either received an ILND alone or NAC plus ILND have disease with high-risk features, they will receive adjuvant chemoradiotherapy or a pelvic lymph node dissection. This study is designed to help to determine the optimal sequencing of chemotherapy, chemoradiotherapy and surgery, as well as examine the utility of chemoradiotherapy in penile squamous cell carcinoma (PSCC), particularly given its efficacy in other SCCs38,39,40,41.
Interest in defining the immune landscape of penile cancer and developing immune-based therapies has grown. Currently, the recommended use of immune-based therapy per NCCN guidelines is limited to immune-checkpoint inhibitor therapy in the form of second-line pembrolizumab for patients with relapsed or metastatic disease that is unresectable, microsatellite instability high, or mismatch repair deficient17. Prospective evidence is lacking, but studies of the immune landscape provide support for further investigating immune-checkpoint inhibitor therapy for penile cancer42,43. Furthermore, HPV-positive and HPV-negative penile tumours exhibit distinct molecular characteristics, making HPV a focal point in tumour profiling and developing novel lines of immune-based treatments, such as therapeutic vaccines44.
In this Review, studies investigating the immune landscape of penile cancer are summarized, existing and novel immune-based therapeutic targets are examined, and the future directions of immune-based therapies in penile cancer based on preclinical and clinical studies are explored.
The tumour immune microenvironment in penile cancer
In any malignancy, understanding the underlying biological processes that drive and correlate with the disease is fundamental in enabling the development of effective therapies. In penile cancer, characterizing dysregulated genes and genetic pathways, defining the immune landscape and understanding the complex interactions within the tumour microenvironment (TME) will create opportunities for new treatment strategies that rely on taking advantage of these genetic and immune-related processes.
The genetic landscape of penile cancer
Given the rarity of penile cancer, the molecular aetiology of this disease was not well understood — especially for HPV-negative penile cancers. Molecular studies, such as whole-exome sequencing, genome-wide methylation arrays and DNA copy number alteration arrays, have been performed to characterize the genetic landscape of penile cancer44,45. These techniques have been used to distinguish the molecular mechanisms underlying HPV-positive and HPV-negative PSCC, identify potential novel drug targets and determine whether these genetic alterations have prognostic value. Frequently altered genes in penile cancer (such as NOTCH1, CDK2NA and PI3KCA), the frequent mutual exclusivity of TP53 mutations and HPV-positive status, and altered molecular pathways in PSCC that might contribute to tumorigenesis and disease progression such as the DNA damage response and mTOR pathways have been discovered46,47,48,49,50,51,52,53,54,55,56,57,58. Given that some of the current recommendations for PSCC treatment are extrapolated from other SCCs, molecular comparisons between PSCC and other SCCs might yield important information regarding the utility of novel immune-based therapies in penile cancer. For example, a whole-exome sequencing analysis of 34 primary PSCC tissue samples showed that the tumour mutational burden (TMB) of penile cancer is significantly lower than that of lung squamous carcinoma (P < 0.0001) and bladder carcinoma (P = 0.001), whereas it is comparable with the TMB of cervical, oesophageal, and head and neck squamous cell carcinoma (HNSCC)58. A higher TMB is associated with an improved response to immune-based therapies59. Comparison of mutational signatures between PSCC and other SCCs showed considerable molecular similarities between PSCC, HNSCC and oesophageal SCC. Specifically, the PSCC tumours were enriched for mutational signatures that were constructed using cophenetic analysis from the other squamous carcinomas analysed. These signatures matched closely to either the SBS2 (APOBEC cytidine deaminase activity) or SBS6 (defective DNA mismatch repair) signatures within the Catalogue of Somatic Mutations in Cancer (COSMIC) database. In this analysis, PSCC, HNSC and oesophageal SCC shared a similar distribution of these mutational signatures within each tumour-type cohort58. At the present time, these findings help to group penile cancer with other SCCs and might inspire studies that use therapies that have been successful in treating molecularly similar SCCs for the treatment of penile cancer.
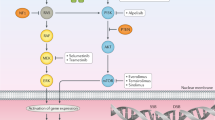
Overall, these comparisons have improved our understanding of PSCC and enabled the identification of frequently mutated genes and molecular pathways with existing targeted therapy agents. These studies were limited to small retrospective cohorts, but they have an important role in advancing our understanding of the molecular drivers of penile cancer. However, further work must be done to validate these studies and determine whether these molecular findings are targetable by therapy. Thus, within the context of immune-based therapies, understanding the specific role of the immune system in penile cancer is first necessary in order to properly assess the potential utility of immune-based therapies in this disease. In tandem, characterization of the genetic changes and cellular processes that drive penile cancer must continue to fully understand the tumour biology of penile cancer. Taken together, an advanced knowledge of both the genetic and immune landscapes of penile cancer and the interplay between the two will inform how to best use immune-based therapies in this disease (Fig. 1).
Defining the penile squamous cell carcinoma (PSCC) immune landscape for human papilloma virus (HPV)-positive and HPV-negative tumours might be important as the implications are not currently fully understood. In HPV-positive tumours, recruitment of CD8+ cytotoxic T cells and CD4+ T helper cells to the TME might be enhanced owing to HPV-antigen presentation on MHC-class-I molecules. Given that HPV-negative tumours more frequently express PD-L1 than HPV-positive ones69,99, they could be associated with a more immunosuppressive TME. However, differences in tumour infiltration by CD8+ cytotoxic T cells between HPV-positive and HPV-negative penile cancers have not been observed69. Notably, HPV-positive tumours have been shown to have greater densities of intratumoural CD163+ macrophages than HPV-negative tumours75, suggesting the existence of differing immunosuppressive mechanisms in HPV-positive versus HPV-negative tumours. CTL, cytotoxic T lymphocyte; MDSC, myeloid-derived suppressor cell; NK cell, natural killer cell; PDL1, programmed death-ligand 1; TME, tumour microenvironment.
Tumour-infiltrating immune cells in penile cancer
Components of the innate and adaptive immune systems infiltrate the TME in many solid tumours and can suppress or promote tumour progression60,61. For example, CD8+ cytotoxic T cells, CD4+ T helper cells and natural killer (NK) cells generally demonstrate antitumour activity, but other tumour-infiltrating immune cells — such as regulatory T (Treg) cells, tumour-associated macrophages (TAMs) and myeloid-derived suppressor cells (MDSCs) — usually dampen the antitumour immune response62,63,64,65. Given that several of these immune cells are present in the tumour immune microenvironment (TIME), the specific pattern of immune infiltration is an important indicator of whether the immune system is working against or synergistically with the tumour62.
Multiple studies66,67,68 have been conducted to characterize tumour-infiltrating lymphocytes (TILs) and other immune cell populations in penile cancer and determine whether these populations correlate with specific prognostic outcomes owing to the prognostic value of tumour-infiltrating immune cells in other SCCs. For example, evaluation of the presence and prognostic value of CD8+ cytotoxic T cells, FOXP3+ Treg cells and TAMs in 213 patients with penile cancer demonstrated that a low stromal CD8+ cytotoxic T cell count was significantly associated with lymph node metastasis (LNM) incidence on univariate analysis (OR 0.60, CI 0.37–0.98; P = 0.04)69. A high level of intratumoural CD163+ TAMs, which are M2-type macrophages associated with poor survival in lung squamous carcinoma70 and oral squamous cell carcinoma71 as well as advanced histopathological features in HNSCC72, were also found to be significantly associated with LNM incidence on univariate analysis (OR 2.45, CI 1.35–4.43; P = <0.01)69. However, in a study in which CD206 was used as a marker of M2-type macrophages73, the results demonstrated that a high density of intratumoural CD206+ macrophages was associated with improved disease-specific survival (DSS) on univariate analysis (OR 0.76, 95% CI 0.62–0.94; P = 0.01)74. Additionally, a high density of stromal or intratumoural CD206+ macrophages was associated with improved DSS on univariate Cox regression analysis when using optimal cut-off points (HR 0.446, 95% CI 0.24–0.81; P = 0.008 and HR 0.35, 95% CI 0.19–0.63; P = 0.001, respectively)74. The optimal cut-off points were 4.38 and 0.85 for stromal and intratumoural CD206+ macrophages, respectively, and were determined using the ‘survminer’ R package.
In light of these contradictory findings about the prognostic value of M2-type macrophages, a spatial approach was used to determine whether the localization of CD14+, CD68+ and CD163+ cells relative to the tumour contributed to differing associations with clinicopathological features75. Upon pooling HPV-negative and high-risk HPV-positive specimens, a significant positive correlation was found between increased tumour grade and the infiltration of CD14+, CD68+ and CD163+ cells in the intratumoural and peritumoural compartments. Additionally, in the high-risk HPV-positive subgroup, CD68+ or CD163+ cells in the peritumoural compartment was significantly associated with LNM (P = 0.031 and P = 0.026, respectively). The association between CD163+ cells and LNM in this study is concordant with the findings of another study69; although it must be noted that both studies69,75 probably derived their respective sample cohorts from the same group of patients treated at the Netherlands Cancer Institute (Amsterdam, Netherlands), leading to similar findings between the two studies. In one study75, the presence of CD163+ cells in the peritumoural compartment also correlated with increased tumour stage (P = 0.030) and increased tumour grade (P = 0.021) within the high-risk HPV-positive subgroup. However, a multiple covariates model did not reveal a significant association between the densities of CD14+, CD68+ or CD163+ cells and DSS in the HPV-negative or the high-risk HPV-positive subgroups. Collectively, these studies suggest a complex relationship between macrophages and penile cancer. This relationship is probably further complicated by the high plasticity of macrophages and the limitations associated with classifying macrophages as either pro-inflammatory or immunosuppressive based on single markers such as CD206 or CD163 (ref.76). Future single-cell analysis will need to be conducted to improve understanding of the role of each macrophage subtype at each stage of disease.
Neither stromal nor intratumoural FOXP3+ Treg cells showed prognostic value in one study69; however, in another study, presence of FOXP3+ lymphocytes in the peri-tumoural inflammatory infiltrate was shown to be independently associated with poor 5-year DSS (HR 2.50, 95% CI 0.79–7.92; P = 0.02)77. However, results of a study involving 178 patients with invasive penile cancer showed that a high density of stromal FOXP3+ Treg cells was associated with improved DSS on multivariate analysis (HR 0.74, 95% CI 0.58–0.94; P = 0.013)74. These results are contradictory, but these findings support existing evidence that suggests that FOXP3+ can be a predictor of either good or poor prognosis depending on the cancer type, and perhaps in this case, in the broader TIME context78. Overall, similar to the limitations surrounding our knowledge of the role of macrophages, the role of FOXP3+ Treg cells in the penile TIME needs be further examined in order to determine their role in this disease.
MDSCs are well-established immunosuppressors in the TIME79. MDSCs are a broad range of phenotypically heterogeneous myeloid-derived cell populations that lack the ability to terminally differentiate into monocytes, macrophages and dendritic cells80. The phenotypic diversity of MDSCs makes them difficult to study, as they can express a wide range of cell-surface markers that can sometimes overlap between different MDSC populations81. MDSCs express a diverse array of cell-surface markers, but can frequently be defined as CD11b+ and/or CD33+ and/or HLA-DR-myeloid-derived cells that can be stimulated in the TME to produce immunosuppressive factors such as reactive oxygen species and arginase80,82. Within the context of penile cancer, MDSCs (CD11b+ Gr1+) have been shown to account for 50% of all immune cells in a mouse model of penile cancer versus only 1% in non-malignant penile tissue (P < 0.01)83. Notably, significant reductions in CD8+ T cells (P < 0.05), NK cells (P < 0.05), B cells (P < 0.01) and CD68+ TAMs (P < 0.05) as a total percentage of all immune cells in the same tumours have been demonstrated, suggesting an immunosuppressive TME lacking these immune cell types that are involved in the antitumour immune response. Indeed, the observed reduction in CD8+ T cells and NK cells could possibly be driven by the downstream immunosuppressive effects of MDSC activity such as reactive oxygen species-driven T cell unresponsiveness84, arginase-driven T cell suppression85, induction of FOXP3+ Treg cells86 and nitric oxide-driven NK-cell suppression87. Together, these findings suggest a need to further define MDSC populations in human-derived penile cancer tumour samples alongside other immune cell populations in the TIME, and examine potential mechanisms by which MDSC populations might exert immunosuppressive effects on other immune cells in the TIME. In addition, increased numbers of MDSCs have been shown to correlate with poor clinicopathological features, dampen response to chemotherapy and immune-checkpoint blockade (ICB), and serve as indicators of worse prognosis across multiple cancer types, suggesting a need to evaluate the role of MDSCs in penile cancer88,89,90,91.
Overall, these studies collectively suggest that TILs and other immune cells could have prognostic value within the setting of penile cancer and are relevant to our understanding of the role of the innate and adaptive immune systems in the TME (Table 1). Knowledge of the role of the immune components of penile tumour tissue and stroma is still in its infancy and the capacity to manipulate these immune components to advance treatment strategies and clinical outcomes is limited. To overcome these limitations and increase knowledge, multicentre, multinational tissue, blood, urine and stool collection cores should be developed with clear collection protocols and advanced integrated immune, molecular and viral testing should be undertaken. Establishing such pivotal resources will help to overcome many of the current limitations that scientists and clinical investigators tackling this disease currently face.
The PD1–PDL1 axis in penile cancer
Within the context of cancer, the programmed cell death protein (PD1) and programmed death-ligand 1 (PDL1) axis is crucial in the immunosuppression that results from the interaction of the PD1 receptor on CD8+ cytotoxic T cells with its ligand PDL1 on tumour cell membranes92,93,94. The PD1–PDL1 axis has a key role in tumour immune evasion and, therefore, blockade of this interaction via ICB using anti-PD1 and anti-PDL1 therapies has become a front-line treatment approach across multiple cancer types95,96,97.
Despite the currently limited use of ICB in penile cancer, several studies have been conducted to characterize PDL1 expression in this disease. In one study, 69.2% of patients with LNM demonstrated PDL1 positivity in the primary tumour, whereas results of another study demonstrated PDL1 positivity in 40% of the primary tumour samples analysed42,43. Overall, the current literature suggests a PDL1 positivity between 40% and 69% among primary penile cancer samples42,43,74,98,99.
The prognostic value of PDL1 expression in penile cancer has also been investigated. In a study involving 213 patients with penile cancer, diffuse PDL1 expression in primary tumour tissue was positively associated with the presence of LNM (OR 2.81, CI 1.01–7.81; P = 0.05) and was predictive of worse DSS on multivariate analysis (HR 2.78, CI 1.10–6.98; P = 0.03) compared with tumours with negative or margin PDL1 expression69. Additionally, results of another study demonstrated a similar result among 178 primary penile cancer samples, in which diffuse tumoural PDL1 expression correlated significantly with worse DSS on multivariate analysis compared with tumours with negative or marginal expression of PDL1 (HR 2.38, 95% CI 1.11–5.10; P = 0.025)74. Finally, another study including 200 primary penile cancer samples showed a significant association between diffuse tumoural PDL1 expression and both a higher incidence of LNM and worse DSS (HR 2.58; P = 0.04) on multivariate analysis99. However, in contrast to these studies, two studies did not show a significant correlation between PDL1 positivity and increased incidence of LNM and death42,43. Of note, a positive association was observed between PDL1 positivity and LNM in one of these studies but was not statistically significant (50% of patients with PDL1-positive disease had LNM versus 23.5% of those with PDL1-negative disease, P = 0.164)42. The lack of statistical significance might be a result of the small sample size in the study (n = 35 patients)42. In another study, no association was observed between PDL1 positivity and LNM. Fewer patients (n = 53) were involved in this study than others in which a significantly positive correlation between PDL1 positivity and LNM was reported.
Overall, these studies collectively suggest a positive correlation between PDL1 positivity and LNM incidence, as well as a negative correlation between PDL1 positivity and DSS. These findings underscore the prognostic value of PDL1-positive status as an indicator of worse prognosis in penile cancer and suggest a need for studies that examine the role of therapies targeting the PD1–PDL1 axis.
With respect to PDL1 positivity and its correlative relationship with other clinicopathological features, in one study, a statistically significantly positive association between primary tumours with usual-type histology and PDL1-positive status (P = 0.040) was found, whereas none of the primary tumours exhibiting warty-type or verrucous-type histology were PDL1 positive (P < 0.001)98. This observation is notable, as tumours of warty-type or verrucous-type histology are associated with better outcomes than those with usual-type histology18, further highlighting the positive association between PDL1 positivity and worse prognosis in penile cancer. With respect to histological or pathological grade and stage, no significant positive association has been observed between PDL1 positivity and increased histological or pathological grade and stage.
Overall, the current literature suggests that PDL1 is frequently expressed in primary penile cancer tissue and is associated with poor prognosis, including worse survival and a greater incidence of LNM. These data highlight the clinical utility and relevance of the PD1–PDL1 axis in the management of penile cancer.
HPV status and the tumour immune microenvironment
According to the WHO guidelines, penile cancers are classified as either HPV related or HPV unrelated100. HPV-related penile cancers mainly include the basaloid, warty-basaloid and warty histological subtypes, whereas HPV-unrelated penile cancers frequently include the usual, verrucous, papillary and sarcomatoid subtypes100. Penile intraepithelial neoplasia — the precursor lesions of penile cancer — are classified as HPV related or HPV unrelated100. In a meta-analysis involving 4,199 patients with penile cancer, the results showed that 50.8% of the total cohort was HPV positive3. In addition, the majority of HPV-positive tumours were histologically HPV-related penile cancers, with 72.7% of HPV-related tumours being HPV positive versus only 19.4% of HPV-unrelated tumours being HPV positive. Nearly one-third (32.2%) of the usual histological subtype of penile cancers in this cohort were HPV positive; this observation is notable considering that 48–65% of PSCCs are of the usual histological subtype101. Additionally, 79.8% of patients with penile intraepithelial neoplasia were HPV positive in this meta-analysis3. Results of a 2018 meta-analysis of seven studies including 649 patients with penile cancer revealed a significantly improved DSS among HPV-positive versus HPV-negative patients with a pooled HR of 0.61 (95% CI, 0.38–0.98)102. Results from one study showed that patients with HPV-positive PSCC had a longer median OS (P = 0.015) following perioperative radiotherapy than patients with HPV-negative PSCC103. Overall, these data suggest the high relevance of HPV pathogenesis and how it relates to penile cancer.
The distinction between HPV-related and HPV-unrelated tumours largely stems from the unique role that HPV can have in penile cancer carcinogenesis44,104. Specifically, the viral E6 and E7 oncoproteins disrupt cell-cycle regulation and enable autonomous cell proliferation104; the E7 oncoprotein disrupts the cell cycle by binding to and inactivating the human retinoblastoma (Rb) protein105, whereas the E6 oncoprotein induces P53 degradation106,107. This disruption causes neoplasia; furthermore, the resulting downstream upregulation of cell-cycle signalling and downregulation of apoptotic signalling leads to an accumulation of the tumour suppressor cyclin-dependent kinase inhibitor 2A (CDKN2A), which is now used as a surrogate immunohistochemical marker for an active, high-risk HPV infection108,109,110. In contrast, HPV-unrelated penile cancers have been shown to harbour P53 mutations with little CDKN2A expression104.
Thus, owing to these histological and molecular differences, studies have been conducted to delineate the TIMEs in HPV-positive and HPV-negative tumours, as well as to further elucidate the prognostic effect of HPV status. For example, in a study of 200 primary penile cancers, PDL1 positivity was observed significantly more frequently in HPV-negative tumours than in high-risk HPV-positive tumours (P = 0.03)99. The prognostic effect of diffuse tumoural PDL1 expression on DSS was greater in HPV-negative than in HPV-positive tumours (HR 3.92 versus HR 2.58)99. In another study, incidence of PDL1 positivity in HPV-negative tumours was greater than in HPV-positive tumours (P = 0.03)69. Diffuse PDL1 expression (HR 5.03, CI 1.81–13.99; P < 0.01) and LNM (HR 82.22, CI 14.99–450.90; P < 0.01) were predictive of worse DSS on multivariate subgroup analysis of patients negative for HPV. In addition, patients negative for HPV had worse DSS (HR 9.73, CI 2.12–44.72; P < 0.01) than patients with high-risk HPV-positive disease. Overall, these data might suggest a greater degree of immunosuppression and T cell exhaustion in HPV-negative tumours than in HPV-positive tumours, perhaps contributing to the worse survival outcomes observed69. Regarding survival, results of a study showed worse DSS in patients with HPV-negative tumours than those with high-risk HPV-positive disease, with 18% of HPV-negative patients succumbing to their disease versus only 2% of HPV-positive patients (P = 0.038)75, which is concordant with previous studies showing worse outcomes among HPV-negative patients108,111,112.
With respect to the relationship between immune infiltration and HPV-status, densities of CD8, Granzyme B (GrB), FOXP3, PD1, CTLA4, CD68 (M1-type TAMs), CD206 (M2-type TAMs), PDL1 and Siglec-15, between HPV-positive and HPV-negative tumours have been compared74; greater densities of intratumoural PD1 (P = 0.002) and stromal GrB (P = 0.049) were observed in HPV-positive tumours, with no significant differences observed across the remainder of the immune marker panel74. Similarly, no significant differences in stromal or intratumoural numbers of CD163+ (M2-type) TAMs, CD8+ T cells, FOXP3+ Treg cells, or the ratio of CD8+ T cells to FOXP3+ Treg cells was found in another study69. Interestingly, spatial distribution of different myeloid cells was explored in a cohort of 103 patients and greater densities of intratumoural CD14+, CD68+ and CD163+ cells in high-risk HPV-positive tumours than in HPV-negative tumours (P < 0.001 for all three cell types) were observed. Similarly, CD14+ and CD68+ cells were found in greater numbers in the peritumoural compartment of high-risk HPV-positive tumours than in HPV-negative tumours (P = 0.004 and P = 0.026, respectively)75. These findings suggest considerably enhanced infiltration of myeloid cell populations in HPV-positive tumours compared with HPV-negative tumours. However, because this study did not identify specific myeloid cell subtypes, ascertaining whether these myeloid cells are immunosuppressive or immunostimulatory is difficult. Thus, further studies to characterize these myeloid cell subtypes are warranted.
Overall, these data show some differences in immune markers between HPV-positive and HPV-negative tumours, but whether greater tumoural activated immune cell infiltration occurs in HPV-positive tumours than in HPV-positive tumours is unclear. Rather, the increased expression of PDL1 in HPV-negative penile cancers and the improved survival observed in patients with HPV-positive tumours might collectively suggest an immunostimulatory capacity of high-risk HPV infection, with its absence enabling increased tumour-mediated immunosuppression and immune evasion.
Collectively, these studies have improved our understanding of the TIME in penile cancer. For example, TILs and other immune cells are actively present in the TME in both HPV-negative and HPV-positive penile cancers. Furthermore, the relative expression of these immune cells — such as CD8+ cytotoxic T cells, FOXP3+ Treg cells and TAMs — have considerable prognostic value with respect to LNM and DSS. These markers could be helpful in prognostication and maybe for treatment selection in the future. Similarly, PDL1 is frequently observed in penile cancer samples, suggesting that immunosuppression probably has a key role in tumour immune evasion and disease progression, and offers the possibility of targeting this immune modulator. In addition, the relative expression and spatial distribution of PDL1 is an independent prognostic indicator, particularly in HPV-negative disease. However, understanding of the TIME remains incomplete. First, owing to the reliance on small retrospective studies, the predictive value of these immune components has not been determined. Second, high-throughput studies that enable visualization of the immune microenvironment at increased resolution — such as single-cell RNA-sequencing, proteomics and metabolomics — are ongoing but have not yet been published. Finally, the paucity of cell culture and animal models of penile cancer limit the ability to study the real-time interplay between immune cells and the TME in live-cell, dynamic contexts.
Immune-based therapies in penile cancer
Immune-based therapies are seldom used as standard of care in the management of penile cancer. Based on current understanding of the TIME in penile cancer, an unmet need for immune-based therapies in penile cancer exists and these therapies could be relevant in the setting of penile cancer (Fig. 2).
Currently, multiple promising options exist for current and future immune-based therapies for penile squamous cell carcinoma (PSCC). a | Immune checkpoint blockade (ICB) via anti-PDL1, anti-PD1, or anti-CTLA4 agents either alone or in combination. b | Human papilloma virus (HPV)-targeting therapeutic vaccines to elicit immune responses against HPV-positive tumours. c | Adoptive T cell therapies to enhance T cell-mediated destruction of tumours, such as tumour-infiltrating lymphocyte (TIL) therapy, chimeric antigen receptor therapy (CAR-T) and T cell receptor (TCR) therapy, in combination with preparative lymphodepletion using chemotherapy or full-body radiotherapy. d | Combination therapy approaches with ICB that either strengthen the antitumour immune response through enhanced tumour-associated antigen release via chemotherapy, stimulate the immune system via an HPV-directed vaccine, or inhibit the activity of immune cells that dampen the immune response (such as myeloid-derived suppressor cells (MDSCs)). CTL, cytotoxic T lymphocyte; CTLA4, cytotoxic T lymphocyte-associated protein 4; PD1, programmed cell death protein 1; PDK1, pyruvate dehydrogenase kinase 1; PDL1, programmed death ligand 1; PI3K, phosphoinositide 3-kinase; PKB, protein kinase B; TKI, tyrosine kinase inhibitor.
Immune checkpoint blockade
Anti-PD1, anti-PDL1 and anti-CTLA4 inhibitors have become a mainstay among contemporary systemic treatment modalities and have been approved by the FDA for a range of metastatic SCCs such as HNSCC, oesophageal SCC, squamous non-small-cell lung cancer (NSCLC) and cutaneous SCC97. However, in the USA and in Europe, ICB is not currently approved for use in the neoadjuvant setting for these SCCs but several trials are ongoing for SCCs such as HNSCC and NSCLC113,114.
In penile cancer, ICB approval is limited to the second-line setting in patients with relapsed or metastatic disease. To date, prospective studies on ICB use in penile cancer have only investigated these inhibitors for a very small number of patients in the setting of relapsed and/or metastatic disease115,116,117,118. For example, in a basket trial (in which an investigational drug is tested across multiple malignancies) a combined regimen of nivolumab (anti-PD1) and ipilimumab (anti-CTLA4) was investigated and included five patients with penile cancer115. None of these patients responded to the combined regimen, with 2/5 patients exhibiting stable disease and 3/5 patients exhibiting progressive disease115. In another trial, three patients with penile cancer who progressed on TIP were treated with pembrolizumab (anti-PD1), two of whom progressed after 3 months and one patient with a microsatellite instability-high tumour exhibited a partial response116. In a single case report, a patient with HPV-negative, p16-negative advanced chemoradiation-refractory penile cancer showed a response to nivolumab (<80% reduction in tumour volume), as well as an increase in CD8+ TILs and a decrease in tumoural PDL1 expression117. In another case report of including two patients with chemotherapy-refractory metastatic disease, a durable complete response at 38 months was reported in one patient and a partial response at 18 months was seen in the second patient118. Currently, interest in using ICB to address the unmet treatment need for locally advanced, relapsed and/or metastatic penile cancer is growing; therefore, a number of ongoing clinical trials are being conducted to investigate ICB in the first-line setting for unresectable disease in combination with chemotherapy (NCT04224740 (ref.119)), progressive disease after platinum-based chemotherapy (NCT03391479 (ref.120)) and maintenance therapy following chemotherapy (NCT03774901 (ref.121)). In addition, NCT03686332 (ref.122) is being undertaken to examine ICB in combination with radiotherapy for unresectable disease. ICB combined with either radiotherapy (that is, immunoradiotherapy) or chemoradiotherapy has been investigated in a broad range of other malignancies, including HNSCC122. In the neoadjuvant setting, results of a phase I study of SBRT plus nivolumab in 10 patients with HPV-associated HNSCC or cancer of unknown primary origin demonstrated a pathological complete response (pCR) in 90% of patients with no grade 4 toxic effects. However, when combining chemoradiotherapy with ICB for HNSCC, early results have been mixed with concerns raised surrounding tolerability, and most studies are still ongoing123,124. In general, the rationale underlying the combination of immune-based therapies with radiotherapy is a potentially enhanced antitumour immune response owing to irradiation of the tumour, as well as increased sensitization of the tumour to radiation with ICB, which has been demonstrated in preclinical models125,126,127. Thus, within the context of penile cancer, a combination therapy approach involving ICB plus radiotherapy has the rationale to be explored in ongoing and future trials. Furthermore, a number of basket trials in genitourinary malignancies and other SCCs in general are also being undertaken (Table 2).
Outcomes associated with recurrent and metastatic penile cancer are dismal30; thus, aggressive management early in the disease course is vital to minimize the chance of recurrence and improve survival outcomes. NAC is currently recommended in NCCN and EAU guidelines for locally advanced disease17,18, but only approximately half of patients who are eligible for chemotherapy benefit from it24. In addition, research has demonstrated a lack of adherence to guidelines on chemotherapy for penile cancer by less-experienced physicians and among physicians who have limited experience directly providing chemotherapy128. In addition, community medical practices that lack resources might have difficulty administering the TIP regimen because of the need to administer TIP over a 5-day period owing to the inclusion of ifosfamide, often in the inpatient setting. In this context, ICB could have promise as an alternative or a complementary agent to TIP, in which anti-PD1 or anti-PDL1 therapies can be a substitute for ifosfamide. This substitution would enable taxol, cisplatin and anti-PD1 or anti-PDL1 therapies to be delivered in the outpatient setting in a single day. The safety and efficacy of this combination approach was demonstrated in the setting of squamous NSCLC in the phase III KEYNOTE-407 trial, in which patients receiving platinum-based chemotherapy in combination with pembrolizumab demonstrated a significant overall survival benefit compared with patients receiving platinum-based chemotherapy plus placebo (15.3 months versus 11.3 months, respectively, P < 0.001). No significant difference in grade 3 or higher adverse events was observed between the two groups129.
Overall, the use of ICB leads to fewer adverse effects than chemotherapy; in fact, a meta-analysis of 22 trials, including a total of 12,727 patients with solid organ malignancies comparing the incidence of adverse events between ICBs (anti-CTLA4, anti-PDL1 and anti-PD1) and standard-of-care chemotherapy revealed that patients receiving ICBs reported fewer grade 3 or higher adverse events, were less likely to die secondary to an adverse event and were less likely to discontinue treatment130. Thus, ICB might be a preferred modality in patients with penile cancer who might have an increased risk of chemotherapy-related toxic effects and a limited capacity to manage potential adverse effects. However, ICBs notably carry a risk of ICB-specific, immune-related adverse events (irAEs)131,132,133. Results of a meta-analysis of irAEs related to anti-PD1 and anti-PDL1 therapies in 20,128 patients revealed that the most frequent all-grade irAEs observed were diarrhoea (9.5%), an increase in aspartate aminotransferase (AST) (3.4%), and vitiligo (3.3%). In addition, the most frequent grade 3 or higher irAEs were an increase in AST (0.75%), an increase in alanine aminotransferase (ALT) (0.70%) and pneumonitis (0.67%)132. Of note, results show that a greater proportion of patients experience irAEs following treatment with anti-CTLA4 agents than anti-PD1 or anti-PDL1 agents133,134,135, suggesting that anti-PD1 and anti-PDL1 agents are safer ICB therapies than anti-CTLA4 agents. Moreover, given the high frequency at which primary penile cancer lesions express PDL1 — especially HPV-negative tumours — and the overall worse prognosis associated with diffuse tumoural PDL1 expression, the rationale for the use of anti-PDL1 and anti-PD1 therapies in penile cancer might be strong, especially in the neoadjuvant setting in patients who have PDL1-positive and/or HPV-negative disease. Evidence supporting the use of neoadjuvant ICB has emerged from the phase II NEOSTAR trial in NSCLC, in which 44 patients with resectable disease were treated with either nivolumab alone or in combination with ipilimumab136. In this study, 38% of patients in the combination arm achieved a major pathological response (MPR), and pCR was observed in 38% of these patients; in comparison, historical data show an MPR rate to NAC ranging between 7% and 27% in the same setting137,138,139,140,141. Additionally, tumoural PDL1 expression was significantly reduced following treatment in patients who achieved MPR or pCR (P = 0.017)136. Similar regimens of either combined neoadjuvant nivolumab and ipilimumab or neoadjuvant nivolumab alone in 29 patients with locally advanced SCC of the oral cavity were shown to result in pathological response rates of 54% and 73%, respectively, with an overall OS of 89% at a median follow-up duration of 14.2 months across the entire cohort142. These findings, along with others in glioblastoma143, bladder cancer144 and melanoma145, might provide support for the use of neoadjuvant ICB in penile cancer. Broadly, neoadjuvant ICB use might be beneficial owing to a reduction in disease burden before surgery leading to improved surgical outcomes. Additionally, delivery of ICB in the presence of the primary tumour could lead to increased recruitment of cytotoxic T cells compared with adjuvant ICB in which the supply of tumour-associated antigens is reduced owing to the absence of the primary tumour146,147,148. Moreover, a rationale for the use of neoadjuvant ICB in combination with neoadjuvant cisplatin-based chemotherapy in a select group of patients with penile cancer might exist. From a molecular perspective, neoadjuvant ICB combined with chemotherapy might be more effective than neoadjuvant ICB alone owing to increased tumour-associated antigens being released as a result of the cytotoxic activity of chemotherapy, leading to enhanced recruitment of cytotoxic immune cells to the TME and depletion of immunosuppressive molecules such as MDSCs and Treg cells149,150. For example, combination neoadjuvant pembrolizumab and chemotherapy is approved by the FDA for use in triple-negative breast cancer151 — this approval is largely based on the results of the KEYNOTE-522 trial demonstrating a significant increase in the number of patients who had a complete response compared with the chemotherapy plus placebo group (64.8% versus 51.2%, P < 0.001)152. However, within the setting of penile cancer, no trials investigating the use of neoadjuvant ICB alone or in combination with chemotherapy are currently being conducted.
In the locally advanced PSCC setting, both neoadjuvant and adjuvant ICB could have an important role in limiting the deleterious effects of the surgical stress response on disease recurrence. Surgery can produce perturbations in neuroendocrine, paracrine, immune, inflammatory and angiogenic signalling that promote the survival of cancer cells and the development of metastatic disease153,154,155. The use of ICB in the perioperative setting might help to reverse surgery-induced immunosuppression and T cell dysfunction156. This phenomenon has been demonstrated in a mouse model of surgical stress, in which blockade of PD1 and the immunosuppressive factor PGE2 reversed CD8+ T cell exhaustion157,158. Thus, within the context of penile cancer, an unmet need exists for ICB earlier in the disease course and its use should be expanded beyond patients with relapsed and/or metastatic disease.
Therapeutic HPV vaccines
HPV infection confers an increased risk of developing penile cancer, and in some high-risk HPV-positive histological subtypes, contributes to carcinogenesis through the activity of the E6 and E7 oncoproteins104,105,106,107. Owing to the continued HPV viral activity that persists throughout the tumour lifecycle and disease course159, therapeutic HPV vaccines that stimulate the immune system and enhance the CD8+ cytotoxic T cell response to HPV-positive tumours have become a new experimental treatment approach for HPV-associated malignancies. Therapeutic vaccines rely on the presentation of mutated versions of the E6 and/or E7 oncoproteins on antigen-presenting cells owing to their constitutive activity in high-risk HPV-positive cells, especially following integration of the viral genome into the host genome159,160,161,162. Several experimental vaccine delivery strategies exist: bacterial live vector vaccines, viral live vector vaccines, peptide vaccines, protein-based vaccines, DNA vaccines, RNA replicon-based DNA vaccines, dendritic cell-based vaccines and adoptive T cell transfer vaccines163,164,165. These delivery strategies are diverse, but the underlying aim of all therapeutic vaccines is to drive immunogenicity and promote cytotoxic T cell activity, as well as promote the development of immune memory against the HPV-positive tumour cells.
In the clinical setting, therapeutic vaccines have been tested against multiple HPV-driven neoplasms and malignancies in clinical trials and have shown some efficacy. For example, a bacterial live vector vaccine that secretes the E7 antigen, called Lm-LLO-E7, given intravenously, resulted in partial responses in 4/15 patients with advanced cervical cancer166. The vaccine was well tolerated, with no grade 4 adverse events reported and pyrexia being the most common grade 2–3 adverse event, which was experienced by all patients. A recombinant modified vaccinia virus, Ankara, a viral vector expressing E2 (a negative regulator of E6 and E7), was used to treat intraepithelial lesions in 1,176 women with cervical intraepithelial neoplasia or condyloma lesions and 180 men with urethral condyloma lesions or anal lesions. Complete elimination of the lesions was observed in 89.3% and 100% of women and men, respectively167. In this study, cold symptoms (69%), chills (63%) and fever (55%) were the most common adverse events and were all deemed to be grade 1. In another study in which a therapeutic synthetic DNA vaccine, VGX-3100, was administered intramuscularly to treat cervical intraepithelial neoplasia, no serious adverse events were reported with erythema at the injection site (78%) and fatigue (54%) being the most commonly reported adverse events168. Thus, it seems that HPV-directed therapeutic vaccines are generally safe based on available data.
In the context of penile cancer, no human studies investigating therapeutic vaccines have been published. However, several basket trials for HPV-associated malignancies, including HPV-positive penile cancer, are ongoing (Table 3). Interestingly, some of these trials involve a combination therapy approach involving HPV therapeutic vaccines and ICB. In principle, this combination approach shows promise in its potential to both stimulate the immune system using HPV neoantigens and downregulate tumour-mediated immunosuppression through ICB, and could prove more effective than either agent alone. For example, in NCT04432597 (ref.169) a gorilla adenovirus HPV vaccine in combination with bintrafusp alfa, a novel bifunctional fusion immunotherapeutic agent that targets both PDL1 and transforming growth factor-β (TGF-β) trap, is being investigated. Bintrafusp alfa has already demonstrated some success as a monotherapy in HPV-associated malignancies, with an objective response rate of 30.5% in a study involving 59 patients170. Other combination therapy approaches involving therapeutic vaccines and chemotherapy or radiotherapy are not currently being evaluated in penile cancer, but they have been tested in patients with vulvar intraepithelial neoplasia, leading to lesion regression and improved immune cell counts171.
Overall, HPV-directed vaccines provide an opportunity to specifically target HPV-positive penile cancer tumours and counteract immunosuppressive factors within the TIME by eliciting a strong antitumour immune response either alone or in combination with other immune-based therapies such as ICB.
Adoptive T cell therapies
The potential of translating immunotherapeutic strategies used to treat other malignancies into treatment approaches for penile cancer has been investigated in preclinical studies. For example, based on studies in melanoma172,173, cervical cancer174,175 and ovarian cancer176, the feasibility of generating TILs from PSCC-positive lymph nodes for adoptive T cell therapy (ACT) has been investigated177. ACT involves the isolation and expansion of tumour-specific TILs from tumour samples followed by autologous reinfusion. CD3+, CD8+ and CD4+ TILs were generated from 11/12 PSCC samples and no significant differences were reported between patients who were HPV positive versus HPV negative or between those who received NAC and those who did not. Additionally, antitumour reactivity of the expanded TILs was observed against 5/11 autologous tumour samples from which TILs were generated177.
These data support the potential feasibility of TIL expansion from a broad range of patients with penile cancer and demonstrate the in vitro efficacy of ACT therapy in this disease context. However, limitations associated with TIL-based therapy for PSCC include the identification of tumour-associated antigens widely expressed by PSCC and not expressed by nonmalignant cells, the relatively lower mutational burden of PSCC than other genitourinary cancers and SCCs49,178, the toxic effects associated with preparative lymphodepletion using full-body radiation or chemotherapy, and an inability to predict responders versus non-responders.
Engineering T cells to contain T cell receptors against tumour-associated HLA-restricted antigens (engineered T cell receptor (TCR) therapy) or non-HLA-restricted antigens (chimeric antigen receptor therapy (CAR-T)) could augment the capabilities of ACT. The engineering of T cells enables the development of T cells that have optimal affinity for a variety of tumour-specific antigens and modifications to T cells that prevent cell death strengthen proliferation, stimulation and inflammatory signalling179. Within the context of HPV-positive penile cancer, engineered T cells can be modified to target HPV-specific oncoproteins such as E6 or E7, the safety and efficacy of which has been demonstrated in a phase I trial, in which 12 patients (11 with SCC and 1 with adenocarcinoma) were intravenously administered TCRs targeting the HPV-16 E7 oncoprotein180. In this study, no dose-limiting toxic effects at doses 1 or 2, reactivity with healthy tissue, or treatment-related deaths were observed. With respect to efficacy, 6/12 and 5/12 patients demonstrated objective responses or stable disease, respectively. Additionally, the serum of these patients did not show the presence of anti-E7 TCR antibodies and the E7-TCRs were not found to express increased levels of PD1 6 weeks post-infusion or in comparison with endogenous T cells. In a phase I/II basket trial, an objective response was observed in 2/12 patients treated with an HPV-16 E6-targeting TCR181. Comparing results across these small, heterogenous cohorts is difficult, but the difference in efficacy between the two trials suggests some of challenges associated with engineered T cell therapy, such as the optimal selection of tumour-associated antigens. For example, the selection of the E7 versus E6 oncoprotein as the TCR target might have contributed to the observed difference in response rates between the two trials. Other challenges include monoclonal specificity, T cell fitness, efficient trafficking of engineered T cells to tumour sites in solid cancer types such as PSCC, and the harsh TIME182,183. CAR-T is unexplored in penile cancer, but has similar challenges in solid tumours and has historically demonstrated limited efficacy184,185.
With respect to the safety profile of ACT therapies, adverse effects can include cytokine release syndrome (CRS), neurotoxicity, infection and off-target tissue destruction as a result of potential cross-reactivity186; some of these adverse effects could be mitigated through improvements in T cell engineering and receptor avidity187. Notably, limited available data suggest differences in the prevalence of certain adverse effects between patients treated with ACT therapies for haematological malignancies versus solid malignancies. For example, a meta-analysis of safety data from CAR-T therapy trials revealed a pooled prevalence of 5% and 12% for CRS and neurotoxicity, respectively, in patients with solid malignancies; for patients with haematological malignancies, the pooled prevalence of CRS and neurotoxicity was 55% and 37%, respectively188. Given that penile cancer is a solid malignancy, prevalence rates of CRS and neurotoxicity in response to CAR-T therapy might be expected to be similar to those observed for solid malignancies.
Within the context of penile cancer, the safety, efficacy and molecular implications of engineered TCR-based therapy remain to be tested and validated in large, prospective studies. NCT02379520 (ref.189) is a prospective trial investigating HPV-16 E7-targeting TCR T cells189 and will provide further insight into the potential utility of this therapeutic approach for penile cancer. Adoptive cellular therapy could be of high value in patients with PSCC who are younger than 65 years old, as they are less likely to have pre-existing comorbid conditions and are at a reduced risk of fatal febrile neutropenia as a result of preparative lymphodepletion for ACT therapies compared with patients older than 65 years190,191,192.
Challenges and future directions
Advances have been made in characterizing immune cell populations in penile cancer and their potential prognostic value, but considerable gaps in our knowledge of the broader immune landscape and its interaction with the TME exist. In addition, opportunities to use immune-based therapies exist in principle based on our current understanding of penile cancer, but data on the utility of immune-based therapies in penile cancer are limited. Thus, improving outcomes for patients with penile cancer requires major efforts in the translational and clinical research settings to enhance our knowledge of this disease and develop effective immune-based therapeutic options.
Development of preclinical models
The rarity of penile cancer creates a challenging environment for studying this disease, both preclinically and clinically. In the preclinical setting, a paucity of PSCC cell lines and genetically engineered mouse models (GEMs) has historically existed, limiting the ability to study the molecular and immune landscape of penile cancer. Varying histological subtypes and their association (or lack thereof) with HPV further necessitate an improved understanding of penile cancer in all its forms and the key differences between them.
The creation of cell lines and GEMs has been undertaken to assist in characterizing the tumour immune landscape and testing novel therapeutic approaches. To date, two GEM models of penile cancer have been developed and tumour immune infiltration, concordance between the GEM models’ transcriptomes and human penile cancer, and the efficacy of combination targeted therapy and immunotherapy was investigated in these models83. To model HPV-driven oncogenesis, the downstream effects of E6 and E7 signalling through co-deletion of Smad4 and Apc in the penile epithelium to generate the SA GEM model were recapitulated, and then a Pten deletion was added to generate the cisplatin-resistant SAP GEM model. A reduction in CD8+ T cells and an increase in MDSCs were demonstrated in the SA GEM model compared with nonmalignant mouse penile tissue. In addition, COX2 overexpression, a potent upregulator of inflammatory processes in penile cancer158, was identified83. Treating tumours in the SA GEM model of penile cancer with a combination of ICB and either a COX2 inhibitor (celecoxib) or a tyrosine kinase inhibitor (TKI) that has been shown to suppress PI3K signalling in MDSCs (cabozantinib)193, resulted in tumour regression83.
Overall, these efforts led to the development of penile cancer GEM models that can be used for further elucidation of the TME as well as platforms for drug discovery and testing. Patient-derived cell lines and patient-derived xenografts (PDXs) have also been developed in penile cancer194,195,196. These patient-derived cell lines and PDX models will enable us to define the tumour biology of penile cancer, create models of drug resistance to current lines of systemic and targeted therapies and find novel therapeutic targets197.
Considerations for future clinical trials on immune-based therapies
The design of future trials of immune-based therapies in penile cancer should focus on three areas: first, ICB use should be investigated in the neoadjuvant setting, either alone or in combination with standard-of-care NAC, owing to the potential synergistic effects of these two therapies when used together as well as existing evidence regarding the benefits of neoadjuvant immunotherapy146,148,198; second, patients should be stratified into HPV-positive and HPV-negative groups, and safety profiles and treatment efficacy in each group should be compared to characterize any differences in outcomes between the two groups. For example, stratification into HPV-positive and HPV-negative groups could help to determine whether patients with HPV-negative disease — in which PDL1 positivity is a stronger prognostic indicator — will benefit more from ICB therapies than those with HPV-positive disease. Finally, combination therapy approaches that combine immune-based therapies (such as HPV vaccines plus ICB agents) or immune-based therapies with targeted agents such as TKIs should be investigated to determine whether they produce treatment responses beyond what could be achieved via any single monotherapy alone. For example, in a mouse model of metastatic castration-resistant prostate cancer, targeting MDSCs with cabozantinib plus ICB resulted in robust antitumour activity compared with ICB alone193. Additionally, superior efficacy of a COX2 inhibitor plus ICB to ICB alone was demonstrated in a mouse model of PSCC83. Thus, future clinical studies on immune-based therapies should be aimed at improving clinicopathological outcomes by depleting or suppressing immunosuppressive actors in the TIME (such as MDSCs, FOXP3+ Treg cells and M2-type TAMs)79,199 when using ICB (Fig. 2). In addition, in each of these scenarios, laboratory-based tools such as multi-omic technologies (transcriptomics, epigenomics and metabolomics), PDX models and high-throughput molecular pathology should be used to characterize the molecular landscape to improve understanding of the TME.
Immune-based therapies for penile cancer are being examined in several basket trials in parallel with other SCCs (Tables 2 and 3). When possible, further molecular studies comparing PSCC with other SCCs should be conducted to further elucidate the immune-related cellular mechanisms that are shared between SCCs of different primary origin. At the present time, studies have been limited to genomic analyses investigating gene mutations, copy number alterations and changes in genomic pathway activity. Thus, detailed single-cell analyses comparing the TIME between PSCC and other SCCs will inform future clinical trials in which immune-based therapeutics with demonstrated effectiveness in more common SCCs, such as lung and HNSCC, can potentially be evaluated in PSCC.
The role of centralization of care and collaborative science
In order to advance clinical investigations and improve penile cancer care, the challenges that arise from the rarity and severity of the disease must be managed. These challenges include under-use of organ-sparing surgery (OSS), leading to poor quality of life and oncological outcomes at non-academic centres200,201,202, non-uniform management owing to the low-level evidence supporting most NCCN and EAU recommendations, under-use of LND at non-academic centres203 and difficulties running large-scale clinical trials to test various investigational treatment modalities. Centralization of care (COC) is a potential solution to these challenges and has been implemented in parts of Europe over the past two decades. For example, COC for penile cancer has existed since 2002 in the UK based on guidance from the National Institute of Clinical Excellence204.
Within the context of advancing immune-based therapies for use in treating penile cancer, COC also improves our ability to research penile cancer, both preclinically and clinically, share knowledge between academic centres and offer patients the option to enrol in a clinical trial early in the disease course205. COC also enables the formation of academic societies focused on the treatment of rare malignancies such as penile cancer, for example, the Global Society of Rare Genitourinary Tumors (GSRGT).
In a disease in which standard-of-care treatment options are largely non-curative for advanced disease, clinical trials present the greatest opportunity to improve outcomes for patients. The International Penile Advanced Cancer Trial (InPACT) is a case study on the implementation of an international, randomized controlled trial for PSCC206. Given the scale and scope of the study, the InPACT trial will be a source of guidance on the successes and limitations associated with facilitating an international, randomized controlled trial for a rare genitourinary cancer involving cross-disciplinary collaboration, multiple treatment modalities and local differences in patient care. This guidance will ultimately help to inform trial design for prospective studies investigating immune-based therapies either alone or in combination with other treatments. Furthermore, the specimens collected through the InPACT trial will become a rich resource for collaborative translational research studies and will enable performance of correlative studies linking the molecular landscape of penile cancer to the clinical outcomes observed in the trial. In this context as well, COC is a key component in efforts to improve understanding of the molecular landscape of penile cancer as well as treat patients in a uniform manner with an expanded range of treatment options that include experimental therapies207.
Conclusions
Immune-based therapies have great potential to improve outcomes for patients with penile cancer, but the immune landscape of penile cancer has not been fully characterized, limiting our ability to accurately assess the optimal immune-based therapies in this disease. Thus, future work should use high-throughput techniques such as genomics, proteomics, metabolomics and single-cell technologies to further define the immune landscape of penile cancer at a high resolution and at different stages of this disease. Furthermore, rare cancers require optimization of the efficacy and translatability of preclinical models — especially those that rely on human-derived tumour samples.
At the present time, evidence that shows the prognostic value of immune markers such as PDL1 across both HPV-positive and HPV-negative cancers suggests that conventional immune-based therapies such as ICB might be effective in penile cancer, particularly in the perioperative setting and in the early treatment of high-risk, localized disease. However, the majority of currently ongoing clinical trials involving ICB are focused on relapsed, metastatic, and/or treatment-refractory disease (Table 2). Thus, efforts must be undertaken to explore the potential benefits of shifting the use of ICB to earlier in the disease course. For example, combining ICB with standard-of-care treatments such as NAC or neoadjuvant chemoradiotherapy followed by surgery could enhance the efficacy of ICB and improve surgical outcomes. Additionally, given the protumorigenic role of HPV in penile cancer and the presence of HPV infection in half of all penile cancers, therapeutic HPV vaccines and ACTs provide an additional means of stimulating the antitumour immune response. Finally, preclinical and clinical studies must explore opportunities to combine immune-based therapies with other novel therapeutic approaches, such as targeted therapies, in order to enhance the efficacy of immune-based therapies that are driven by the activity of cytotoxic T lymphocytes. The rarity of penile cancer necessitates that these preclinical and clinical studies be conducted via international collaborations with COC in mind.
References
Siegel, R. L., Miller, K. D., Fuchs, H. E. & Jemal, A. Cancer statistics, 2021. CA Cancer J. Clin. 71, 7–33 (2021).
Sung, H. et al. Global Cancer Statistics 2020: GLOBOCAN estimates of Incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249 (2021).
Olesen, T. B. et al. Prevalence of human papillomavirus DNA and p16(INK4a) in penile cancer and penile intraepithelial neoplasia: a systematic review and meta-analysis. Lancet Oncol. 20, 145–158 (2019).
Kidd, L. C. et al. Relationship between human papillomavirus and penile cancer-implications for prevention and treatment. Transl. Androl. Urol. 6, 791–802 (2017).
Torbrand, C. et al. Socioeconomic factors and penile cancer risk and mortality; a population-based study. BJU Int. 119, 254–260 (2017).
Favorito, L. A. et al. Epidemiologic study on penile cancer in Brazil. Int. Braz. J. Urol. 34, 587–591 discussion 591–593 (2008).
Madsen, B. S., van den Brule, A. J., Jensen, H. L., Wohlfahrt, J. & Frisch, M. Risk factors for squamous cell carcinoma of the penis — population-based case-control study in Denmark. Cancer Epidemiol. Biomark. Prev. 17, 2683–2691 (2008).
Morris, B. J. et al. The strong protective effect of circumcision against cancer of the penis. Adv. Urol. 2011, 812368 (2011).
Morris, B. J. et al. Early infant male circumcision: systematic review, risk-benefit analysis, and progress in policy. World J. Clin. Pediatr. 6, 89–102 (2017).
Harish, K. & Ravi, R. The role of tobacco in penile carcinoma. Br. J. Urol. 75, 375–377 (1995).
Tward, J. The case for nonsurgical therapy of nonmetastatic penile cancer. Nat. Rev. Urol. 15, 574–584 (2018).
Ficarra, V., Akduman, B., Bouchot, O., Palou, J. & Tobias-Machado, M. Prognostic factors in penile cancer. Urology 76, S66–S73 (2010).
Srinivas, V., Morse, M. J., Herr, H. W., Sogani, P. C. & Whitmore, W. F. Jr. Penile cancer: relation of extent of nodal metastasis to survival. J. Urol. 137, 880–882 (1987).
Horenblas, S. & van Tinteren, H. Squamous cell carcinoma of the penis. IV. Prognostic factors of survival: analysis of tumor, nodes and metastasis classification system. J. Urol. 151, 1239–1243 (1994).
Djajadiningrat, R. S. et al. Contemporary management of regional nodes in penile cancer — improvement of survival? J. Urol. 191, 68–73 (2014).
Pagliaro, L. C. & Crook, J. Multimodality therapy in penile cancer: when and which treatments? World J. Urol. 27, 221–225 (2009).
National Comprehensive Cancer Network. Penile Cancer. nccn.org https://www.nccn.org/professionals/physician_gls/pdf/penile.pdf (2021).
Hakenberg, O.W. et al. EAU Guidelines: Penile Cancer. https://uroweb.org/guidelines/penile-cancer (2020).
Soodana-Prakash, N. et al. Lymph node yield as a predictor of overall survival following inguinal lymphadenectomy for penile cancer. Urol. Oncol. 36, 471 e419–471.e427 (2018).
Li, Z. S. et al. Disease-specific survival after radical lymphadenectomy for penile cancer: prediction by lymph node count and density. Urol. Oncol. 32, 893–900 (2014).
Zargar-Shoshtari, K. et al. Extent of pelvic lymph node dissection in penile cancer may impact survival. World J. Urol. 34, 353–359 (2016).
Ahmed, M. E. et al. in Penile Carcinoma 97–107 (Springer International Publishing, 2021).
Pagliaro, L. C. et al. Neoadjuvant paclitaxel, ifosfamide, and cisplatin chemotherapy for metastatic penile cancer: a phase II study. J. Clin. Oncol. 28, 3851–3857 (2010).
Azizi, M. et al. Systematic review and meta-analysis-is there a benefit in using neoadjuvant systemic chemotherapy for locally advanced penile squamous cell carcinoma? J. Urol. 203, 1147–1155 (2020).
Chahoud, J., Tamil, M. & Necchi, A. Second line salvage systemic therapy for advanced penile cancer. Urol. Oncol. https://doi.org/10.1016/j.urolonc.2020.08.001 (2020).
Nicolai, N. et al. A combination of cisplatin and 5-fluorouracil with a taxane in patients who underwent lymph node dissection for nodal metastases from squamous cell carcinoma of the penis: treatment outcome and survival analyses in neoadjuvant and adjuvant settings. Clin. Genitourin. Cancer 14, 323–330 (2016).
Necchi, A. et al. Nomogram-based prediction of overall survival after regional lymph node dissection and the role of perioperative chemotherapy in penile squamous cell carcinoma: a retrospective multicenter study. Urol. Oncol. 37, 531.e7–531.e15 (2019).
Sharma, P. et al. Adjuvant chemotherapy is associated with improved overall survival in pelvic node-positive penile cancer after lymph node dissection: a multi-institutional study. Urol. Oncol. 33, 496.e17–e23 (2015).
Han, S. C., Kim, D. H., Higgins, S. A., Carcangiu, M. L. & Kacinski, B. M. Chemoradiation as primary or adjuvant treatment for locally advanced carcinoma of the vulva. Int. J. Radiat. Oncol. Biol. Phys. 47, 1235–1244 (2000).
Wang, J., Pettaway, C. A. & Pagliaro, L. C. Treatment for metastatic penile cancer after first-line chemotherapy failure: analysis of response and survival outcomes. Urology 85, 1104–1110 (2015).
Challapalli, A. et al. A phase II trial of cabazitaxel as second line chemotherapy in relapsed locally advanced and/or metastatic carcinoma of the penis. J. Int. Med. Res. 47, 4664–4672 (2019).
Nicholson, S. et al. Phase II trial of docetaxel, cisplatin and 5FU chemotherapy in locally advanced and metastatic penis cancer (CRUK/09/001). Br. J. Cancer 109, 2554–2559 (2013).
Di Lorenzo, G. et al. Cisplatin and 5-fluorouracil in inoperable, stage IV squamous cell carcinoma of the penis. BJU Int. 110, E661–E666 (2012).
Di Lorenzo, G. et al. Paclitaxel in pretreated metastatic penile cancer: final results of a phase 2 study. Eur. Urol. 60, 1280–1284 (2011).
Theodore, C. et al. A phase II multicentre study of irinotecan (CPT 11) in combination with cisplatin (CDDP) in metastatic or locally advanced penile carcinoma (EORTC PROTOCOL 30992). Ann. Oncol. 19, 1304–1307 (2008).
Chahoud, J., Kohli, M. & Spiess, P. E. Management of advanced penile cancer. Mayo Clin. Proc. 96, 720–732 (2021).
US National Library of Medicine. ClinicalTrials.gov, https://clinicaltrials.gov/ct2/show/NCT02305654 (2019).
Bartelink, H. et al. Concomitant radiotherapy and chemotherapy is superior to radiotherapy alone in the treatment of locally advanced anal cancer: results of a phase III randomized trial of the European Organization for Research and Treatment of Cancer Radiotherapy and Gastrointestinal Cooperative Groups. J. Clin. Oncol. 15, 2040–2049 (1997).
UKCCCR Anal Cancer Trial Working Party. Epidermoid anal cancer: results from the UKCCCR randomised trial of radiotherapy alone versus radiotherapy, 5-fluorouracil, and mitomycin. UK Co-ordinating Committee on Cancer Research. Lancet 348, 1049–1054 (1996).
van Doorn, H. C., Ansink, A., Verhaar-Langereis, M. & Stalpers, L. Neoadjuvant chemoradiation for advanced primary vulvar cancer. Cochrane Database Syst. Rev. https://doi.org/10.1002/14651858.CD003752.pub2 (2006).
Moore, D. H. et al. A phase II trial of radiation therapy and weekly cisplatin chemotherapy for the treatment of locally-advanced squamous cell carcinoma of the vulva: a gynecologic oncology group study. Gynecol. Oncol. 124, 529–533 (2012).
De Bacco, M. W. et al. PD-L1 and p16 expression in penile squamous cell carcinoma from an endemic region. Clin. Genitourin. Cancer 18, e254–e259 (2020).
Cocks, M. et al. Immune-checkpoint status in penile squamous cell carcinoma: a North American cohort. Hum. Pathol. 59, 55–61 (2017).
Chahoud, J., Pickering, C. R. & Pettaway, C. A. Genetics and penile cancer: recent developments and implications. Curr. Opin. Urol. 29, 364–370 (2019).
Aydin, A. M. et al. Understanding genomics and the immune environment of penile cancer to improve therapy. Nat. Rev. Urol. 17, 555–570 (2020).
Ali, S. M. et al. Comprehensive genomic profiling of advanced penile carcinoma suggests a high frequency of clinically relevant genomic alterations. Oncologist 21, 33–39 (2016).
Busso-Lopes, A. F. et al. Genomic profiling of human penile carcinoma predicts worse prognosis and survival. Cancer Prev. Res. 8, 149–156 (2015).
Feber, A. et al. CSN1 somatic mutations in penile squamous cell carcinoma. Cancer Res. 76, 4720–4727 (2016).
Jacob, J. M. et al. Comparative genomic profiling of refractory and metastatic penile and nonpenile cutaneous squamous cell carcinoma: implications for selection of systemic therapy. J. Urol. 201, 541–548 (2019).
La-Touche, S. et al. DNA copy number aberrations, and human papillomavirus status in penile carcinoma. clinico-pathological correlations and potential driver genes. PLoS One 11, e0146740 (2016).
Marchi, F. A. et al. Multidimensional integrative analysis uncovers driver candidates and biomarkers in penile carcinoma. Sci. Rep. 7, 6707 (2017).
McDaniel, A. S. et al. Genomic profiling of penile squamous cell carcinoma reveals new opportunities for targeted therapy. Cancer Res. 75, 5219–5227 (2015).
Feber, A. et al. Epigenetics markers of metastasis and HPV-induced tumorigenesis in penile cancer. Clin. Cancer Res. 21, 1196–1206 (2015).
Hartz, J. M. et al. Integrated loss of miR-1/miR-101/miR-204 discriminates metastatic from nonmetastatic penile carcinomas and can predict patient outcome. J. Urol. 196, 570–578 (2016).
Kuasne, H. et al. Integrative miRNA and mRNA analysis in penile carcinomas reveals markers and pathways with potential clinical impact. Oncotarget 8, 15294–15306 (2017).
Kuasne, H. et al. Genome-wide methylation and transcriptome analysis in penile carcinoma: uncovering new molecular markers. Clin. Epigenetics 7, 46 (2015).
Necchi, A. et al. Gene expression profiling of advanced penile squamous cell carcinoma receiving cisplatin-based chemotherapy improves prognostication and identifies potential therapeutic targets. Eur. Urol. Focus. 4, 733–736 (2018).
Chahoud, J. et al. Whole-exome sequencing in penile squamous cell carcinoma uncovers novel prognostic categorization and drug targets similar to head and neck squamous cell carcinoma. Clin. Cancer Res. 27, 2560–2570 (2021).
Jardim, D. L., Goodman, A., de Melo Gagliato, D. & Kurzrock, R. The challenges of tumor mutational burden as an immunotherapy biomarker. Cancer Cell 39, 154–173 (2021).
Schreiber, R. D., Old, L. J. & Smyth, M. J. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science 331, 1565–1570 (2011).
Hendry, S. et al. Assessing tumor-infiltrating lymphocytes in solid tumors: a practical review for pathologists and proposal for a standardized method from the international immunooncology biomarkers working group: part 1: assessing the host immune response, tils in invasive breast carcinoma and ductal carcinoma in situ, metastatic tumor deposits and areas for further research. Adv. Anat. Pathol. 24, 235–251 (2017).
Fridman, W. H., Pages, F., Sautes-Fridman, C. & Galon, J. The immune contexture in human tumours: impact on clinical outcome. Nat. Rev. Cancer 12, 298–306 (2012).
Tay, R. E., Richardson, E. K. & Toh, H. C. Revisiting the role of CD4+ T cells in cancer immunotherapy-new insights into old paradigms. Cancer Gene Ther. 28, 5–17 (2021).
Ohue, Y. & Nishikawa, H. Regulatory T (Treg) cells in cancer: can Treg cells be a new therapeutic target? Cancer Sci. 110, 2080–2089 (2019).
Mantovani, A., Marchesi, F., Malesci, A., Laghi, L. & Allavena, P. Tumour-associated macrophages as treatment targets in oncology. Nat. Rev. Clin. Oncol. 14, 399–416 (2017).
Xu, Q., Wang, C., Yuan, X., Feng, Z. & Han, Z. Prognostic value of tumor-infiltrating lymphocytes for patients with head and neck squamous cell carcinoma. Transl. Oncol. 10, 10–16 (2017).
Spector, M. E. et al. Prognostic value of tumor-infiltrating lymphocytes in head and neck squamous cell carcinoma. JAMA Otolaryngol. Head. Neck Surg. 145, 1012–1019 (2019).
Jiang, D. et al. Tumour infiltrating lymphocytes correlate with improved survival in patients with esophageal squamous cell carcinoma. Sci. Rep. 7, 44823 (2017).
Ottenhof, S. R. et al. The prognostic value of immune factors in the tumor microenvironment of penile squamous cell carcinoma. Front. Immunol. 9, 1253 (2018).
Cao, L. et al. M2 macrophage infiltration into tumor islets leads to poor prognosis in non-small-cell lung cancer. Cancer Manag. Res. 11, 6125–6138 (2019).
Alves, A. M., Diel, L. F. & Lamers, M. L. Macrophages and prognosis of oral squamous cell carcinoma: a systematic review. J. Oral. Pathol. Med. 47, 460–467 (2018).
Kumar, A. T. et al. Prognostic significance of tumor-associated macrophage content in head and neck squamous cell carcinoma: a meta-analysis. Front. Oncol. 9, 656 (2019).
Wang, Y., Smith, W., Hao, D., He, B. & Kong, L. M1 and M2 macrophage polarization and potentially therapeutic naturally occurring compounds. Int. Immunopharmacol. 70, 459–466 (2019).
Chu, C. et al. Immunophenotypes based on the tumor immune microenvironment allow for unsupervised penile cancer patient stratification. Cancers https://doi.org/10.3390/cancers12071796 (2020).
Rafael, T. S. et al. Distinct patterns of myeloid cell infiltration in patients with hrHPV-positive and hrHPV-negative penile squamous cell carcinoma: the importance of assessing myeloid cell densities within the spatial context of the tumor. Front. Immunol. 12, 682030 (2021).
Cheng, S. et al. A pan-cancer single-cell transcriptional atlas of tumor infiltrating myeloid cells. Cell 184, 792–809.e23 (2021).
Vassallo, J. et al. Pathologic and imunohistochemical characterization of tumoral inflammatory cell infiltrate in invasive penile squamous cell carcinomas: Fox-P3 expression is an independent predictor of recurrence. Tumour Biol. 36, 2509–2516 (2015).
Szylberg, L., Karbownik, D. & Marszalek, A. The role of FOXP3 in human cancers. Anticancer. Res. 36, 3789–3794 (2016).
Gabrilovich, D. I., Ostrand-Rosenberg, S. & Bronte, V. Coordinated regulation of myeloid cells by tumours. Nat. Rev. Immunol. 12, 253–268 (2012).
Gabrilovich, D. I. & Nagaraj, S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 9, 162–174 (2009).
Bronte, V. et al. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat. Commun. 7, 12150 (2016).
Greten, T. F., Manns, M. P. & Korangy, F. Myeloid derived suppressor cells in human diseases. Int. Immunopharmacol. 11, 802–807 (2011).
Huang, T. et al. Effective combinatorial immunotherapy for penile squamous cell carcinoma. Nat. Commun. 11, 2124 (2020).
Kusmartsev, S., Nefedova, Y., Yoder, D. & Gabrilovich, D. I. Antigen-specific inhibition of CD8+ T cell response by immature myeloid cells in cancer is mediated by reactive oxygen species. J. Immunol. 172, 989–999 (2004).
Bronte, V. & Zanovello, P. Regulation of immune responses by L-arginine metabolism. Nat. Rev. Immunol. 5, 641–654 (2005).
Huang, B. et al. Gr-1+CD115+ immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Res. 66, 1123–1131 (2006).
Stiff, A. et al. Nitric oxide production by myeloid-derived suppressor cells plays a role in impairing Fc receptor-mediated natural killer cell function. Clin. Cancer Res. 24, 1891–1904 (2018).
Diaz-Montero, C. M. et al. Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy. Cancer Immunol. Immunother. 58, 49–59 (2009).
Alizadeh, D. et al. Doxorubicin eliminates myeloid-derived suppressor cells and enhances the efficacy of adoptive T-cell transfer in breast cancer. Cancer Res. 74, 104–118 (2014).
Meyer, C. et al. Frequencies of circulating MDSC correlate with clinical outcome of melanoma patients treated with ipilimumab. Cancer Immunol. Immunother. 63, 247–257 (2014).
Ai, L. et al. Prognostic role of myeloid-derived suppressor cells in cancers: a systematic review and meta-analysis. BMC Cancer 18, 1220 (2018).
Iwai, Y. et al. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc. Natl Acad. Sci. USA 99, 12293–12297 (2002).
Parsa, A. T. et al. Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nat. Med. 13, 84–88 (2007).
Taube, J. M. et al. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci. Transl. Med. 4, 127ra137 (2012).
Ribas, A. & Wolchok, J. D. Cancer immunotherapy using checkpoint blockade. Science 359, 1350–1355 (2018).
Pardoll, D. M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 12, 252–264 (2012).
Vaddepally, R. K., Kharel, P., Pandey, R., Garje, R. & Chandra, A. B. Review of indications of FDA-approved immune checkpoint inhibitors per NCCN guidelines with the level of evidence. Cancers https://doi.org/10.3390/cancers12030738 (2020).
Udager, A. M. et al. Frequent PD-L1 expression in primary and metastatic penile squamous cell carcinoma: potential opportunities for immunotherapeutic approaches. Ann. Oncol. 27, 1706–1712 (2016).
Ottenhof, S. R. et al. Expression of programmed death ligand 1 in penile cancer is of prognostic value and associated with HPV status. J. Urol. 197, 690–697 (2017).
Moch, H., Cubilla, A. L., Humphrey, P. A., Reuter, V. E. & Ulbright, T. M. The 2016 WHO classification of tumours of the urinary system and male genital organs-part A: renal, penile, and testicular tumours. Eur. Urol. 70, 93–105 (2016).
Chaux, A. & Cubilla, A. L. Advances in the pathology of penile carcinomas. Hum. Pathol. 43, 771–789 (2012).
Sand, F. L., Rasmussen, C. L., Frederiksen, M. H., Andersen, K. K. & Kjaer, S. K. Prognostic significance of HPV and p16 status in men diagnosed with penile cancer: a systematic review and meta-analysis. Cancer Epidemiol. Biomark. Prev. 27, 1123–1132 (2018).
Bandini, M. et al. Association between human papillomavirus infection and outcome of perioperative nodal radiotherapy for penile carcinoma. Eur. Urol. Oncol. 4, 802–810 (2021).
Mannweiler, S., Sygulla, S., Winter, E. & Regauer, S. Two major pathways of penile carcinogenesis: HPV-induced penile cancers overexpress p16ink4a, HPV-negative cancers associated with dermatoses express p53, but lack p16ink4a overexpression. J. Am. Acad. Dermatol. 69, 73–81 (2013).
Dyson, N., Howley, P. M., Munger, K. & Harlow, E. The human papilloma virus-16 E7 oncoprotein is able to bind to the retinoblastoma gene product. Science 243, 934–937 (1989).
Scheffner, M., Werness, B. A., Huibregtse, J. M., Levine, A. J. & Howley, P. M. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell 63, 1129–1136 (1990).
Werness, B. A., Levine, A. J. & Howley, P. M. Association of human papillomavirus types 16 and 18 E6 proteins with p53. Science 248, 76–79 (1990).
Steinestel, J. et al. The role of histologic subtype, p16(INK4a) expression, and presence of human papillomavirus DNA in penile squamous cell carcinoma. BMC Cancer 15, 220 (2015).
Cubilla, A. L. et al. Value of p16INK4a in the pathology of invasive penile squamous cell carcinomas: a report of 202 cases. Am. J. Surg. Pathol. 35, 253–261 (2011).
Romagosa, C. et al. p16Ink4a overexpression in cancer: a tumor suppressor gene associated with senescence and high-grade tumors. Oncogene 30, 2087–2097 (2011).
Djajadiningrat, R. S. et al. Human papillomavirus prevalence in invasive penile cancer and association with clinical outcome. J. Urol. 193, 526–531 (2015).
Lont, A. P. et al. Presence of high-risk human papillomavirus DNA in penile carcinoma predicts favorable outcome in survival. Int. J. Cancer 119, 1078–1081 (2006).
Stafford, M. & Kaczmar, J. The neoadjuvant paradigm reinvigorated: a review of pre-surgical immunotherapy in HNSCC. Cancers Head. Neck 5, 4 (2020).
Uprety, D., Mandrekar, S. J., Wigle, D., Roden, A. C. & Adjei, A. A. Neoadjuvant immunotherapy for NSCLC: current concepts and future approaches. J. Thorac. Oncol. 15, 1281–1297 (2020).
McGregor, B. A. et al. Results of a multicenter, phase 2 study of nivolumab and ipilimumab for patients with advanced rare genitourinary malignancies. Cancer 127, 840–849 (2021).
Hahn, A. W. et al. Pembrolizumab for advanced penile cancer: a case series from a phase II basket trial. Invest. N. Drugs https://doi.org/10.1007/s10637-021-01100-x (2021).
Trafalis, D. T. et al. Evidence for efficacy of treatment with the anti-PD-1 mab nivolumab in radiation and multichemorefractory advanced penile squamous cell carcinoma. J. Immunother. 41, 300–305 (2018).
Chahoud, J. et al. Case report: two cases of chemotherapy refractory metastatic penile squamous cell carcinoma with extreme durable response to pembrolizumab. Front. Oncol. 10, 615298 (2020).
US National Library of Medicine. ClinicalTrials.gov, https://ClinicalTrials.gov/show/NCT04224740 (2022).
US National Library of Medicine. ClinicalTrials.gov, https://ClinicalTrials.gov/show/NCT03391479 (2021).
US National Library of Medicine. ClinicalTrials.gov, https://ClinicalTrials.gov/show/NCT03774901 (2022).
Deutsch, E., Chargari, C., Galluzzi, L. & Kroemer, G. Optimising efficacy and reducing toxicity of anticancer radioimmunotherapy. Lancet Oncol. 20, e452–e463 (2019).
Plavc, G. & Strojan, P. Combining radiotherapy and immunotherapy in definitive treatment of head and neck squamous cell carcinoma: review of current clinical trials. Radiol. Oncol. 54, 377–393 (2020).
Xing, D. T. et al. Recent research on combination of radiotherapy with targeted therapy or immunotherapy in head and neck squamous cell carcinoma: a review for radiation oncologists. Cancers https://doi.org/10.3390/cancers13225716 (2021).
Karam, S. D. & Raben, D. Radioimmunotherapy for the treatment of head and neck cancer. Lancet Oncol. 20, e404–e416 (2019).
Oweida, A. et al. Ionizing radiation sensitizes tumors to PD-L1 immune checkpoint blockade in orthotopic murine head and neck squamous cell carcinoma. Oncoimmunology 6, e1356153 (2017).
Twyman-Saint Victor, C. et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature 520, 373–377 (2015).
Distler, F. A. et al. Adherence to the EAU guideline recommendations for systemic chemotherapy in penile cancer: results of the E-PROPS study group survey. World J. Urol. 38, 2523–2530 (2020).
Paz-Ares, L. et al. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N. Engl. J. Med. 379, 2040–2051 (2018).
Magee, D. E. et al. Adverse event profile for immunotherapy agents compared with chemotherapy in solid organ tumors: a systematic review and meta-analysis of randomized clinical trials. Ann. Oncol. 31, 50–60 (2020).
Michot, J. M. et al. Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur. J. Cancer 54, 139–148 (2016).
Wang, Y. et al. Treatment-related adverse events of PD-1 and PD-L1 inhibitors in clinical trials: a systematic review and meta-analysis. JAMA Oncol. 5, 1008–1019 (2019).
Horvat, T. Z. et al. Immune-related adverse events, need for systemic immunosuppression, and effects on survival and time to treatment failure in patients with melanoma treated with ipilimumab at Memorial Sloan Kettering Cancer Center. J. Clin. Oncol. 33, 3193–3198 (2015).
Weber, J. S. et al. Safety profile of nivolumab monotherapy: a pooled analysis of patients with advanced melanoma. J. Clin. Oncol. 35, 785–792 (2017).
Naidoo, J. et al. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann. Oncol. 26, 2375–2391 (2015).
Cascone, T. et al. Neoadjuvant nivolumab or nivolumab plus ipilimumab in operable non-small cell lung cancer: the phase 2 randomized NEOSTAR trial. Nat. Med. 27, 504–514 (2021).
Pataer, A. et al. Histopathologic response criteria predict survival of patients with resected lung cancer after neoadjuvant chemotherapy. J. Thorac. Oncol. 7, 825–832 (2012).
Chaft, J. E. et al. Phase II trial of neoadjuvant bevacizumab plus chemotherapy and adjuvant bevacizumab in patients with resectable nonsquamous non-small-cell lung cancers. J. Thorac. Oncol. 8, 1084–1090 (2013).
Cascone, T. et al. Induction cisplatin docetaxel followed by surgery and erlotinib in non-small cell lung cancer. Ann. Thorac. Surg. 105, 418–424 (2018).
Weissferdt, A. et al. Agreement on major pathological response in NSCLC patients receiving neoadjuvant chemotherapy. Clin. Lung Cancer 21, 341–348 (2020).
Cascone, T. et al. A phase I/II study of neoadjuvant cisplatin, docetaxel, and nintedanib for resectable non-small cell lung cancer. Clin. Cancer Res. 26, 3525–3536 (2020).
Schoenfeld, J. D. et al. Neoadjuvant nivolumab or nivolumab plus ipilimumab in untreated oral cavity squamous cell carcinoma: a phase 2 open-label randomized clinical trial. JAMA Oncol. 6, 1563–1570 (2020).
Schalper, K. A. et al. Neoadjuvant nivolumab modifies the tumor immune microenvironment in resectable glioblastoma. Nat. Med. 25, 470–476 (2019).
Necchi, A. et al. Pembrolizumab as neoadjuvant therapy before radical cystectomy in patients with muscle-invasive urothelial bladder carcinoma (PURE-01): an open-label, single-arm, phase II study. J. Clin. Oncol. 36, 3353–3360 (2018).
Amaria, R. N. et al. Neoadjuvant immune checkpoint blockade in high-risk resectable melanoma. Nat. Med. 24, 1649–1654 (2018).
Liu, J. et al. Improved efficacy of neoadjuvant compared to adjuvant immunotherapy to eradicate metastatic disease. Cancer Discov. 6, 1382–1399 (2016).
Blank, C. U. et al. Neoadjuvant versus adjuvant ipilimumab plus nivolumab in macroscopic stage III melanoma. Nat. Med. 24, 1655–1661 (2018).
O’Donnell, J. S., Hoefsmit, E. P., Smyth, M. J., Blank, C. U. & Teng, M. W. L. The promise of neoadjuvant immunotherapy and surgery for cancer treatment. Clin. Cancer Res. 25, 5743–5751 (2019).
Yu, W. D., Sun, G., Li, J., Xu, J. & Wang, X. Mechanisms and therapeutic potentials of cancer immunotherapy in combination with radiotherapy and/or chemotherapy. Cancer Lett. 452, 66–70 (2019).
Chen, G. & Emens, L. A. Chemoimmunotherapy: reengineering tumor immunity. Cancer Immunol. Immunother. 62, 203–216 (2013).
FDA. FDA approves pembrolizumab for high-risk early-stage triple-negative breast cancer. fda.gov https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-pembrolizumab-high-risk-early-stage-triple-negative-breast-cancer (2021).
Schmid, P. et al. Pembrolizumab for early triple-negative breast cancer. N. Engl. J. Med. 382, 810–821 (2020).
Hiller, J. G., Perry, N. J., Poulogiannis, G., Riedel, B. & Sloan, E. K. Perioperative events influence cancer recurrence risk after surgery. Nat. Rev. Clin. Oncol. 15, 205–218 (2018).
Horowitz, M., Neeman, E., Sharon, E. & Ben-Eliyahu, S. Exploiting the critical perioperative period to improve long-term cancer outcomes. Nat. Rev. Clin. Oncol. 12, 213–226 (2015).
Chen, Z. et al. Surgical stress and cancer progression: the twisted tango. Mol. Cancer 18, 132 (2019).
Bakos, O., Lawson, C., Rouleau, S. & Tai, L. H. Combining surgery and immunotherapy: turning an immunosuppressive effect into a therapeutic opportunity. J. Immunother. Cancer 6, 86 (2018).
Sun, Z. et al. Treatment with anti-programmed cell death 1 (PD-1) antibody restored postoperative CD8+ T cell dysfunction by surgical stress. Biomed. Pharmacother. 89, 1235–1241 (2017).
Golijanin, D. et al. Cyclooxygenase-2 and microsomal prostaglandin E synthase-1 are overexpressed in squamous cell carcinoma of the penis. Clin. Cancer Res. 10, 1024–1031 (2004).
zur Hausen, H. Papillomaviruses and cancer: from basic studies to clinical application. Nat. Rev. Cancer 2, 342–350 (2002).
Morrow, M. P., Yan, J. & Sardesai, N. Y. Human papillomavirus therapeutic vaccines: targeting viral antigens as immunotherapy for precancerous disease and cancer. Expert. Rev. Vaccines 12, 271–283 (2013).
Lin, K., Doolan, K., Hung, C. F. & Wu, T. C. Perspectives for preventive and therapeutic HPV vaccines. J. Formos. Med. Assoc. 109, 4–24 (2010).
van der Burg, S. H. & Melief, C. J. Therapeutic vaccination against human papilloma virus induced malignancies. Curr. Opin. Immunol. 23, 252–257 (2011).
Garbuglia, A. R., Lapa, D., Sias, C., Capobianchi, M. R. & Del Porto, P. The use of both therapeutic and prophylactic vaccines in the therapy of papillomavirus disease. Front. Immunol. 11, 188 (2020).
Chabeda, A. et al. Therapeutic vaccines for high-risk HPV-associated diseases. Papillomavirus Res. 5, 46–58 (2018).
Yang, A. et al. Current state in the development of candidate therapeutic HPV vaccines. Expert. Rev. Vaccines 15, 989–1007 (2016).
Maciag, P. C., Radulovic, S. & Rothman, J. The first clinical use of a live-attenuated Listeria monocytogenes vaccine: a phase I safety study of Lm-LLO-E7 in patients with advanced carcinoma of the cervix. Vaccine 27, 3975–3983 (2009).
Rosales, R. et al. Regression of human papillomavirus intraepithelial lesions is induced by MVA E2 therapeutic vaccine. Hum. Gene Ther. 25, 1035–1049 (2014).
Trimble, C. L. et al. Safety, efficacy, and immunogenicity of VGX-3100, a therapeutic synthetic DNA vaccine targeting human papillomavirus 16 and 18 E6 and E7 proteins for cervical intraepithelial neoplasia 2/3: a randomised, double-blind, placebo-controlled phase 2b trial. Lancet 386, 2078–2088 (2015).
US National Library of Medicine. ClinicalTrials.gov, https://ClinicalTrials.gov/show/NCT04432597 (2022).
Strauss, J. et al. Bintrafusp alfa, a bifunctional fusion protein targeting TGF-β and PD-L1, in patients with human papillomavirus-associated malignancies. J. Immunother. Cancer https://doi.org/10.1136/jitc-2020-001395 (2020).
Daayana, S. et al. Phase II trial of imiquimod and HPV therapeutic vaccination in patients with vulval intraepithelial neoplasia. Br. J. Cancer 102, 1129–1136 (2010).
Rosenberg, S. A. et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin. Cancer Res. 17, 4550–4557 (2011).
Besser, M. J. et al. Clinical responses in a phase II study using adoptive transfer of short-term cultured tumor infiltration lymphocytes in metastatic melanoma patients. Clin. Cancer Res. 16, 2646–2655 (2010).
Stevanovic, S. et al. Complete regression of metastatic cervical cancer after treatment with human papillomavirus-targeted tumor-infiltrating T cells. J. Clin. Oncol. 33, 1543–1550 (2015).
Stevanovic, S. et al. A phase II study of tumor-infiltrating lymphocyte therapy for human papillomavirus-associated epithelial cancers. Clin. Cancer Res. 25, 1486–1493 (2019).
Fujita, K. et al. Prolonged disease-free period in patients with advanced epithelial ovarian cancer after adoptive transfer of tumor-infiltrating lymphocytes. Clin. Cancer Res. 1, 501–507 (1995).
Aydin, A. M. et al. Expansion of tumor-infiltrating lymphocytes (TIL) from penile cancer patients. Int. Immunopharmacol. 94, 107481 (2021).
Chalmers, Z. R. et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med. 9, 34 (2017).
Restifo, N. P., Dudley, M. E. & Rosenberg, S. A. Adoptive immunotherapy for cancer: harnessing the T cell response. Nat. Rev. Immunol. 12, 269–281 (2012).
Nagarsheth, N. B. et al. TCR-engineered T cells targeting E7 for patients with metastatic HPV-associated epithelial cancers. Nat. Med. 27, 419–425 (2021).
Doran, S. L. et al. T-cell receptor gene therapy for human papillomavirus-associated epithelial cancers: a first-in-human, phase I/II study. J. Clin. Oncol. 37, 2759–2768 (2019).
Kunert, A. et al. TCR-engineered T cells meet new challenges to treat solid tumors: choice of antigen, T cell fitness, and sensitization of tumor milieu. Front. Immunol. 4, 363 (2013).
Zhao, L. & Cao, Y. J. Engineered T cell therapy for cancer in the clinic. Front. Immunol. 10, 2250 (2019).
Wagner, J., Wickman, E., DeRenzo, C. & Gottschalk, S. CAR T cell therapy for solid tumors: bright future or dark reality? Mol. Ther. 28, 2320–2339 (2020).
Newick, K., O’Brien, S., Moon, E. & Albelda, S. M. CAR T cell therapy for solid tumors. Annu. Rev. Med. 68, 139–152 (2017).
Wolf, B. et al. Safety and tolerability of adoptive cell therapy in cancer. Drug Saf. 42, 315–334 (2019).
D’Ippolito, E., Schober, K., Nauerth, M. & Busch, D. H. T cell engineering for adoptive T cell therapy: safety and receptor avidity. Cancer Immunol. Immunother. 68, 1701–1712 (2019).
Grigor, E. J. M. et al. Risks and benefits of chimeric antigen receptor T-cell (CAR-T) therapy in cancer: a systematic review and meta-analysis. Transfus. Med. Rev. 33, 98–110 (2019).
US National Library of Medicine. ClinicalTrials.gov, https://ClinicalTrials.gov/show/NCT02379520 (2022).
Gyurkocza, B. & Sandmaier, B. M. Conditioning regimens for hematopoietic cell transplantation: one size does not fit all. Blood 124, 344–353 (2014).
Smith, T. J. et al. Recommendations for the use of WBC growth factors: American Society of Clinical Oncology clinical practice guideline update. J. Clin. Oncol. 33, 3199–3212 (2015).
Urban, D. et al. Mortality among neutropenic cancer patients within the United States: the association with hospital volume. JCO Oncol. Pract. 17, e582–e592 (2021).
Lu, X. et al. Effective combinatorial immunotherapy for castration-resistant prostate cancer. Nature 543, 728–732 (2017).
Naumann, C. M. et al. Establishment and characterization of primary cell lines of squamous cell carcinoma of the penis and its metastasis. J. Urol. 187, 2236–2242 (2012).
Munoz, J. J. et al. A comprehensive characterization of cell cultures and xenografts derived from a human verrucous penile carcinoma. Tumour Biol. 37, 11375–11384 (2016).
Zhou, Q. H. et al. Molecular characterization and integrative genomic analysis of a panel of newly established penile cancer cell lines. Cell Death Dis. 9, 684 (2018).
Hernandez, M. C. et al. Patient-derived xenografts in surgical oncology: a short research review. Surgery 168, 1021–1025 (2020).
Forde, P. M. et al. Neoadjuvant PD-1 blockade in resectable lung cancer. N. Engl. J. Med. 378, 1976–1986 (2018).
Kastenmuller, W. et al. Regulatory T cells selectively control CD8+ T cell effector pool size via IL-2 restriction. J. Immunol. 187, 3186–3197 (2011).
Kamel, M. H. et al. Survival outcomes of organ sparing surgery, partial penectomy, and total penectomy in pathological T1/T2 penile cancer: report from the National Cancer Data Base. Urol. Oncol. 36, 82.e7–82.e15 (2018).
Zukiwskyj, M., Daly, P. & Chung, E. Penile cancer and phallus preservation strategies: a review of current literature. BJU Int. 112, 21–26 (2013).
Bayles, A. C. & Sethia, K. K. The impact of Improving outcomes guidance on the management and outcomes of patients with carcinoma of the penis. Ann. R. Coll. Surg. Engl. 92, 44–45 (2010).
Chipollini, J., Tang, D. H., Sharma, P., Baumgarten, A. S. & Spiess, P. E. Patterns of regional lymphadenectomy for clinically node-negative patients with penile carcinoma: analysis from the national cancer database from 1998 to 2012. Clin. Genitourin. Cancer 15, 670–677.e1 (2017).
Kamel, M. H. Should the care of penile cancer be confined to centralized centers of excellence? Eur. Urol. Focus. 5, 735–736 (2019).
Jakobsen, J. K., Pettaway, C. A. & Ayres, B. Centralization and equitable care in rare urogenital malignancies: the case for penile cancer. Eur. Urol. Focus. https://doi.org/10.1016/j.euf.2021.09.019 (2021).
Canter, D. J. et al. The international penile advanced cancer trial (InPACT): rationale and current status. Eur. Urol. Focus. 5, 706–709 (2019).
Vanthoor, J. et al. Making surgery safer by centralization of care: impact of case load in penile cancer. World J. Urol. 38, 1385–1390 (2020).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/show/NCT03686332 (2022).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/show/NCT04231981 (2022).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/show/NCT04718584 (2021).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/show/NCT02496208 (2022).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/show/NCT04357873 (2021).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/show/NCT03866382 (2022).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/show/NCT02721732 (2022).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/show/NCT03333616 (2021).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/show/NCT03517488 (2022).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/show/NCT02834013 (2022).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/show/NCT03427411 (2022).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/show/NCT03439085 (2022).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/show/NCT04287868 (2022).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/show/NCT03418480 (2022).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/show/NCT02858310 (2022).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/show/NCT04180215 (2022).
Author information
Authors and Affiliations
Contributions
V.B.J. and J.C. researched data for the article and wrote the manuscript. All authors contributed substantially to discussion of the content and reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
P.E.S. maintains leadership positions of relevance as a member of the NCCN penile cancer panel, President of the Global Society of Rare GU Tumors and ASCO/EAU Penile Cancer Panel Member. A.N. serves as Vice-President of the Global Society of Rare GU Tumors (GSRGT) and is an ASCO/EAU Penile Cancer Panel Member. C.A.P. is an Editorial Consultant for the ‘UpToDate’ penile cancer series published by Wolters Kluwer. J.C. reports that he provided advisory board consultations for Pfizer, Aveo and Exelixis. V.B.J. declares no competing interests.
Peer review
Peer review information
Nature Reviews Urology thanks Oliver Hakenberg, Juanita Crook and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
GSRGT: https://www.gsrgt.com
Rights and permissions
About this article
Cite this article
Joshi, V.B., Spiess, P.E., Necchi, A. et al. Immune-based therapies in penile cancer. Nat Rev Urol 19, 457–474 (2022). https://doi.org/10.1038/s41585-022-00617-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41585-022-00617-x