Abstract
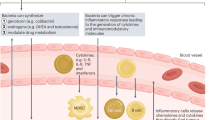
Ageing is correlated with elevated bladder cancer incidence, morbidity and mortality. Advanced age is also associated with elevated markers of chronic inflammation and perturbations in gut and urinary tract microbiota. One reason for the increased incidence and mortality of bladder cancer in the elderly might be that age-associated changes in multiple microbiomes induce systemic metabolic changes that contribute to immune dysregulation with potentially tumorigenic effects. The gut and urinary microbiomes could be dysregulated in bladder cancer, although the effect of these changes is poorly understood. Each of these domains — the immune system, gut microbiome and urinary microbiome — might also influence the response of patients with bladder cancer to treatment. Improved understanding of age-related alterations to the immune system and gut and urinary microbiomes could provide possible insight into the risk of bladder cancer development and progression in the elderly. In patients with bladder cancer, improved understanding of microbiota might also provide potential targets for therapeutic intervention.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
National Cancer Institute. Cancer stat facts: bladder cancer. SEER https://seer.cancer.gov/statfacts/html/urinb.html (2020).
Taylor, J. A. III & Kuchel, G. A. Bladder cancer in the elderly: clinical outcomes, basic mechanisms, and future research direction. Nat. Clin. Pract. Urol. 6, 135–144 (2009).
Babjuk, M. Bladder cancer in the elderly. Eur. Urol. 73, 51–52 (2018).
Feng, H., Zhang, W., Li, J. & Lu, X. Different patterns in the prognostic value of age for bladder cancer-specific survival depending on tumor stages. Am. J. Cancer Res. 5, 2090–2097 (2015).
Shariat, S. F. et al. The effect of age and gender on bladder cancer: a critical review of the literature. BJU Int. 105, 300–308 (2010).
Luzzago, S. et al. Effect of age on cancer-specific mortality in patients with urothelial carcinoma of the urinary bladder: a population-based competing-risks analysis across disease stages. Am. J. Clin. Oncol. 43, 880–888 (2020).
Stanton, M. L., Xiao, L., Czerniak, B. A. & Guo, C. C. Urothelial tumors of the urinary bladder in young patients: a clinicopathologic study of 59 cases. Arch. Pathol. Lab. Med. 137, 1337–1341 (2013).
Wang, Q. H. et al. Clinicopathologic comparison of urothelial bladder carcinoma in young and elder patients. Pathol. Oncol. Res. 22, 67–70 (2016).
Wang, Z. H. et al. Does urothelial cancer of bladder behave differently in young patients? Chin. Med. J. 125, 2643–2648 (2012).
Konety, B. R. & Joslyn, S. A. Factors influencing aggressive therapy for bladder cancer: an analysis of data from the SEER program. J. Urol. 170, 1765–1771 (2003). Crucial analysis of the SEER database demonstrating differences in ageing and bladder cancer.
Briggs, N. C., Young, T. B., Gilchrist, K. W., Vaillancourt, A. M. & Messing, E. M. Age as a predictor of an aggressive clinical course for superficial bladder cancer in men. Cancer 69, 1445–1451 (1992).
Tan, T. Z., Rouanne, M., Tan, K. T., Huang, R. Y. & Thiery, J. P. Molecular subtypes of urothelial bladder cancer: results from a meta-cohort analysis of 2411 tumors. Eur. Urol. 75, 423–432 (2019). Robust analysis of tumour subtyping and how it relates to bladder cancer outcomes. Early molecular subtyping data showing differences in tumours derived from young and old patients.
Knowles, M. A. & Hurst, C. D. Molecular biology of bladder cancer: new insights into pathogenesis and clinical diversity. Nat. Rev. Cancer 15, 25–41 (2015).
Pietzak, E. J. et al. Next-generation sequencing of nonmuscle invasive bladder cancer reveals potential biomarkers and rational therapeutic targets. Eur. Urol. 72, 952–959 (2017).
Sung, J. Y. et al. FGFR3 overexpression is prognostic of adverse outcome for muscle-invasive bladder carcinoma treated with adjuvant chemotherapy. Urol. Oncol. 32, 49.e23–31 (2014).
Kacew, A. & Sweis, R. F. FGFR3 alterations in the era of immunotherapy for urothelial bladder cancer. Front. Immunol. 11, 575258 (2020).
Chappidi, M. R. et al. Frailty as a marker of adverse outcomes in patients with bladder cancer undergoing radical cystectomy. Urol. Oncol. 34, 256.e1–6 (2016).
Palumbo, C. et al. Patient frailty predicts worse perioperative outcomes and higher cost after radical cystectomy. Surg. Oncol. 32, 8–13 (2020).
Sathianathen, N. J., Jarosek, S., Lawrentschuk, N., Bolton, D. & Konety, B. R. A simplified frailty index to predict outcomes after radical cystectomy. Eur. Urol. Focus. 5, 658–663 (2019).
Horovitz, D. et al. Does patient age affect survival after radical cystectomy? BJU Int. 110, E486–E493 (2012).
Gray, P. J. et al. Use of potentially curative therapies for muscle-invasive bladder cancer in the United States: results from the national cancer data base. Eur. Urol. 63, 823–829 (2013).
Resorlu, B., Beduk, Y., Baltaci, S., Ergun, G. & Talas, H. The prognostic significance of advanced age in patients with bladder cancer treated with radical cystectomy. BJU Int. 103, 480–483 (2009).
Franceschi, C. & Bonafe, M. Centenarians as a model for healthy aging. Biochem. Soc. Trans. 31, 457–461 (2003).
Franceschi, C., Garagnani, P., Parini, P., Giuliani, C. & Santoro, A. Inflammaging: a new immune-metabolic viewpoint for age-related diseases. Nat. Rev. Endocrinol. 14, 576–590 (2018).
Franceschi, C., Garagnani, P., Vitale, G., Capri, M. & Salvioli, S. Inflammaging and ‘Garb-aging’. Trends Endocrinol. Metab. 28, 199–212 (2017).
Guedj, A. et al. Gut microbiota shape ‘inflamm-ageing’ cytokines and account for age-dependent decline in DNA damage repair. Gut 69, 1064–1075 (2020).
Guedj, A. et al. Early age decline in DNA repair capacity in the liver: in depth profile of differential gene expression. Aging 8, 3131–3146 (2016).
Biragyn, A. & Ferrucci, L. Gut dysbiosis: a potential link between increased cancer risk in ageing and inflammaging. Lancet Oncol. 19, e295–e304 (2018).
Ghosh, T. S., Das, M., Jeffery, I. B. & O’Toole, P. W. Adjusting for age improves identification of gut microbiome alterations in multiple diseases. eLife 9, e50240 (2020).
Gopalakrishnan, V. et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science 359, 97–103 (2018).
Routy, B. et al. The gut microbiota influences anticancer immunosurveillance and general health. Nat. Rev. Clin. Oncol. 15, 382–396 (2018).
Routy, B. et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 359, 91–97 (2018).
Baruch, E. N. et al. Fecal microbiota transplant promotes response in immunotherapy-refractory melanoma patients. Science 371, 602–609 (2021). Report demonstrating early clinical efficacy of faecal microbiota transplant.
Aso, Y. et al. Preventive effect of a Lactobacillus casei preparation on the recurrence of superficial bladder cancer in a double-blind trial. The BLP Study Group. Eur. Urol. 27, 104–109 (1995).
Aso, Y. & Akazan, H. Prophylactic effect of a Lactobacillus casei preparation on the recurrence of superficial bladder cancer. BLP Study Group. Urol. Int. 49, 125–129 (1992).
He, C. et al. Sulforaphane normalizes intestinal flora and enhances gut barrier in mice with BBN-induced bladder cancer. Mol. Nutr. Food Res. 62, e1800427 (2018). First analysis of gut microbiome in patients with bladder cancer.
Naito, S. et al. Prevention of recurrence with epirubicin and lactobacillus casei after transurethral resection of bladder cancer. J. Urol. 179, 485–490 (2008).
Bektas, A., Schurman, S. H., Sen, R. & Ferrucci, L. Human T cell immunosenescence and inflammation in aging. J. Leukoc. Biol. 102, 977–988 (2017).
Franceschi, C. et al. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann. NY Acad. Sci. 908, 244–254 (2000).
Fulop, T. et al. Immunosenescence and cancer. Crit. Rev. Oncog. 18, 489–513 (2013).
Albayrak, S. et al. Can the neutrophil-to-lymphocyte ratio be used to predict recurrence and progression of non-muscle-invasive bladder cancer? Kaohsiung J. Med. Sci. 32, 327–333 (2016).
Gondo, T. et al. Prognostic value of neutrophil-to-lymphocyte ratio and establishment of novel preoperative risk stratification model in bladder cancer patients treated with radical cystectomy. Urology 79, 1085–1091 (2012).
Hermanns, T. et al. Pre-treatment neutrophil-to-lymphocyte ratio as predictor of adverse outcomes in patients undergoing radical cystectomy for urothelial carcinoma of the bladder. Br. J. Cancer 111, 444–451 (2014).
Kim, J. & Bae, J. S. Tumor-associated macrophages and neutrophils in tumor microenvironment. Mediat. Inflamm. 2016, 6058147 (2016).
Liu, K., Zhao, K., Wang, L. & Sun, E. The prognostic values of tumor-infiltrating neutrophils, lymphocytes and neutrophil/lymphocyte rates in bladder urothelial cancer. Pathol. Res. Pract. 214, 1074–1080 (2018).
Mbeutcha, A. et al. Prognostic significance of markers of systemic inflammatory response in patients with non-muscle-invasive bladder cancer. Urol. Oncol. 34, 483 e417–483 e424 (2016).
Zhou, L. et al. Tumor-infiltrating neutrophils predict benefit from adjuvant chemotherapy in patients with muscle invasive bladder cancer. Oncoimmunology 6, e1293211 (2017).
Sadighi Akha, A. A. Aging and the immune system: an overview. J. Immunol. Methods 463, 21–26 (2018).
Fulop, T. et al. Immunosenescence and inflamm-aging as two sides of the same coin: friends or foes? Front. Immunol. 8, 1960 (2017).
Linton, P. J. & Dorshkind, K. Age-related changes in lymphocyte development and function. Nat. Immunol. 5, 133–139 (2004).
Rock, K. L., Latz, E., Ontiveros, F. & Kono, H. The sterile inflammatory response. Annu. Rev. Immunol. 28, 321–342 (2010).
Rock, K. L. & Kono, H. The inflammatory response to cell death. Annu. Rev. Pathol. 3, 99–126 (2008).
Leidal, A. M., Levine, B. & Debnath, J. Autophagy and the cell biology of age-related disease. Nat. Cell Biol. 20, 1338–1348 (2018).
Ershler, W. B. et al. Interleukin-6 and aging: blood levels and mononuclear cell production increase with advancing age and in vitro production is modifiable by dietary restriction. Lymphokine Cytokine Res. 12, 225–230 (1993).
Sharma, B., Nannuru, K. C., Varney, M. L. & Singh, R. K. Host Cxcr2-dependent regulation of mammary tumor growth and metastasis. Clin. Exp. Metastasis 32, 65–72 (2015).
Sharma, B., Nawandar, D. M., Nannuru, K. C., Varney, M. L. & Singh, R. K. Targeting CXCR2 enhances chemotherapeutic response, inhibits mammary tumor growth, angiogenesis, and lung metastasis. Mol. Cancer Ther. 12, 799–808 (2013).
Hoeijmakers, J. H. DNA damage, aging, and cancer. N. Engl. J. Med. 361, 1475–1485 (2009).
Sjödahl, G. et al. Infiltration of CD3+ and CD68+ cells in bladder cancer is subtype specific and affects the outcome of patients with muscle-invasive tumors. Urol. Oncol. 32, 791–797 (2014).
Wu, S. Q., Xu, R., Li, X. F., Zhao, X. K. & Qian, B. Z. Prognostic roles of tumor associated macrophages in bladder cancer: a system review and meta-analysis. Oncotarget 9, 25294–25303 (2018).
Roszer, T. Understanding the mysterious M2 macrophage through activation markers and effector mechanisms. Mediators Inflamm. 2015, 816460 (2015).
Vartolomei, M. D. et al. Prognostic role of pretreatment neutrophil-to-lymphocyte ratio (NLR) in patients with non-muscle-invasive bladder cancer (NMIBC): a systematic review and meta-analysis. Urol. Oncol. 36, 389–399 (2018).
Viers, B. R. et al. Pretreatment neutrophil-to-lymphocyte ratio is associated with advanced pathologic tumor stage and increased cancer-specific mortality among patients with urothelial carcinoma of the bladder undergoing radical cystectomy. Eur. Urol. 66, 1157–1164 (2014). Initial large study demonstrating usefulness of the NLR in muscle-invasive bladder cancer.
Parada, B. et al. Anti-inflammatory, anti-proliferative and antioxidant profiles of selective cyclooxygenase-2 inhibition as chemoprevention for rat bladder carcinogenesis. Cancer Biol. Ther. 8, 1615–1622 (2009).
Suttmann, H. et al. Neutrophil granulocytes are required for effective Bacillus Calmette-Guérin immunotherapy of bladder cancer and orchestrate local immune responses. Cancer Res. 66, 8250–8257 (2006).
Kawai, K. et al. Enhancement of rat urinary bladder tumorigenesis by lipopolysaccharide-induced inflammation. Cancer Res. 53, 5172–5175 (1993).
Tamatani, T., Turk, P., Weitzman, S. & Oyasu, R. Tumorigenic conversion of a rat urothelial cell line by human polymorphonuclear leukocytes activated by lipopolysaccharide. Jpn. J. Cancer Res. 90, 829–836 (1999).
Yu, L. X. et al. Endotoxin accumulation prevents carcinogen-induced apoptosis and promotes liver tumorigenesis in rodents. Hepatology 52, 1322–1333 (2010).
Elchuri, S. et al. CuZnSOD deficiency leads to persistent and widespread oxidative damage and hepatocarcinogenesis later in life. Oncogene 24, 367–380 (2005).
Dhawan, D., Jeffreys, A. B., Zheng, R., Stewart, J. C. & Knapp, D. W. Cyclooxygenase-2 dependent and independent antitumor effects induced by celecoxib in urinary bladder cancer cells. Mol. Cancer Ther. 7, 897–904 (2008).
Daley, J. M., Thomay, A. A., Connolly, M. D., Reichner, J. S. & Albina, J. E. Use of Ly6G-specific monoclonal antibody to deplete neutrophils in mice. J. Leukoc. Biol. 83, 64–70 (2008).
Wilson, C. L. et al. NFκB1 is a suppressor of neutrophil-driven hepatocellular carcinoma. Nat. Commun. 6, 6818 (2015).
Coffelt, S. B. et al. IL-17-producing γδ T cells and neutrophils conspire to promote breast cancer metastasis. Nature 522, 345–348 (2015).
Jamieson, T. et al. Inhibition of CXCR2 profoundly suppresses inflammation-driven and spontaneous tumorigenesis. J. Clin. Invest. 122, 3127–3144 (2012).
Yu, A. et al. Presence of lymphocytic infiltrate cytotoxic T lymphocyte CD3+, CD8+, and immunoscore as prognostic marker in patients after radical cystectomy. PLoS ONE 13, e0205746 (2018).
Faraj, S. F. et al. Assessment of tumoral PD-L1 expression and intratumoral CD8+ T cells in urothelial carcinoma. Urology 85, 703.e701–706 (2015).
Sharma, P. et al. CD8 tumor-infiltrating lymphocytes are predictive of survival in muscle-invasive urothelial carcinoma. Proc. Natl Acad. Sci. USA 104, 3967–3972 (2007).
Holland, B. C. et al. Age and sex have no impact on expression levels of markers of immune cell infiltration and immune checkpoint pathways in patients with muscle-invasive urothelial carcinoma of the bladder treated with radical cystectomy. Cancer Immunol. Immunother. 68, 991–997 (2019).
Gilbert, J. A. et al. Current understanding of the human microbiome. Nat. Med. 24, 392–400 (2018).
Buchta Rosean, C. M. & Rutkowski, M. R. The influence of the commensal microbiota on distal tumor-promoting inflammation. Semin. Immunol. 32, 62–73 (2017).
Chen, J. et al. The microbiome and breast cancer: a review. Breast Cancer Res. Treat. 178, 493–496 (2019).
Koliarakis, I. et al. Oral bacteria and intestinal dysbiosis in colorectal cancer. Int. J. Mol. Sci. 20, 4146 (2019).
Zhuang, H. et al. Dysbiosis of the gut microbiome in lung cancer. Front. Cell Infect. Microbiol. 9, 112 (2019).
Rajagopala, S. V. et al. The human microbiome and cancer. Cancer Prev. Res. 10, 226–234 (2017).
Spadoni, I. et al. A gut-vascular barrier controls the systemic dissemination of bacteria. Science 350, 830–834 (2015).
Mouries, J. et al. Microbiota-driven gut vascular barrier disruption is a prerequisite for non-alcoholic steatohepatitis development. J. Hepatol. 71, 1216–1228 (2019).
Grander, C. et al. The role of gut vascular barrier in experimental alcoholic liver disease and A. muciniphila supplementation. Gut Microbes 12, 1851986 (2020).
Mármol, I., Sánchez-de-Diego, C., Pradilla Dieste, A., Cerrada, E. & Rodriguez Yoldi, M. J. Colorectal carcinoma: a general overview and future perspectives in colorectal cancer. Int. J. Mol. Sci. 18, 197 (2017).
Allam-Ndoul, B., Castonguay-Paradis, S. & Veilleux, A. Gut microbiota and intestinal trans-epithelial permeability. Int. J. Mol. Sci. 21, 6402 (2020).
He, C. et al. Gut microbial composition changes in bladder cancer patients: a case-control study in Harbin, China. Asia Pac. J. Clin. Nutr. 29, 395–403 (2020).
Chopin, V., Toillon, R. A., Jouy, N. & Le Bourhis, X. Sodium butyrate induces P53-independent, Fas-mediated apoptosis in MCF-7 human breast cancer cells. Br. J. Pharmacol. 135, 79–86 (2002).
Nastasi, C. et al. Butyrate and propionate inhibit antigen-specific CD8+ T cell activation by suppressing IL-12 production by antigen-presenting cells. Sci. Rep. 7, 14516 (2017).
Wang, F. et al. Sodium butyrate inhibits migration and induces AMPK-mTOR pathway-dependent autophagy and ROS-mediated apoptosis via the miR-139-5p/Bmi-1 axis in human bladder cancer cells. FASEB J. 34, 4266–4282 (2020).
Tan, J. et al. The role of short-chain fatty acids in health and disease. Adv. Immunol. 121, 91–119 (2014).
Thevaranjan, N. et al. Age-associated microbial dysbiosis promotes intestinal permeability, systemic inflammation, and macrophage dysfunction. Cell Host Microbe 21, 455–466 e454 (2017).
Bian, G. et al. The gut microbiota of healthy aged Chinese is similar to that of the healthy young. mSphere 2, e00327-17 (2017).
Badal, V. D. et al. The gut microbiome, aging, and longevity: a systematic review. Nutrients 12, 3759 (2020).
Derrien, M., Belzer, C. & de Vos, W. M. Akkermansia muciniphila and its role in regulating host functions. Microb. Pathog. 106, 171–181 (2017).
Biagi, E. et al. The gut microbiota of centenarians: signatures of longevity in the gut microbiota profile. Mech. Ageing Dev. 165, 180–184 (2017). Important analysis of the microbiome in centenarians and other age groups.
Derosa, L. et al. Intestinal Akkermansia muciniphila predicts clinical response to PD-1 blockade in patients with advanced non-small-cell lung cancer. Nat. Med. 28, 315–324 (2022). Seminal paper demonstrating the potential benefit of Akkermansia in cancer therapy.
Biagi, E. et al. Gut microbiota and extreme longevity. Curr. Biol. 26, 1480–1485 (2016).
Shin, N. R. et al. An increase in the Akkermansia spp. population induced by metformin treatment improves glucose homeostasis in diet-induced obese mice. Gut 63, 727–735 (2014).
Grajeda-Iglesias, C. et al. Oral administration of Akkermansia muciniphila elevates systemic antiaging and anticancer metabolites. Aging 13, 6375–6405 (2021).
Scheppach, W., Bartram, H. P. & Richter, F. Role of short-chain fatty acids in the prevention of colorectal cancer. Eur. J. Cancer 31A, 1077–1080 (1995).
Donohoe, D. R. et al. The Warburg effect dictates the mechanism of butyrate-mediated histone acetylation and cell proliferation. Mol. Cell 48, 612–626 (2012).
Cai, D. et al. Nutrient intake is associated with longevity characterization by metabolites and element profiles of healthy centenarians. Nutrients 8, 564 (2016).
Wu, L. et al. A cross-sectional study of compositional and functional profiles of gut microbiota in Sardinian centenarians. mSystems 4, e00325-19 (2019).
Salazar, N. et al. Age-associated changes in gut microbiota and dietary components related with the immune system in adulthood and old age: a cross-sectional study. Nutrients 11, 1765 (2019).
Iraporda, C. et al. Lactate and short chain fatty acids produced by microbial fermentation downregulate proinflammatory responses in intestinal epithelial cells and myeloid cells. Immunobiology 220, 1161–1169 (2015).
Cox, M. A. et al. Short-chain fatty acids act as antiinflammatory mediators by regulating prostaglandin E2 and cytokines. World J. Gastroenterol. 15, 5549–5557 (2009).
Wang, F. et al. The inflammation induced by lipopolysaccharide can be mitigated by short-chain fatty acid, butyrate, through upregulation of IL-10 in septic shock. Scand. J. Immunol. 85, 258–263 (2017).
Luu, M. et al. Regulation of the effector function of CD8+ T cells by gut microbiota-derived metabolite butyrate. Sci. Rep. 8, 14430 (2018).
Coutzac, C. et al. Systemic short chain fatty acids limit antitumor effect of CTLA-4 blockade in hosts with cancer. Nat. Commun. 11, 2168 (2020).
Krantz, D. et al. Neoadjuvant chemotherapy reinforces antitumour T cell response in urothelial urinary bladder cancer. Eur. Urol. 74, 688–692 (2018).
Eruslanov, E., Daurkin, I., Vieweg, J., Daaka, Y. & Kusmartsev, S. Aberrant PGE2 metabolism in bladder tumor microenvironment promotes immunosuppressive phenotype of tumor-infiltrating myeloid cells. Int. Immunopharmacol. 11, 848–855 (2011).
Kurtova, A. V. et al. Blocking PGE2-induced tumour repopulation abrogates bladder cancer chemoresistance. Nature 517, 209–213 (2015).
Vinolo, M. A. et al. Short-chain fatty acids stimulate the migration of neutrophils to inflammatory sites. Clin. Sci. 117, 331–338 (2009).
Sonowal, R. et al. Indoles from commensal bacteria extend healthspan. Proc. Natl Acad. Sci. USA 114, E7506–E7515 (2017).
Barcena, C. et al. Healthspan and lifespan extension by fecal microbiota transplantation into progeroid mice. Nat. Med. 25, 1234–1242 (2019).
Antushevich, H. Fecal microbiota transplantation in disease therapy. Clin. Chim. Acta 503, 90–98 (2020).
Chen, D., Wu, J., Jin, D., Wang, B. & Cao, H. Fecal microbiota transplantation in cancer management: current status and perspectives. Int. J. Cancer 145, 2021–2031 (2019).
Rothschild, D. et al. Environment dominates over host genetics in shaping human gut microbiota. Nature 555, 210–215 (2018).
Clark, R. I. et al. Distinct shifts in microbiota composition during drosophila aging impair intestinal function and drive mortality. Cell Rep. 12, 1656–1667 (2015).
Curty, G., de Carvalho, P. S. & Soares, M. A. The role of the cervicovaginal microbiome on the genesis and as a biomarker of premalignant cervical intraepithelial neoplasia and invasive cervical cancer. Int. J. Mol. Sci. 21, 222 (2019).
Mani, S. Microbiota and breast cancer. Prog. Mol. Biol. Transl. Sci. 151, 217–229 (2017).
Tran, L. & Greenwood-Van Meerveld, B. Age-associated remodeling of the intestinal epithelial barrier. J. Gerontol. A Biol. Sci. Med. Sci. 68, 1045–1056 (2013).
Kühn, F. et al. Intestinal alkaline phosphatase targets the gut barrier to prevent aging. JCI Insight 5, e134049 (2020).
Schirmer, M. et al. Linking the human gut microbiome to inflammatory cytokine production capacity. Cell 167, 1125–1136.e8 (2016).
Champer, M. et al. The role of the vaginal microbiome in gynaecological cancer. BJOG 125, 309–315 (2018).
Wu, P. et al. Profiling the urinary microbiota in male patients with bladder cancer in China. Front. Cell Infect. Microbiol. 8, 167 (2018).
Zeng, J. et al. Alterations in urobiome in patients with bladder cancer and implications for clinical outcome: a single-institution study. Front. Cell Infect. Microbiol. 10, 555508 (2020).
Aragón, I. M. et al. The urinary tract microbiome in health and disease. Eur. Urol. Focus 4, 128–138 (2018).
Lewis, D. A. et al. The human urinary microbiome; bacterial DNA in voided urine of asymptomatic adults. Front. Cell Infect. Microbiol. 3, 41 (2013).
Fouts, D. E. et al. Integrated next-generation sequencing of 16 S rDNA and metaproteomics differentiate the healthy urine microbiome from asymptomatic bacteriuria in neuropathic bladder associated with spinal cord injury. J. Transl Med. 10, 174 (2012).
Pohl, H. G. et al. The urine microbiome of healthy men and women differs by urine collection method. Int. Neurourol. J. 24, 41–51 (2020).
Burnett, L. A., Hochstedler, B. R., Weldon, K., Wolfe, A. J. & Brubaker, L. Recurrent urinary tract infection: association of clinical profiles with urobiome composition in women. Neurourol. Urodyn. 40, 1479–1489 (2021).
Zandbergen, L. E., Halverson, T., Brons, J. K., Wolfe, A. J. & de Vos, M. G. J. The good and the bad: ecological interaction measurements between the urinary microbiota and uropathogens. Front. Microbiol. 12, 659450 (2021).
Bajic, P., Wolfe, A. J. & Gupta, G. N. The urinary microbiome: implications in bladder cancer pathogenesis and therapeutics. Urology 126, 10–15 (2019).
Pearce, M. M. et al. The female urinary microbiome: a comparison of women with and without urgency urinary incontinence. mBio 5, e01283-14 (2014).
Abernethy, M. G. et al. Urinary microbiome and cytokine levels in women with interstitial cystitis. Obstet. Gynecol. 129, 500–506 (2017).
Bučević Popović, V. et al. The urinary microbiome associated with bladder cancer. Sci. Rep. 8, 12157 (2018). Early analysis of the urinary microbiome in patients with bladder cancer.
Markowski, M. C. et al. The microbiome and genitourinary cancer: a collaborative review. Eur. Urol. 75, 637–646 (2019).
Porter, C. M., Shrestha, E., Peiffer, L. B. & Sfanos, K. S. The microbiome in prostate inflammation and prostate cancer. Prostate Cancer Prostatic Dis. 21, 345–354 (2018).
Liu, F. et al. Dysbiosis signatures of the microbial profile in tissue from bladder cancer. Cancer Med. 8, 6904–6914 (2019).
Parra-Grande, M. et al. Profiling the bladder microbiota in patients with bladder cancer. Front. Microbiol. 12, 718776 (2021).
Ching, C. B. et al. Interleukin-6/Stat3 signaling has an essential role in the host antimicrobial response to urinary tract infection. Kidney Int. 93, 1320–1329 (2018).
Samuelsson, P., Hang, L., Wullt, B., Irjala, H. & Svanborg, C. Toll-like receptor 4 expression and cytokine responses in the human urinary tract mucosa. Infect. Immun. 72, 3179–3186 (2004).
McDermott, C. et al. Alterations in acetylcholine, PGE2 and IL6 release from urothelial cells following treatment with pyocyanin and lipopolysaccharide. Toxicol. Vitr. 27, 1693–1698 (2013).
Yeh, J., Lu, M., Alvarez-Lugo, L. & Chai, T. C. Bladder urothelial BK channel activity is a critical mediator for innate immune response in urinary tract infection pathogenesis. Am. J. Physiol. Renal Physiol. 316, F617–F623 (2019).
Ying, H. et al. TLR4 mediates MAPK-STAT3 axis activation in bladder epithelial cells. Inflammation 36, 1064–1074 (2013).
Mirzaei, S. et al. Pre-clinical investigation of STAT3 pathway in bladder cancer: paving the way for clinical translation. Biomed. Pharmacother. 133, 111077 (2021).
Gatta, L. B. et al. Hyper-activation of STAT3 sustains progression of non-papillary basal-type bladder cancer via FOSL1 regulome. Cancers 11, 1219 (2019).
Korac-Prlic, J. et al. Targeting Stat3 signaling impairs the progression of bladder cancer in a mouse model. Cancer Lett. 490, 89–99 (2020).
Reis, S. T. et al. Increased expression of MMP-9 and IL-8 are correlated with poor prognosis of bladder cancer. BMC Urol. 12, 18 (2012).
Huang, C. P., Liu, L. X. & Shyr, C. R. Tumor-associated macrophages facilitate bladder cancer progression by increasing cell growth, migration, invasion and cytokine expression. Anticancer. Res. 40, 2715–2724 (2020).
Urakami, S. et al. Combination analysis of hypermethylated Wnt-antagonist family genes as a novel epigenetic biomarker panel for bladder cancer detection. Clin. Cancer Res. 12, 2109–2116 (2006).
Lin, C. et al. Constitutive β-catenin activation induces male-specific tumorigenesis in the bladder urothelium. Cancer Res. 73, 5914–5925 (2013).
Shin, K. et al. Hedgehog signaling restrains bladder cancer progression by eliciting stromal production of urothelial differentiation factors. Cancer Cell 26, 521–533 (2014).
Shin, K. et al. Hedgehog/Wnt feedback supports regenerative proliferation of epithelial stem cells in bladder. Nature 472, 110–114 (2011).
Mysorekar, I. U., Isaacson-Schmid, M., Walker, J. N., Mills, J. C. & Hultgren, S. J. Bone morphogenetic protein 4 signaling regulates epithelial renewal in the urinary tract in response to uropathogenic infection. Cell Host Microbe 5, 463–475 (2009).
Shin, K. et al. Cellular origin of bladder neoplasia and tissue dynamics of its progression to invasive carcinoma. Nat. Cell Biol. 16, 469–478 (2014). Establishment of the cellular origin of bladder cancer and crucial pathways involved in the proliferation of those cells.
Van Batavia, J. et al. Bladder cancers arise from distinct urothelial sub-populations. Nat. Cell Biol. 16, 982–991 (2014). Establishment of the cellular origin of bladder cancer and crucial pathways involved in proliferation of those cells.
Kaneko, S. & Li, X. X chromosome protects against bladder cancer in females via a KDM6A-dependent epigenetic mechanism. Sci. Adv. 4, eaar5598 (2018).
Bertram, J. S. & Craig, A. W. Specific induction of bladder cancer in mice by butyl-(4-hydroxybutyl)-nitrosamine and the effects of hormonal modifications on the sex difference in response. Eur. J. Cancer 8, 587–594 (1972).
Bayne, C. E., Farah, D., Herbst, K. W. & Hsieh, M. H. Role of urinary tract infection in bladder cancer: a systematic review and meta-analysis. World J. Urol. 36, 1181–1190 (2018).
Kamat, A. M. et al. Bladder cancer. Lancet 388, 2796–2810 (2016).
Gontero, P. et al. Prognostic factors and risk groups in T1G3 non-muscle-invasive bladder cancer patients initially treated with Bacillus Calmette-Guerin: results of a retrospective multicenter study of 2451 patients. Eur. Urol. 67, 74–82 (2015).
Joudi, F. N., Smith, B. J., O’Donnell, M. A. & Konety, B. R. The impact of age on the response of patients with superficial bladder cancer to intravesical immunotherapy. J. Urol. 175, 1634–1639; discussion 175, 1639–1640 (2006).
Herr, H. W. Age and outcome of superficial bladder cancer treated with bacille Calmette-Guérin therapy. Urology 70, 65–68 (2007).
Sweis, R. F. et al. Association of the commensal urinary microbiome with response to Bacillus Calmette-Guérin (BCG) immunotherapy in nonmuscle invasive bladder cancer. J. Clin. Oncol. 37 (Suppl. 7), 423 (2019).
Sivan, A. et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science 350, 1084–1089 (2015).
Morales, A., Eidinger, D. & Bruce, A. W. Intracavitary Bacillus Calmette-Guerin in the treatment of superficial bladder tumors. J. Urol. 116, 180–183 (1976).
Pettenati, C. & Ingersoll, M. A. Mechanisms of BCG immunotherapy and its outlook for bladder cancer. Nat. Rev. Urol. 15, 615–625 (2018).
Pirzada, M. T. et al. Outcomes of BCG induction in high-risk non-muscle-invasive bladder cancer patients (NMIBC): a retrospective cohort study. Cureus 9, e957 (2017).
Kates, M. et al. Adaptive immune resistance to intravesical BCG in non-muscle invasive bladder cancer: implications for prospective BCG-unresponsive trials. Clin. Cancer Res. 26, 882–891 (2020).
Kato, I., Endo, K. & Yokokura, T. Effects of oral administration of Lactobacillus casei on antitumor responses induced by tumor resection in mice. Int. J. Immunopharmacol. 16, 29–36 (1994).
Ohashi, Y. et al. Habitual intake of lactic acid bacteria and risk reduction of bladder cancer. Urol. Int. 68, 273–280 (2002).
Zhang, Y. et al. Lactobacillus casei protects dextran sodium sulfate- or rapamycin-induced colonic inflammation in the mouse. Eur. J. Nutr. 59, 1443–1451 (2020).
Lee, T. H. et al. Lactobacillus salivarius BP121 prevents cisplatin‑induced acute kidney injury by inhibition of uremic toxins such as indoxyl sulfate and p‑cresol sulfate via alleviating dysbiosis. Int. J. Mol. Med. 45, 1130–1140 (2020).
Munday, R. et al. Inhibition of urinary bladder carcinogenesis by broccoli sprouts. Cancer Res. 68, 1593–1600 (2008).
Iida, K. et al. Nrf2 and p53 cooperatively protect against BBN-induced urinary bladder carcinogenesis. Carcinogenesis 28, 2398–2403 (2007).
Xie, H., Chun, F. K., Rutz, J. & Blaheta, R. A. Sulforaphane impact on reactive oxygen species (ROS) in bladder carcinoma. Int. J. Mol. Sci. 22, 5938 (2021).
Teo, M. Y. & Rosenberg, J. E. Nivolumab for the treatment of urothelial cancers. Expert Rev. Anticancer. Ther. 18, 215–221 (2018).
Miller, P. L. & Carson, T. L. Mechanisms and microbial influences on CTLA-4 and PD-1-based immunotherapy in the treatment of cancer: a narrative review. Gut Pathog. 12, 43 (2020).
Vetizou, M. & Trinchieri, G. Anti-PD1 in the wonder-gut-land. Cell Res. 28, 263–264 (2018).
Matson, V. et al. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science 359, 104–108 (2018).
Byrd, A. L. et al. Gut microbiome stability and dynamics in healthy donors and patients with non-gastrointestinal cancers. J. Exp. Med. 218, e20200606 (2021).
Biagi, E. et al. Ageing and gut microbes: perspectives for health maintenance and longevity. Pharmacol. Res. 69, 11–20 (2013).
Acknowledgements
This work is funded in part by the Albert Charitable Trust (J.A.T. III), American Urological Association Research Scholar Award (B.L.W.) and National Institutes of Health (R01CA185322 (S.U.)).
Author information
Authors and Affiliations
Contributions
A.M., B.L.W. and J.A.T. III researched data for the article. All authors contributed substantially to discussion of the content. A.M., B.L.W. and J.A.T. III wrote the article. All authors reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Urology thanks Randy Sweis and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Martin, A., Woolbright, B.L., Umar, S. et al. Bladder cancer, inflammageing and microbiomes. Nat Rev Urol 19, 495–509 (2022). https://doi.org/10.1038/s41585-022-00611-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41585-022-00611-3
This article is cited by
-
Aging induces changes in cancer formation and microbial content in a murine model of bladder cancer
GeroScience (2024)
-
A sialyltransferases-related gene signature serves as a potential predictor of prognosis and therapeutic response for bladder cancer
European Journal of Medical Research (2023)
-
Causal associations between gut microbiota and urological tumors: a two-sample mendelian randomization study
BMC Cancer (2023)
-
Bladder cancer
Nature Reviews Disease Primers (2023)
-
Short-chain fatty acids in cancer pathogenesis
Cancer and Metastasis Reviews (2023)