Abstract
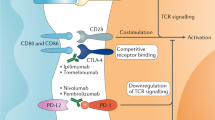
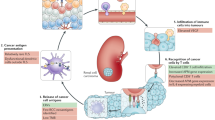
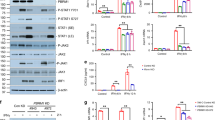
The treatment of advanced and metastatic kidney cancer has entered a golden era with the addition of more therapeutic options, improved survival and new targeted therapies. Tyrosine kinase inhibitors, mammalian target of rapamycin (mTOR) inhibitors and immune checkpoint blockade have all been shown to be promising strategies in the treatment of renal cell carcinoma (RCC). However, little is known about the best therapeutic approach for individual patients with RCC and how to combat therapeutic resistance. Cancers, including RCC, rely on sustained replicative potential. The cyclin-dependent kinases CDK4 and CDK6 are involved in cell-cycle regulation with additional roles in metabolism, immunogenicity and antitumour immune response. Inhibitors of CDK4 and CDK6 are now commonly used as approved and investigative treatments in breast cancer, as well as several other tumours. Furthermore, CDK4/6 inhibitors have been shown to work synergistically with other kinase inhibitors, including mTOR inhibitors, as well as with immune checkpoint inhibitors in preclinical cancer models. The effect of CDK4/6 inhibitors in kidney cancer is relatively understudied compared with other cancers, but the preclinical studies available are promising. Collectively, growing evidence suggests that targeting CDK4 and CDK6 in kidney cancer, alone and in combination with current therapeutics including mTOR and immune checkpoint inhibitors, might have therapeutic benefit and should be further explored.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Siegel, R. L., Miller, K. D. & Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 70, 7–30 (2020).
Hsieh, F. S. et al. Palbociclib induces activation of AMPK and inhibits hepatocellular carcinoma in a CDK4/6-independent manner. Mol. Oncol. 11, 1035–1049 (2017).
McDermott, D. F. et al. Randomized phase III trial of high-dose interleukin-2 versus subcutaneous interleukin-2 and interferon in patients with metastatic renal cell carcinoma. J. Clin. Oncol. 23, 133–141 (2005).
Motzer, R. J. et al. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet 372, 449–456 (2008).
Motzer, R. J. et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N. Engl. J. Med. 356, 115–124 (2007).
Shen, C. & Kaelin, W. G. Jr The VHL/HIF axis in clear cell renal carcinoma. Semin. Cancer Biol. 23, 18–25 (2013).
Linehan, W. M. et al. The metabolic basis of kidney cancer. Cancer Discov. 9, 1006–1021 (2019).
Rini, B. I. et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N. Engl. J. Med. 380, 1116–1127 (2019).
Motzer, R. J. et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N. Engl. J. Med. 373, 1803–1813 (2015).
Motzer, R. J. et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N. Engl. J. Med. 378, 1277–1290 (2018).
Topalian, S. L., Drake, C. G. & Pardoll, D. M. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell 27, 450–461 (2015).
Turner, N. C. et al. Palbociclib in hormone-receptor-positive advanced breast cancer. N. Engl. J. Med. 373, 209–219 (2015).
Hortobagyi, G. N. et al. Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N. Engl. J. Med. 375, 1738–1748 (2016).
Sledge, G. W. Jr et al. MONARCH 2: abemaciclib in combination with fulvestrant in women with HR+/HER2- advanced breast cancer who had progressed while receiving endocrine therapy. J. Clin. Oncol. 35, 2875–2884 (2017).
Baker, S. J. & Reddy, E. P. CDK4: a key player in the cell cycle, development, and cancer. Genes Cancer 3, 658–669 (2012).
Schopf, F. H., Biebl, M. M. & Buchner, J. The HSP90 chaperone machinery. Nat. Rev. Mol. Cell Biol. 18, 345–360 (2017).
Bai, J., Li, Y. & Zhang, G. Cell cycle regulation and anticancer drug discovery. Cancer Biol. Med. 14, 348–362 (2017).
Knudsen, E. S. & Witkiewicz, A. K. The strange case of CDK4/6 inhibitors: mechanisms, resistance, and combination strategies. Trends Cancer 3, 39–55 (2017).
Pernas, S., Tolaney, S. M., Winer, E. P. & Goel, S. CDK4/6 inhibition in breast cancer: current practice and future directions. Ther. Adv. Med. Oncol. 10, 1758835918786451 (2018).
O’Leary, B., Finn, R. S. & Turner, N. C. Treating cancer with selective CDK4/6 inhibitors. Nat. Rev. Clin. Oncol. 13, 417–430 (2016).
Finn, R. S. et al. Palbociclib and letrozole in advanced breast cancer. N. Engl. J. Med. 375, 1925–1936 (2016).
Yardley, D. A. MONALEESA clinical program: a review of ribociclib use in different clinical settings. Future Oncol. 15, 2673–2686 (2019).
Klein, M. E., Kovatcheva, M., Davis, L. E., Tap, W. D. & Koff, A. CDK4/6 inhibitors: the mechanism of action may not be as simple as once thought. Cancer Cell 34, 9–20 (2018).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/show/NCT04751929 (2021).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/show/NCT04438824 (2021).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/show/NCT04360941 (2021).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/show/NCT04213404 (2021).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/show/NCT03294694 (2020).
Cretella, D. et al. The anti-tumor efficacy of CDK4/6 inhibition is enhanced by the combination with PI3K/AKT/mTOR inhibitors through impairment of glucose metabolism in TNBC cells. J. Exp. Clin. Cancer Res. 37, 72 (2018).
Gopalan, P. K. et al. CDK4/6 inhibition stabilizes disease in patients with p16-null non-small cell lung cancer and is synergistic with mTOR inhibition. Oncotarget 9, 37352–37366 (2018).
Michaloglou, C. et al. Combined inhibition of mTOR and CDK4/6 is required for optimal blockade of E2F function and long-term growth inhibition in estrogen receptor-positive breast cancer. Mol. Cancer Ther. 17, 908–920 (2018).
Yamamoto, T., Kanaya, N., Somlo, G. & Chen, S. Synergistic anti-cancer activity of CDK4/6 inhibitor palbociclib and dual mTOR kinase inhibitor MLN0128 in pRb-expressing ER-negative breast cancer. Breast Cancer Res. Treat. 174, 615–625 (2019).
Song, X. et al. Combined CDK4/6 and Pan-mTOR inhibition is synergistic against intrahepatic cholangiocarcinoma. Clin. Cancer Res. 25, 403–413 (2019).
Olmez, I. et al. Combined CDK4/6 and mTOR inhibition is synergistic against glioblastoma via multiple mechanisms. Clin. Cancer Res. 23, 6958–6968 (2017).
Asby, D. J. et al. Combined use of CDK4/6 and mTOR inhibitors induce synergistic growth arrest of diffuse intrinsic pontine glioma cells via mutual downregulation of mTORC1 activity. Cancer Manag. Res. 10, 3483–3500 (2018).
Goel, S. et al. CDK4/6 inhibition triggers anti-tumour immunity. Nature 548, 471–475 (2017).
Deng, J. et al. CDK4/6 inhibition augments antitumor immunity by enhancing T-cell activation. Cancer Discov. 8, 216–233 (2018).
Schaer, D. A. et al. The CDK4/6 inhibitor abemaciclib induces a T cell inflamed tumor microenvironment and enhances the efficacy of PD-L1 checkpoint blockade. Cell Rep. 22, 2978–2994 (2018).
Yu, J. et al. Genetic aberrations in the CDK4 pathway are associated with innate resistance to PD-1 blockade in Chinese patients with non-cutaneous melanoma. Clin. Cancer Res. 25, 6511–6523 (2019).
Zhang, J. et al. Cyclin D-CDK4 kinase destabilizes PD-L1 via cullin 3-SPOP to control cancer immune surveillance. Nature 553, 91–95 (2018).
Malumbres, M. & Barbacid, M. Cell cycle, CDKs and cancer: a changing paradigm. Nat. Rev. Cancer 9, 153–166 (2009).
Satyanarayana, A. & Kaldis, P. Mammalian cell-cycle regulation: several Cdks, numerous cyclins and diverse compensatory mechanisms. Oncogene 28, 2925–2939 (2009).
Sherr, C. J. D-type cyclins. Trends Biochem. Sci. 20, 187–190 (1995).
Sherr, C. J. & Roberts, J. M. Living with or without cyclins and cyclin-dependent kinases. Genes Dev. 18, 2699–2711 (2004).
Malumbres, M. et al. Mammalian cells cycle without the D-type cyclin-dependent kinases CDK4 and CDK6. Cell 118, 493–504 (2004).
Rane, S. G. et al. Loss of CDK4 expression causes insulin-deficient diabetes and CDK4 activation results in beta-islet cell hyperplasia. Nat. Genet. 22, 44–52 (1999).
Kato, J., Matsushime, H., Hiebert, S. W., Ewen, M. E. & Sherr, C. J. Direct binding of cyclin D to the retinoblastoma gene product (pRb) and pRb phosphorylation by the cyclin D-dependent kinase CDK4. Genes Dev. 7, 331–342 (1993).
Pan, W., Cox, S., Hoess, R. H. & Grafstrom, R. H. A cyclin D1/cyclin-dependent kinase 4 binding site within the C domain of the retinoblastoma protein. Cancer Res. 61, 2885–2891 (2001).
Wallace, M. & Ball, K. L. Docking-dependent regulation of the Rb tumor suppressor protein by Cdk4. Mol. Cell Biol. 24, 5606–5619 (2004).
Braden, W. A., McClendon, A. K. & Knudsen, E. S. Cyclin-dependent kinase 4/6 activity is a critical determinant of pre-replication complex assembly. Oncogene 27, 7083–7093 (2008).
Ruas, M. et al. CDK4 and CDK6 delay senescence by kinase-dependent and p16INK4a-independent mechanisms. Mol. Cell Biol. 27, 4273–4282 (2007).
Brookes, S. et al. Evidence for a CDK4-dependent checkpoint in a conditional model of cellular senescence. Cell Cycle 14, 1164–1173 (2015).
Matthews, H. K., Bertoli, C. & de Bruin, R. A. M. Cell cycle control in cancer. Nat. Rev. Mol. Cell Biol. 23, 74–88 (2021).
Haas, K. et al. Mutual requirement of CDK4 and Myc in malignant transformation: evidence for cyclin D1/CDK4 and p16INK4A as upstream regulators of Myc. Oncogene 15, 179–192 (1997).
Hermeking, H. et al. Identification of CDK4 as a target of c-MYC. Proc. Natl Acad. Sci. USA 97, 2229–2234 (2000).
Obaya, A. J., Kotenko, I., Cole, M. D. & Sedivy, J. M. The proto-oncogene c-myc acts through the cyclin-dependent kinase (Cdk) inhibitor p27Kip1 to facilitate the activation of CDK4 and CDK6 and early G1 phase progression. J. Biol. Chem. 277, 31263–31269 (2002).
Tsutsui, T. et al. Targeted disruption of CDK4 delays cell cycle entry with enhanced p27(Kip1) activity. Mol. Cell Biol. 19, 7011–7019 (1999).
Meyer, C. A. et al. Drosophila CDK4 is required for normal growth and is dispensable for cell cycle progression. EMBO J. 19, 4533–4542 (2000).
Jaakkola, P. et al. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 292, 468–472 (2001).
Frei, C. & Edgar, B. A. Drosophila cyclin D/Cdk4 requires Hif-1 prolyl hydroxylase to drive cell growth. Dev. Cell 6, 241–251 (2004).
Lee, Y. et al. Cyclin D1-Cdk4 controls glucose metabolism independently of cell cycle progression. Nature 510, 547–551 (2014).
Wang, H. et al. The metabolic function of cyclin D3-CDK6 kinase in cancer cell survival. Nature 546, 426–430 (2017).
Romero-Pozuelo, J., Figlia, G., Kaya, O., Martin-Villalba, A. & Teleman, A. A. CDK4 and CDK6 couple the cell-cycle machinery to cell growth via mTORC1. Cell Rep. 31, 107504 (2020).
Krall, A. S. & Christofk, H. R. Cell cycle: division enzyme regulates metabolism. Nature 546, 357–358 (2017).
Serrano, M., Hannon, G. J. & Beach, D. A new regulatory motif in cell-cycle control causing specific inhibition of cyclin D/CDK4. Nature 366, 704–707 (1993).
Annicotte, J. S. et al. The CDK4-pRB-E2F1 pathway controls insulin secretion. Nat. Cell Biol. 11, 1017–1023 (2009).
Martinez-Carreres, L. et al. CDK4 regulates lysosomal function and mTORC1 activation to promote cancer cell survival. Cancer Res. 79, 5245–5259 (2019).
Hardie, D. G., Ross, F. A. & Hawley, S. A. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat. Rev. Mol. Cell Biol. 13, 251–262 (2012).
Lopez-Mejia, I. C. et al. CDK4 phosphorylates AMPKalpha2 to inhibit its activity and repress fatty acid oxidation. Mol. Cell 68, 336–349.e6 (2017).
Nebenfuehr, S., Kollmann, K. & Sexl, V. The role of CDK6 in cancer. Int. J. Cancer 147, 2988–2995 (2020).
Dai, K., Kobayashi, R. & Beach, D. Physical interaction of mammalian CDC37 with CDK4. J. Biol. Chem. 271, 22030–22034 (1996).
Stepanova, L., Leng, X., Parker, S. B. & Harper, J. W. Mammalian p50Cdc37 is a protein kinase-targeting subunit of Hsp90 that binds and stabilizes Cdk4. Genes Dev. 10, 1491–1502 (1996).
Gu, Y., Turck, C. W. & Morgan, D. O. Inhibition of CDK2 activity in vivo by an associated 20K regulatory subunit. Nature 366, 707–710 (1993).
Harper, J. W., Adami, G. R., Wei, N., Keyomarsi, K. & Elledge, S. J. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell 75, 805–816 (1993).
Xiong, Y. et al. p21 is a universal inhibitor of cyclin kinases. Nature 366, 701–704 (1993).
Noda, A., Ning, Y., Venable, S. F., Pereira-Smith, O. M. & Smith, J. R. Cloning of senescent cell-derived inhibitors of DNA synthesis using an expression screen. Exp. Cell Res. 211, 90–98 (1994).
Polyak, K. et al. p27Kip1, a cyclin-Cdk inhibitor, links transforming growth factor-beta and contact inhibition to cell cycle arrest. Genes Dev. 8, 9–22 (1994).
Polyak, K. et al. Cloning of p27Kip1, a cyclin-dependent kinase inhibitor and a potential mediator of extracellular antimitogenic signals. Cell 78, 59–66 (1994).
Toyoshima, H. & Hunter, T. p27, a novel inhibitor of G1 cyclin-Cdk protein kinase activity, is related to p21. Cell 78, 67–74 (1994).
Lee, M. H., Reynisdottir, I. & Massague, J. Cloning of p57KIP2, a cyclin-dependent kinase inhibitor with unique domain structure and tissue distribution. Genes Dev. 9, 639–649 (1995).
Matsuoka, S. et al. p57KIP2, a structurally distinct member of the p21CIP1 Cdk inhibitor family, is a candidate tumor suppressor gene. Genes Dev. 9, 650–662 (1995).
Hannon, G. J. & Beach, D. p15INK4B is a potential effector of TGF-beta-induced cell cycle arrest. Nature 371, 257–261 (1994).
Guan, K. L. et al. Growth suppression by p18, a p16INK4/MTS1- and p14INK4B/MTS2-related CDK6 inhibitor, correlates with wild-type pRb function. Genes Dev. 8, 2939–2952 (1994).
Hirai, H., Roussel, M. F., Kato, J. Y., Ashmun, R. A. & Sherr, C. J. Novel INK4 proteins, p19 and p18, are specific inhibitors of the cyclin D-dependent kinases CDK4 and CDK6. Mol. Cell Biol. 15, 2672–2681 (1995).
Chan, F. K., Zhang, J., Cheng, L., Shapiro, D. N. & Winoto, A. Identification of human and mouse p19, a novel CDK4 and CDK6 inhibitor with homology to p16ink4. Mol. Cell Biol. 15, 2682–2688 (1995).
LaBaer, J. et al. New functional activities for the p21 family of CDK inhibitors. Genes Dev. 11, 847–862 (1997).
James, M. K., Ray, A., Leznova, D. & Blain, S. W. Differential modification of p27Kip1 controls its cyclin D-cdk4 inhibitory activity. Mol. Cell Biol. 28, 498–510 (2008).
Blain, S. W., Montalvo, E. & Massague, J. Differential interaction of the cyclin-dependent kinase (Cdk) inhibitor p27Kip1 with cyclin A-Cdk2 and cyclin D2-Cdk4. J. Biol. Chem. 272, 25863–25872 (1997).
Russo, A. A., Tong, L., Lee, J. O., Jeffrey, P. D. & Pavletich, N. P. Structural basis for inhibition of the cyclin-dependent kinase Cdk6 by the tumour suppressor p16INK4a. Nature 395, 237–243 (1998).
Roussel, M. F. The INK4 family of cell cycle inhibitors in cancer. Oncogene 18, 5311–5317 (1999).
Takita, J. et al. Deletion map of chromosome 9 and p16 (CDKN2A) gene alterations in neuroblastoma. Cancer Res. 57, 907–912 (1997).
Nobori, T. et al. Deletions of the cyclin-dependent kinase-4 inhibitor gene in multiple human cancers. Nature 368, 753–756 (1994).
Boice, J. A. & Fairman, R. Structural characterization of the tumor suppressor p16, an ankyrin-like repeat protein. Protein Sci. 5, 1776–1784 (1996).
Coleman, K. G. et al. Identification of CDK4 sequences involved in cyclin D1 and p16 binding. J. Biol. Chem. 272, 18869–18874 (1997).
Ceha, H. M., Nasser, I., Medema, R. H. & Slebos, R. J. Several noncontiguous domains of CDK4 are involved in binding to the P16 tumor suppressor protein. Biochem. Biophys. Res. Commun. 249, 550–555 (1998).
Sotillo, R. et al. Wide spectrum of tumors in knock-in mice carrying a Cdk4 protein insensitive to INK4 inhibitors. EMBO J. 20, 6637–6647 (2001).
Rane, S. G., Cosenza, S. C., Mettus, R. V. & Reddy, E. P. Germ line transmission of the Cdk4R24C mutation facilitates tumorigenesis and escape from cellular senescence. Mol. Cell Biol. 22, 644–656 (2002).
Foulkes, W. D., Flanders, T. Y., Pollock, P. M. & Hayward, N. K. The CDKN2A (p16) gene and human cancer. Mol. Med. 3, 5–20 (1997).
Jardim, D. L. et al. Cyclin pathway genomic alterations across 190,247 solid tumors: leveraging large-scale data to inform therapeutic directions. Oncologist 26, e78–e89 (2021).
Ueki, K. et al. CDKN2/p16 or RB alterations occur in the majority of glioblastomas and are inversely correlated. Cancer Res. 56, 150–153 (1996).
Young, R. J. et al. Loss of CDKN2A expression is a frequent event in primary invasive melanoma and correlates with sensitivity to the CDK4/6 inhibitor PD0332991 in melanoma cell lines. Pigment. Cell Melanoma Res. 27, 590–600 (2014).
Maubec, E. et al. Characteristics of the coexistence of melanoma and renal cell carcinoma. Cancer 116, 5716–5724 (2010).
Ricketts, C. J. et al. The cancer genome atlas comprehensive molecular characterization of renal cell carcinoma. Cell Rep. 23, 313–326.e5 (2018).
Latif, F. et al. Identification of the von Hippel-Lindau disease tumor suppressor gene. Science 260, 1317–1320 (1993).
Nickerson, M. L. et al. Mutations in a novel gene lead to kidney tumors, lung wall defects, and benign tumors of the hair follicle in patients with the Birt-Hogg-Dube syndrome. Cancer Cell 2, 157–164 (2002).
Zbar, B. et al. Hereditary papillary renal cell carcinoma: clinical studies in 10 families. J. Urol. 153, 907–912 (1995).
Grubb, R. L. 3rd et al. Hereditary leiomyomatosis and renal cell cancer: a syndrome associated with an aggressive form of inherited renal cancer. J. Urol. 177, 2074–2079; discussion 2079–2080 (2007).
Launonen, V. et al. Inherited susceptibility to uterine leiomyomas and renal cell cancer. Proc. Natl Acad. Sci. USA 98, 3387–3392 (2001).
Jafri, M. et al. Germline mutations in the CDKN2B tumor suppressor gene predispose to renal cell carcinoma. Cancer Discov. 5, 723–729 (2015).
Zygmunt, A., Tedesco, V. C., Udho, E. & Krucher, N. A. Hypoxia stimulates p16 expression and association with cdk4. Exp. Cell Res. 278, 53–60 (2002).
Gump, J., Stokoe, D. & McCormick, F. Phosphorylation of p16INK4A correlates with Cdk4 association. J. Biol. Chem. 278, 6619–6622 (2003).
Jinno, S., Hung, S. C. & Okayama, H. Cell cycle start from quiescence controlled by tyrosine phosphorylation of Cdk4. Oncogene 18, 565–571 (1999).
Kato, J. Y., Matsuoka, M., Strom, D. K. & Sherr, C. J. Regulation of cyclin D-dependent kinase 4 (cdk4) by cdk4-activating kinase. Mol. Cell Biol. 14, 2713–2721 (1994).
Bisteau, X. et al. CDK4 T172 phosphorylation is central in a CDK7-dependent bidirectional CDK4/CDK2 interplay mediated by p21 phosphorylation at the restriction point. PLoS Genet. 9, e1003546 (2013).
Colleoni, B. et al. JNKs function as CDK4-activating kinases by phosphorylating CDK4 and p21. Oncogene 36, 4349–4361 (2017).
Blancquaert, S. et al. cAMP-dependent activation of mammalian target of rapamycin (mTOR) in thyroid cells. Implication in mitogenesis and activation of CDK4. Mol. Endocrinol. 24, 1453–1468 (2010).
Knudsen, E. S. et al. Cell cycle plasticity driven by MTOR signaling: integral resistance to CDK4/6 inhibition in patient-derived models of pancreatic cancer. Oncogene 38, 3355–3370 (2019).
Hartl, F. U., Bracher, A. & Hayer-Hartl, M. Molecular chaperones in protein folding and proteostasis. Nature 475, 324–332 (2011).
Walton-Diaz, A. et al. Contributions of co-chaperones and post-translational modifications towards Hsp90 drug sensitivity. Future Med. Chem. 5, 1059–1071 (2013).
Woodford, M. R. et al. Targeting Hsp90 in non-cancerous maladies. Curr. Top. Med. Chem. 16, 2792–2804 (2016).
Sima, S. & Richter, K. Regulation of the Hsp90 system. Biochim. Biophys. Acta Mol. Cell Res. 1865, 889–897 (2018).
Mayer, M. P. & Le Breton, L. Hsp90: breaking the symmetry. Mol. Cell 58, 8–20 (2015).
Rohl, A., Rohrberg, J. & Buchner, J. The chaperone Hsp90: changing partners for demanding clients. Trends Biochem. Sci. 38, 253–262 (2013).
Czemeres, J., Buse, K. & Verkhivker, G. M. Atomistic simulations and network-based modeling of the Hsp90-Cdc37 chaperone binding with Cdk4 client protein: a mechanism of chaperoning kinase clients by exploiting weak spots of intrinsically dynamic kinase domains. PLoS One 12, e0190267 (2017).
Verba, K. A. & Agard, D. A. How Hsp90 and Cdc37 lubricate kinase molecular switches. Trends Biochem. Sci. 42, 799–811 (2017).
Lamphere, L. et al. Interaction between Cdc37 and Cdk4 in human cells. Oncogene 14, 1999–2004 (1997).
Zhao, Q., Boschelli, F., Caplan, A. J. & Arndt, K. T. Identification of a conserved sequence motif that promotes Cdc37 and cyclin D1 binding to Cdk4. J. Biol. Chem. 279, 12560–12564 (2004).
Truman, A. W. et al. CDK-dependent Hsp70 Phosphorylation controls G1 cyclin abundance and cell-cycle progression. Cell 151, 1308–1318 (2012).
Verba, K. A. et al. Atomic structure of Hsp90-Cdc37-Cdk4 reveals that Hsp90 traps and stabilizes an unfolded kinase. Science 352, 1542–1547 (2016).
Hallett, S. T. et al. Differential regulation of G1 CDK complexes by the Hsp90-Cdc37 chaperone system. Cell Rep. 21, 1386–1398 (2017).
Vaughan, C. K. et al. Structure of an Hsp90-Cdc37-Cdk4 complex. Mol. Cell 23, 697–707 (2006).
Vaughan, C. K. et al. Hsp90-dependent activation of protein kinases is regulated by chaperone-targeted dephosphorylation of Cdc37. Mol. Cell 31, 886–895 (2008).
Mollapour, M. et al. Swe1Wee1-dependent tyrosine phosphorylation of Hsp90 regulates distinct facets of chaperone function. Mol. Cell 37, 333–343 (2010).
Backe, S. J., Sager, R. A., Woodford, M. R., Makedon, A. M. & Mollapour, M. Post-translational modifications of Hsp90 and translating the chaperone code. J. Biol. Chem. 295, 11099–11117 (2020).
Miyata, Y. & Nishida, E. CK2 binds, phosphorylates, and regulates its pivotal substrate Cdc37, an Hsp90-cochaperone. Mol. Cell Biochem. 274, 171–179 (2005).
Oberoi, J. et al. Structural and functional basis of protein phosphatase 5 substrate specificity. Proc. Natl Acad. Sci. USA 113, 9009–9014 (2016).
Sager, R. A., Dushukyan, N., Woodford, M. & Mollapour, M. Structure and function of the co-chaperone protein phosphatase 5 in cancer. Cell Stress. Chaperones 25, 383–394 (2020).
Xu, W. et al. Dynamic tyrosine phosphorylation modulates cycling of the HSP90-P50(CDC37)-AHA1 chaperone machine. Mol. Cell 47, 434–443 (2012).
Bachman, A. B. et al. Phosphorylation induced cochaperone unfolding promotes kinase recruitment and client class-specific Hsp90 phosphorylation. Nat. Commun. 9, 265 (2018).
Smith, J. R. et al. Restricting direct interaction of CDC37 with HSP90 does not compromise chaperoning of client proteins. Oncogene 34, 15–26 (2015).
Lawless, N., Blacklock, K., Berrigan, E. & Verkhivker, G. Structural bioinformatics and protein docking analysis of the molecular chaperone-kinase interactions: towards allosteric inhibition of protein kinases by targeting the hsp90-cdc37 chaperone machinery. Pharmaceuticals 6, 1407–1428 (2013).
Zhu, J. et al. Cdc37 facilitates cell survival of colorectal carcinoma via activating the CDK4 signaling pathway. Cancer Sci. 109, 656–665 (2018).
Wang, Z., Wei, W., Sun, C. K., Chua, M. S. & So, S. Suppressing the CDC37 cochaperone in hepatocellular carcinoma cells inhibits cell cycle progression and cell growth. Liver Int. 35, 1403–1415 (2015).
D’Annessa, I. et al. Design of disruptors of the Hsp90-Cdc37 interface. Molecules 25, 360 (2020).
Paladino, A. et al. Chemical perturbation of oncogenic protein folding: from the prediction of locally unstable structures to the design of disruptors of hsp90-client interactions. Chemistry 26, 9459–9465 (2020).
Steinebach, C. et al. Systematic exploration of different E3 ubiquitin ligases: an approach towards potent and selective CDK6 degraders. Chem. Sci. 11, 3474–3486 (2020).
Sherr, C. J., Beach, D. & Shapiro, G. I. Targeting CDK4 and CDK6: from discovery to therapy. Cancer Discov. 6, 353–367 (2016).
Soni, R. et al. Selective in vivo and in vitro effects of a small molecule inhibitor of cyclin-dependent kinase 4. J. Natl Cancer Inst. 93, 436–446 (2001).
Martin, M. P., Endicott, J. A. & Noble, M. E. M. Structure-based discovery of cyclin-dependent protein kinase inhibitors. Essays Biochem. 61, 439–452 (2017).
De Azevedo, W. F. Jr et al. Structural basis for specificity and potency of a flavonoid inhibitor of human CDK2, a cell cycle kinase. Proc. Natl Acad. Sci. USA 93, 2735–2740 (1996).
Gray, N. S. et al. Exploiting chemical libraries, structure, and genomics in the search for kinase inhibitors. Science 281, 533–538 (1998).
Ikuta, M. et al. Crystallographic approach to identification of cyclin-dependent kinase 4 (CDK4)-specific inhibitors by using CDK4 mimic CDK2 protein. J. Biol. Chem. 276, 27548–27554 (2001).
McInnes, C. et al. Structural determinants of CDK4 inhibition and design of selective ATP competitive inhibitors. Chem. Biol. 11, 525–534 (2004).
Park, H., Yeom, M. S. & Lee, S. Loop flexibility and solvent dynamics as determinants for the selective inhibition of cyclin-dependent kinase 4: comparative molecular dynamics simulation studies of CDK2 and CDK4. Chembiochem 5, 1662–1672 (2004).
Guan, H., Du, Y., Han, W., Shen, J. & Li, Q. Development of selective cyclin-dependent kinase 4 inhibitors for antineoplastic therapies. Anticancer. Agents Med. Chem. 17, 646–657 (2017).
Fry, D. W. et al. Specific inhibition of cyclin-dependent kinase 4/6 by PD 0332991 and associated antitumor activity in human tumor xenografts. Mol. Cancer Ther. 3, 1427–1438 (2004).
Toogood, P. L. et al. Discovery of a potent and selective inhibitor of cyclin-dependent kinase 4/6. J. Med. Chem. 48, 2388–2406 (2005).
Cho, Y. S. et al. 4-(Pyrazol-4-yl)-pyrimidines as selective inhibitors of cyclin-dependent kinase 4/6. J. Med. Chem. 53, 7938–7957 (2010).
Gelbert, L. M. et al. Preclinical characterization of the CDK4/6 inhibitor LY2835219: in-vivo cell cycle-dependent/independent anti-tumor activities alone/in combination with gemcitabine. Invest. N. Drugs 32, 825–837 (2014).
Wright, M. D. & Abraham, M. J. Preclinical discovery and development of abemaciclib used to treat breast cancer. Expert Opin. Drug Discov. 16, 485–496 (2021).
O’Brien, N. et al. Preclinical activity of abemaciclib alone or in combination with antimitotic and targeted therapies in breast cancer. Mol. Cancer Ther. 17, 897–907 (2018).
Torres-Guzman, R. et al. Preclinical characterization of abemaciclib in hormone receptor positive breast cancer. Oncotarget 8, 69493–69507 (2017).
Chen, P. et al. Spectrum and Degree of CDK drug interactions predicts clinical performance. Mol. Cancer Ther. 15, 2273–2281 (2016).
Knudsen, E. S., Hutcheson, J., Vail, P. & Witkiewicz, A. K. Biological specificity of CDK4/6 inhibitors: dose response relationship, in vivo signaling, and composite response signature. Oncotarget 8, 43678–43691 (2017).
Roberto, M. et al. CDK4/6 inhibitor treatments in patients with hormone receptor positive, Her2 negative advanced breast cancer: potential molecular mechanisms, clinical implications and future perspectives. Cancers 13, 332 (2021).
Singer, E. A., Golijanin, D. J., Miyamoto, H. & Messing, E. M. Androgen deprivation therapy for prostate cancer. Expert Opin. Pharmacother. 9, 211–228 (2008).
Finn, R. S. et al. PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor-positive human breast cancer cell lines in vitro. Breast Cancer Res. 11, R77 (2009).
Ertel, A. et al. RB-pathway disruption in breast cancer: differential association with disease subtypes, disease-specific prognosis and therapeutic response. Cell Cycle 9, 4153–4163 (2010).
Altucci, L. et al. 17beta-Estradiol induces cyclin D1 gene transcription, p36D1-p34cdk4 complex activation and p105Rb phosphorylation during mitogenic stimulation of G1-arrested human breast cancer cells. Oncogene 12, 2315–2324 (1996).
Dickler, M. N. et al. MONARCH 1, a phase II study of abemaciclib, a CDK4 and CDK6 inhibitor, as a single agent, in patients with refractory HR+/HER2− metastatic breast cancer. Clin. Cancer Res. 23, 5218–5224 (2017).
Li, J. et al. Cyclin-dependent kinase 4 and 6 inhibitors in hormone receptor-positive, human epidermal growth factor receptor-2 negative advanced breast cancer: a meta-analysis of randomized clinical trials. Breast Cancer Res. Treat. 180, 21–32 (2020).
Wang, L. et al. CDK4/6 inhibitors plus endocrine therapy improve overall survival in advanced HR+/HER2- breast cancer: a meta-analysis of randomized controlled trials. Breast J. 26, 1439–1443 (2019).
Deng, Y. et al. CDK4/6 inhibitors in combination with hormone therapy for HR+/HER2− advanced breast cancer: a systematic review and meta-analysis of randomized controlled trials. Clin. Breast Cancer 18, e943–e953 (2018).
Messina, C. et al. CDK4/6 inhibitors in advanced hormone receptor-positive/HER2-negative breast cancer: a systematic review and meta-analysis of randomized trials. Breast Cancer Res. Treat. 172, 9–21 (2018).
Gao, J. J. et al. CDK4/6 inhibitor treatment for patients with hormone receptor-positive, HER2-negative, advanced or metastatic breast cancer: a US Food and Drug Administration pooled analysis. Lancet Oncol. 21, 250–260 (2020).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/show/NCT03701334 (2022).
Johnston, S. R. D. et al. Abemaciclib combined with endocrine therapy for the adjuvant treatment of HR+, HER2−, node-positive, high-risk, early breast cancer (monarchE). J. Clin. Oncol. 38, 3987–3998 (2020).
Curigliano, G. & Loibl, S. CDK4/6 inhibitors in breast cancer: one more step towards reduced mortality. Lancet Oncol. 21, 191–192 (2020).
Tan, A. R. et al. Trilaciclib plus chemotherapy versus chemotherapy alone in patients with metastatic triple-negative breast cancer: a multicentre, randomised, open-label, phase 2 trial. Lancet Oncol. 20, 1587–1601 (2019).
Al Baghdadi, T. et al. Sunitinib in patients with metastatic colorectal cancer (mCRC) with FLT-3 amplification: results from the targeted agent and profiling utilization registry (TAPUR) study. Target. Oncol. 15, 743–750 (2020).
Alva, A. S. et al. Pembrolizumab in patients with metastatic breast cancer with high tumor mutational burden: results from the targeted agent and profiling utilization registry (TAPUR) study. J. Clin. Oncol. 39, 2443–2451 (2021).
Fisher, J. G. et al. Cetuximab in patients with breast cancer, non-small cell lung cancer, and ovarian cancer without KRAS, NRAS, or BRAF mutations: results from the targeted agent and profiling utilization registry (TAPUR) study. Target. Oncol. 15, 733–741 (2020).
Flaherty, K. T. et al. Molecular landscape and actionable alterations in a genomically guided cancer clinical trial: National Cancer Institute Molecular Analysis for Therapy Choice (NCI-MATCH). J. Clin. Oncol. 38, 3883–3894 (2020).
Tate, S. C. et al. A population pharmacokinetic and pharmacodynamic analysis of abemaciclib in a phase I clinical trial in cancer patients. Clin. Pharmacokinet. 57, 335–344 (2018).
Zhang, J. et al. A randomized phase I study of abemaciclib in Chinese patients with advanced and/or metastatic cancers. Target. Oncol. 16, 177–187 (2021).
Hussussian, C. J. et al. Germline p16 mutations in familial melanoma. Nat. Genet. 8, 15–21 (1994).
Kamb, A. et al. Analysis of the p16 gene (CDKN2) as a candidate for the chromosome 9p melanoma susceptibility locus. Nat. Genet. 8, 23–26 (1994).
Zuo, L. et al. Germline mutations in the p16INK4a binding domain of CDK4 in familial melanoma. Nat. Genet. 12, 97–99 (1996).
Teh, J. L. F. et al. Metabolic adaptations to MEK and CDK4/6 cotargeting in uveal melanoma. Mol. Cancer Ther. 19, 1719–1726 (2020).
Perez-Galan, P., Dreyling, M. & Wiestner, A. Mantle cell lymphoma: biology, pathogenesis, and the molecular basis of treatment in the genomic era. Blood 117, 26–38 (2011).
Leonard, J. P. et al. Selective CDK4/6 inhibition with tumor responses by PD0332991 in patients with mantle cell lymphoma. Blood 119, 4597–4607 (2012).
Martin, P. et al. A phase I trial of palbociclib plus bortezomib in previously treated mantle cell lymphoma. Leuk. Lymphoma 60, 2917–2921 (2019).
Binh, M. B. et al. MDM2 and CDK4 immunostainings are useful adjuncts in diagnosing well-differentiated and dedifferentiated liposarcoma subtypes: a comparative analysis of 559 soft tissue neoplasms with genetic data. Am. J. Surg. Pathol. 29, 1340–1347 (2005).
Dickson, M. A. et al. Phase II trial of the CDK4 inhibitor PD0332991 in patients with advanced CDK4-amplified well-differentiated or dedifferentiated liposarcoma. J. Clin. Oncol. 31, 2024–2028 (2013).
Hadjadj, D. et al. A hypothesis-driven approach identifies CDK4 and CDK6 inhibitors as candidate drugs for treatments of adrenocortical carcinomas. Aging 9, 2695–2716 (2017).
Huang, S. et al. CDK4/6 inhibitor suppresses gastric cancer with CDKN2A mutation. Int. J. Clin. Exp. Med. 8, 11692–11700 (2015).
Frisone, D. et al. Durable response to palbociclib and letrozole in ovarian cancer with CDKN2A loss. Cancer Biol. Ther. 21, 197–202 (2020).
Rose, T. L. et al. Phase II trial of palbociclib in patients with metastatic urothelial cancer after failure of first-line chemotherapy. Br. J. Cancer 119, 801–807 (2018).
Gong, X. et al. Genomic aberrations that activate D-type cyclins are associated with enhanced sensitivity to the CDK4 and CDK6 inhibitor abemaciclib. Cancer Cell 32, 761–776.e6 (2017).
Green, J. L. et al. Direct CDKN2 modulation of CDK4 alters target engagement of CDK4 inhibitor drugs. Mol. Cancer Ther. 18, 771–779 (2019).
Iida, M. et al. The p21 levels have the potential to be a monitoring marker for ribociclib in breast cancer. Oncotarget 10, 4907–4918 (2019).
Vilgelm, A. E. et al. MDM2 antagonists overcome intrinsic resistance to CDK4/6 inhibition by inducing p21. Sci. Transl. Med. 11, eaav7171 (2019).
Pandey, K. et al. Combined CDK2 and CDK4/6 inhibition overcomes palbociclib resistance in breast cancer by enhancing senescence. Cancers 12, 3566 (2020).
Bockstaele, L. et al. Regulated activating Thr172 phosphorylation of cyclin-dependent kinase 4(CDK4): its relationship with cyclins and CDK “inhibitors”. Mol. Cell Biol. 26, 5070–5085 (2006).
Raspe, E. et al. CDK4 phosphorylation status and a linked gene expression profile predict sensitivity to palbociclib. EMBO Mol. Med. 9, 1052–1066 (2017).
Pandey, K. et al. Molecular mechanisms of resistance to CDK4/6 inhibitors in breast cancer: a review. Int. J. Cancer 145, 1179–1188 (2019).
Pancholi, S. et al. Tumour kinome re-wiring governs resistance to palbociclib in oestrogen receptor positive breast cancers, highlighting new therapeutic modalities. Oncogene 39, 4781–4797 (2020).
Tong, Z. et al. Functional genomics identifies predictive markers and clinically actionable resistance mechanisms to CDK4/6 inhibition in bladder cancer. J. Exp. Clin. Cancer Res. 38, 322 (2019).
Hua, H. et al. Targeting mTOR for cancer therapy. J. Hematol. Oncol. 12, 71 (2019).
Voss, M. H., Molina, A. M. & Motzer, R. J. mTOR inhibitors in advanced renal cell carcinoma. Hematol. Oncol. Clin. North. Am. 25, 835–852 (2011).
Ghidini, M. et al. Clinical development of mTor inhibitors for renal cancer. Expert Opin. Investig. Drugs 26, 1229–1237 (2017).
Paternot, S. & Roger, P. P. Combined inhibition of MEK and mammalian target of rapamycin abolishes phosphorylation of cyclin-dependent kinase 4 in glioblastoma cell lines and prevents their proliferation. Cancer Res. 69, 4577–4581 (2009).
Zacharek, S. J., Xiong, Y. & Shumway, S. D. Negative regulation of TSC1–TSC2 by mammalian D-type cyclins. Cancer Res. 65, 11354–11360 (2005).
Kim, J. & Guan, K. L. mTOR as a central hub of nutrient signalling and cell growth. Nat. Cell Biol. 21, 63–71 (2019).
Pikman, Y. et al. Synergistic drug combinations with a CDK4/6 inhibitor in T-cell acute lymphoblastic leukemia. Clin. Cancer Res. 23, 1012–1024 (2017).
Modiano, J. F., Domenico, J., Szepesi, A., Lucas, J. J. & Gelfand, E. W. Differential requirements for interleukin-2 distinguish the expression and activity of the cyclin-dependent kinases Cdk4 and Cdk2 in human T cells. J. Biol. Chem. 269, 32972–32978 (1994).
Modiano, J. F., Mayor, J., Ball, C., Fuentes, M. K. & Linthicum, D. S. CDK4 expression and activity are required for cytokine responsiveness in T cells. J. Immunol. 165, 6693–6702 (2000).
Macian, F. NFAT proteins: key regulators of T-cell development and function. Nat. Rev. Immunol. 5, 472–484 (2005).
Jin, X. et al. Phosphorylated RB promotes cancer immunity by inhibiting NF-kappaB activation and PD-L1 expression. Mol. Cell 73, 22–35 e26 (2019).
Iwai, Y. et al. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc. Natl Acad. Sci. USA 99, 12293–12297 (2002).
Herbst, R. S. et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 515, 563–567 (2014).
Sato, H., Okonogi, N. & Nakano, T. Rationale of combination of anti-PD-1/PD-L1 antibody therapy and radiotherapy for cancer treatment. Int. J. Clin. Oncol. 25, 801–809 (2020).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/show/NCT03905889 (2022).
O’Brien, J., Hayder, H., Zayed, Y. & Peng, C. Overview of MicroRNA biogenesis, mechanisms of actions, and circulation. Front. Endocrinol. 9, 402 (2018).
Xiao, H. et al. MiR-1 downregulation correlates with poor survival in clear cell renal cell carcinoma where it interferes with cell cycle regulation and metastasis. Oncotarget 6, 13201–13215 (2015).
Xiao, H. et al. miR-206 functions as a novel cell cycle regulator and tumor suppressor in clear-cell renal cell carcinoma. Cancer Lett. 374, 107–116 (2016).
Statello, L., Guo, C. J., Chen, L. L. & Huarte, M. Gene regulation by long non-coding RNAs and its biological functions. Nat. Rev. Mol. Cell Biol. 22, 96–118 (2021).
Chen, D., Sun, X., Zhang, X. & Cao, J. Inhibition of the CDK4/6-Cyclin D-Rb pathway by ribociclib augments chemotherapy and immunotherapy in renal cell carcinoma. Biomed. Res. Int. 2020, 9525207 (2020).
Clark, D. J. et al. Integrated proteogenomic characterization of clear cell renal cell carcinoma. Cell 179, 964–983.e31 (2019).
Logan, J. E. et al. PD-0332991, a potent and selective inhibitor of cyclin-dependent kinase 4/6, demonstrates inhibition of proliferation in renal cell carcinoma at nanomolar concentrations and molecular markers predict for sensitivity. Anticancer. Res. 33, 2997–3004 (2013).
Semenza, G. L. Targeting HIF-1 for cancer therapy. Nat. Rev. Cancer 3, 721–732 (2003).
Jonasch, E. et al. Belzutifan for renal cell carcinoma in Von Hippel-Lindau disease. N. Engl. J. Med. 385, 2036–2046 (2021).
Atkins, D. J. et al. Concomitant deregulation of HIF1alpha and cell cycle proteins in VHL-mutated renal cell carcinomas. Virchows Arch. 447, 634–642 (2005).
Schodel, J. et al. Common genetic variants at the 11q13.3 renal cancer susceptibility locus influence binding of HIF to an enhancer of cyclin D1 expression. Nat. Genet. 44, 420–425 (2012).
Bailey, S. T. et al. MYC activation cooperates with Vhl and Ink4a/Arf loss to induce clear cell renal cell carcinoma. Nat. Commun. 8, 15770 (2017).
Bommi-Reddy, A. et al. Kinase requirements in human cells. III. Altered kinase requirements in VHL−/− cancer cells detected in a pilot synthetic lethal screen. Proc. Natl Acad. Sci. USA 105, 16484–16489 (2008).
Zhu, G. et al. Synthesis, structure-activity relationship, and biological studies of indolocarbazoles as potent cyclin D1-CDK4 inhibitors. J. Med. Chem. 46, 2027–2030 (2003).
Nicholson, H. E. et al. HIF-independent synthetic lethality between CDK4/6 inhibition and VHL loss across species. Sci. Signal 12, eaay0482 (2019).
Small, J., Washburn, E., Millington, K., Zhu, J. & Holder, S. L. The addition of abemaciclib to sunitinib induces regression of renal cell carcinoma xenograft tumors. Oncotarget 8, 95116–95134 (2017).
Anand, P., Sundaram, C., Jhurani, S., Kunnumakkara, A. B. & Aggarwal, B. B. Curcumin and cancer: an “old-age” disease with an “age-old” solution. Cancer Lett. 267, 133–164 (2008).
Mukhopadhyay, A. et al. Curcumin-induced suppression of cell proliferation correlates with down-regulation of cyclin D1 expression and CDK4-mediated retinoblastoma protein phosphorylation. Oncogene 21, 8852–8861 (2002).
Debata, P. R. et al. Curcumin potentiates the ability of sunitinib to eliminate the VHL-lacking renal cancer cells 786-O: rapid inhibition of Rb phosphorylation as a preamble to cyclin D1 inhibition. Anticancer. Agents Med. Chem. 13, 1508–1513 (2013).
Wang, Y. et al. Wogonin induces apoptosis and reverses sunitinib resistance of renal cell carcinoma cells via inhibiting CDK4-RB pathway. Front. Pharmacol. 11, 1152 (2020).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/show/NCT04594005 (2021).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/show/NCT02065063 (2018).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/show/NCT02022982 (2022).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/show/NCT03310879 (2022).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/show/NCT02693535 (2022).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/show/NCT02465060 (2022).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/show/NCT03297606 (2021).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/show/NCT03239015 (2020).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/show/NCT03065062 (2022).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/show/NCT01522989 (2020).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/show/NCT03237390 (2021).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/show/NCT04116541 (2021).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/show/NCT04557449 (2022).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/show/NCT02919696 (2020).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/show/NCT01394016 (2021).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/show/NCT03965845 (2021).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/show/NCT04439201 (2022).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/show/NCT02389842 (2019).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/show/NCT04162301 (2021).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/show/NCT04060511 (2019).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/show/NCT04275050 (2020).
Cho, Y. S. et al. Fragment-based discovery of 7-azabenzimidazoles as potent, highly selective, and orally active CDK4/6 inhibitors. ACS Med. Chem. Lett. 3, 445–449 (2012).
Acknowledgements
The authors are grateful to W. Marston Linehan (Urologic Oncology Branch, NCI) for his scientific contribution. This work was partly supported by the National Institute of General Medical Sciences with the NIH (grants R35GM139584, R01GM124256 and DoD KC190038 (M.M.), and R01GM139932 (D.B.)). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. This work was also supported by funds from SUNY Upstate Medical University and the Upstate Foundation (D.B. and M.M.).
Author information
Authors and Affiliations
Contributions
M.M., R.A.S., G.E.S. and I.N. researched data for article. M.M., R.A.S., S.J.B., G.B. and D.B. made substantial contribution to the discussion of content. M.M., R.A.S., S.J.B., E.A. and M.R.W. wrote the article. M.M., R.A.S. and S.J.B. reviewed/edited the manuscript before submission
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Urology thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sager, R.A., Backe, S.J., Ahanin, E. et al. Therapeutic potential of CDK4/6 inhibitors in renal cell carcinoma. Nat Rev Urol 19, 305–320 (2022). https://doi.org/10.1038/s41585-022-00571-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41585-022-00571-8