Abstract
Primary hyperoxalurias are a devastating family of diseases leading to multisystem oxalate deposition, nephrolithiasis, nephrocalcinosis and end-stage renal disease. Traditional treatment paradigms are limited to conservative management, dialysis and combined transplantation of the kidney and liver, of which the liver is the primary source of oxalate production. However, transplantation is associated with many potential complications, including operative risks, graft rejection, post-transplant organ failure, as well as lifelong immunosuppressive medications and their adverse effects. New therapeutics being developed for primary hyperoxalurias take advantage of biochemical knowledge about oxalate synthesis and metabolism, and seek to specifically target these pathways with the goal of decreasing the accumulation and deposition of oxalate in the body.
Key points
-
Primary hyperoxalurias (PHs) are a devastating family of rare, autosomal-recessive genetic disorders that lead to multisystem oxalate deposition, nephrolithiasis, nephrocalcinosis and end-stage renal disease.
-
Traditional treatment paradigms are limited to conservative management, dialysis and inevitably transplantation of the kidney and liver, which is associated with high morbidity and the need for lifelong immunosuppression.
-
New therapeutics being developed for PHs take advantage of biochemical knowledge about oxalate synthesis and metabolism to specifically target these pathways, with the goal of decreasing the accumulation and deposition of plasma oxalate in the body.
-
New therapeutics can be divided into classes, and include substrate reduction therapy, intestinal oxalate degradation, chaperone therapy, enzyme restoration therapy and targeting of the inflammasome.
-
Lumasiran, a mRNA therapeutic targeting glycolate oxidase, was the first primary hyperoxaluria-specific therapeutic approved by the European Medicines Agency and the FDA in 2020.
-
Future work includes further clinical trials for promising therapeutics in the pipeline, identification of biomarkers of response to PH-directed therapy, optimization of drug development and delivery of new therapeutics.
Similar content being viewed by others
Introduction
Primary hyperoxalurias (PHs) are a family of rare, autosomal-recessive genetic disorders first described by Lepoutre in 1925 (ref.1). PH prevalence has been estimated to be <3 in 1,000,000 (ref.2), or as high as 1 in 58,000 (ref.3). PHs involve an overabundance of oxalate, which is synthesized in the liver and excreted renally4. Oxalate and calcium combine and form calcium oxalate (CaOx) salts, which are highly insoluble and organize to form recurrent urolithiasis and nephrocalcinosis, leading to progressive renal insult and eventually end-stage renal disease (ESRD)4. In patients with PHs, inflammation secondary to oxalate-induced tubular toxicity, nephrocalcinosis and renal obstruction by stones are thought to drive chronic kidney disease progression5, which in turn initiates a vicious cycle, as the decrease in glomerular filtration rate (GFR) leads to less oxalate excretion. Eventually, systemic accumulation of calcium oxalate can manifest in extra-renal tissues such as the skin, retina, bones and heart, and this accumulation is often fatal6.
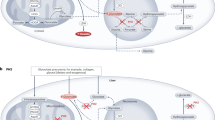
Oxalate is synthesized in the liver from its main precursor glyoxylate7. Cellular oxalate production occurs via three primary steps: first, mitochondrial catabolism of hydroxyproline from collagen turnover and metabolism of animal proteins to produce glycolate; second, peroxisomal metabolism of glycolate from mitochondrial catabolism and vegetables and fruit to glyoxylate; and third, cytosolic transport of glyoxylate leading to oxalate synthesis (Fig. 1).
In mitochondria, 4-hydroxy-2-oxoglutarate (HOG) is converted into glyoxylate and pyruvate by 4-hydroxy-2-oxoglutarate aldolase (HOGA1). Glyoxylate reductase/hydroxypyruvate reductase (GRHPR) converts glyoxylate into glycolate. In peroxisomes, glycolate from mitochondrial catabolism and vegetable and fruit is oxidized into glyoxylate by glycolate oxidase (GO), whereas glyoxylate and l-alanine are transaminated by alanine:glyoxylate aminotransferase (AGT), a pyridoxal 5′-phosphate (PLP)-dependent enzyme, to form pyruvate and glycine, respectively. In the cytosol, glyoxylate can be converted into oxalate by lactate dehydrogenase (LDH). Deficiency in AGT or GRHPR leads to the accumulation of glyoxylate in the cytosol and its increased conversion into oxalate by LDH. Different components of the oxalate pathway are mutated in primary hyperoxaluria (text highlighted in red). PH1, primary hyperoxaluria type 1; PH2, primary hyperoxaluria type 2; PH3, primary hyperoxaluria type 3.
Mitochondrial catabolism of hydroxyproline involves conversion of 4-hydroxy-2-oxoglutarate (HOG) into glyoxylate and pyruvate by 4-hydroxy-2-oxoglutarate aldolase (HOGA1)8. Glyoxylate derived from mitochondrial catabolism and vegetables and fruit is converted into glycolate by glyoxylate reductase/hydroxypyruvate reductase (GRHPR)9,10. Glycolate can then be delivered to the peroxisome, where it is oxidized into glyoxylate by glycolate oxidase11. In peroxisomes, glyoxylate and L-alanine are transaminated by alanine:glyoxylate aminotransferase (AGT), a pyridoxal 5′-phosphate (PLP)-dependent enzyme, to form pyruvate and glycine, respectively12. Glyoxylate in the cytosol can either be converted back into glycolate by GRHPR or into oxalate by lactate dehydrogenase (LDH)13.
Deficiencies in different genes of glyoxylate metabolism lead to three types of PH (Fig. 1). Primary hyperoxaluria type 1 (PH1) involves functional loss of AGT, encoded by AGXT, which leads to accumulation of glyoxylate in the cytosol and its LDH-mediated conversion to oxalate14. Over 200 AGXT mutations have been described (Human Gene Mutation Database). Mutation frequencies differ according to ethnicity, but the three most common worldwide are p.G170R, c.33dupC and p.I244T, which account for ~ 30%, 11% and 6% of AGXT mutant alleles, respectively3. PH1 accounts for ~80% of all PHs, with a prevalence of 1–3 cases per million people and an incidence of 1 in 120,000 live births, although these numbers could be underestimated owing to a lack of widespread genetic screening15,16. PH1 is the most severe form of the disease, with nearly all patients with PH1 progressing to ESRD17. ESRD is diagnosed at the median age of 24 years in patients with PH1 (ref.18), and 20–50% of patients with PH1 have advanced ESRD at the time of diagnosis19,20. ESRD outcomes are also worse in patients with PH1, with a risk of death roughly three times higher than patients with ERSD without PH1 (ref.20).
Primary hyperoxaluria type 2 (PH2) involves functional loss of GRHPR, encoded by GRHPR, which decreases metabolism of glyoxylate and leads to increased LDH-mediated oxalate synthesis14 (Fig. 1). Thirty-nine different mutations in GRHPR have been described (Human Gene Mutation Database, accessed 12 June 2021). The most common mutations reported are c.103delG and c.403_404 + 2delAAGT; in one study, c.103delG accounted for 37% of mutant alleles and c.403_404 + 2delAAGT accounted for 18% worldwide3. The exact disease prevalence is unknown but estimated to be ~10% of PHs21,22. Patients with PH2 tend to have a less severe phenotype than those with PH1, with the absence of infantile oxalosis and ESRD occurring in only 20–25% of patients23,24.
Primary hyperoxaluria type 3 (PH3) involves functional loss of HOGA1, encoded by HOGA1. The exact mechanism through which HOGA1 impairment contributes to downstream oxalate accumulation is unclear but has been hypothesized to involve either HOG-mediated inhibition of GRHPR or direct catabolism of HOG to generate glyoxylate by a cytosolic aldolase14. In total, 33 different HOGA1 mutations have been identified (Human Gene Mutation Database). The most frequently described mutation is c.700 + 5G > T, which accounts for ~50% of all mutant alleles25. PH3 is less severe than PH1 and PH2, and tends to present with early symptomatic nephrolithiasis in the first months to years of life8,25,26.
Broadly, the traditional treatment pathway of PHs involves conservative medical management, followed by dialysis and eventual liver and kidney transplant in the setting of ESRD27. The advent of next-generation sequencing and other genomic testing techniques have brought about an era of precision medicine, in which disease-modifying genes and relevant pathways can be dissected and targeted with novel therapeutics. These new drugs have shown promise in clinical trials; for example, RNA interference therapeutic lumasiran demonstrated both effectiveness and tolerability in phase III clinical trials, leading to approval by both the FDA and European Medicines Agency (EMA) for use in PH1 (refs28,29).
This Review covers current treatment paradigms for PHs, discusses available data on new therapeutics and their mechanisms of action, and examines future directions for novel research in the field.
Traditional management options
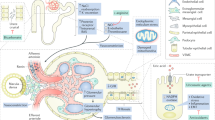
Traditional treatment options for PH are limited (Fig. 2). An aggressive increase in fluid intake (in the order of 3–4 l per day) is recommended in all patients with PH, and in infants who cannot self-improve their fluid intake this treatment could result in the placement of a gastrostomy tube to ensure fluid intake overnight30. Dietary changes have traditionally been thought to be irrelevant in PH1, as the fraction of dietary oxalate excreted into urine is very low (<5%)31. However, a 2018 study demonstrated that decreased oxalate and hydroxyproline dietary intake leads to reduced urinary oxalate excretion in some patients32. Citrate compounds (such as potassium citrate and sodium citrate), commonly prescribed to treat kidney stone disease, inhibit crystallization by binding available free calcium and alkalizing urine33. Similarly, orthophosphates (neutral phosphates) delay stone formation by reducing calcium oxalate crystallization in patients with PH; results from a study in which 25 patients were treated with orthophosphates and pyridoxine showed significantly reduced urinary supersaturation with calcium oxalate (P < 0.001) and increased inhibition of calcium oxalate formation (P < 0.001)34.
Traditional management options for primary hyperoxaluria (PH) include pyridoxine, citrate compounds, neutral phosphates, dialysis, and liver and kidney transplantation. New areas of therapeutic development include substrate reduction therapy, intestinal oxalate degradation, chaperone therapy, enzyme restoration therapy and targeting of the inflammasome. Therapeutics tested clinically in PH are highlighted in green, therapeutics tested in preclinical models of PH are highlighted in yellow, and therapeutics that have only been tested in other diseases but might be promising for PH are highlighted in red. AGT, alanine:glyoxylate aminotransferase; AGT-PGA-AGT, alanine:glyoxylate aminotransferase cross-linked to moieties of polyethylene glycol (PEG) and polyglutamic acid (PGA); DECA, dequalinium chloride; IL-1βRi, interleukin-1β receptor inhibitor; iPSCs, induced pluripotent stem cells; NLRP3i, NOD-, LRR- and pyrin domain-containing protein 3 inhibitor; OxDC CLEC, oxalate decarboxalase cross-linked crystals; TNFRi, tumour necrosis factor receptor inhibitor.
Pyridoxine (vitamin B6), which is metabolized to pyridoxal-5′-phosphate (PLP, the essential cofactor of AGT) was first shown to be effective in decreasing plasma oxalate levels in a subset of patients with PH1 in 1967 (ref.35). Pyridoxine leads to a decrease in urine oxalate in ~30% of patients with PH1, particularly those with Gly170Arg and Phe152Ile genotypes, although pyridoxine-sensitive patients eventually develop ESRD as well19. Results of a small prospective clinical trial of pyridoxine in 12 patients demonstrated adequate response, defined as >30% reduction in mean relative urine oxalate in 50% of the cohort36. The mechanism of action of pyridoxine in PH involves multifactorial activity on AGT: pyridoxine has been shown to increase expression, catalytic activity and peroxisomal import of AGT, restoring the function of the enzyme lost in PH1 (ref.37).
When PH reaches the ESRD stage, dialysis treatment is started. Frequent and short haemodialysis sessions with high flux filters, typically 2–3 h daily, have been shown to be more efficient than longer, less frequent dialysis regimens, such as standard haemodialysis regimens of three times weekly38,39. The addition of nocturnal peritoneal dialysis can increase oxalate elimination further40. However, even daily haemodialysis and peritoneal dialysis sessions are unable to eliminate sufficient levels of oxalate to decrease systemic build-up, and dialysis is usually a temporary stopgap before definitive treatment39.
The only definitive cure for PH is transplantation of the liver, the source of oxalate production4. This procedure is typically performed as combined liver and kidney transplantation (CLKT), which has shown to be superior to isolated kidney transplant (IKT) for kidney graft survival, reported to be 76% for CLKT and 14% for IKT at 5 years20. Survival outcomes and liver graft survival at 5 years for CLKT have been reported to be 80% and 72%, respectively20,39. IKT is an option for specific populations of patients, including patients who are not surgical candidates and patients who respond to pyridoxine therapy41, but is limited by poor graft survival42. The optimal timing of liver transplant relative to kidney transplant and whether transplant is done combined or sequentially remains unknown and the subject of debate43. For example, a study in which 201 patients received liver and kidney transplantation showed no differences in survival, liver graft outcomes or kidney graft outcomes between patients receiving CLKT and those receiving sequential liver and kidney transplantation44. Transplantation is a morbid procedure associated with operative risks, graft rejection, post-transplant organ failure and lifelong immunosuppressive medications and their adverse effects45,46, therefore providing a window of opportunity for the development of new therapeutics.
New clinically available therapeutic options
New therapeutics being developed for PHs take advantage of biochemical knowledge about oxalate synthesis and metabolism to specifically target these pathways, with the goal of decreasing the accumulation and deposition of plasma oxalate in the body. Current therapeutic classes of drugs that have been clinically tested can be divided into three strategies: substrate reduction therapy (SRT), which seeks to target key enzymes responsible for oxalate production; enhanced intestinal oxalate degradation through administration of oral enzymes or probiotics; and chaperone therapy for misfolded proteins (Table 1; Fig. 2).
Substrate reduction therapy
SRT is a definitive therapeutic approach to treating metabolic disorders caused by accumulation of dangerous substrates by targeting key enzymes responsible for their production14. The ideal SRT target for PH must satisfy two primary requirements: the target must be a key step in the oxalate metabolic pathway; and the inhibition of the target must lead to minimal off-target effects. To date, two targets have emerged for the treatment of PH: glycolate oxidase and lactate dehydrogenase (LDH).
Glycolate oxidase (encoded by HAO1) is an enzyme that catalyses the synthesis of glyoxylate, the precursor molecule for oxalate, in hepatocytes10. Patients with inherited loss-of-function mutations in glycolate oxidase do not display any phenotype other than high urine glycolate levels, which suggests that therapeutic inhibition will probably be tolerated47. Multiple inhibitors of glycolate oxidase have been identified, including 4-carboxy-5-dodecylsulfanyl-1,2,3-tiazole (CDST) and 4-carboxy-5-[(4-chlorophenyl) sulfanyl]-1,2,3-thiadiazole (CCPST), although these inhibitors have not made it into clinical trials because of issues with dosing and adverse effects47,48. A therapeutic RNA interference (RNAi) drug against glycolate oxidase (ALN-GO1) was shown to decrease urinary oxalate in mice, rats and non-human primates49. This preclinical success led to the development of lumasiran (Oxlumo), which is ALN-GO1 tethered to N-acetylgalactosamine for targeted delivery to hepatocytes50. Lumasiran demonstrated 65.4% mean reduction in urinary oxalate relative to baseline, with no deaths or serious adverse events, in 26 patients with non-ESRD PH aged ≥6 years in ILLUMINATE-A, a double-blind, placebo-controlled phase III trial51. Initial data from the completed phase III ILLUMINATE-B trial (NCT03905694) presented at the 2020 American Society of Nephrology (ASN) conference indicate promising results of lumasiran treatment in patients with non-ESRD PH1 aged <6 years, reporting a 72% mean reduction in urinary oxalate:creatinine ratios at 6 months52. The phase III ILLUMINATE-C trial (NCT04152200), which involves patients with PH1 and ESRD, completed enrolment in December 2020, but has not released any results yet53. Based on the success of the trials so far, lumasiran was approved by the EMA in October 2020 (ref.28) and the FDA in November 2020 (ref.29), being the first specific treatment for PH1 approved by either agency.
LDH catalyses conversion from glyoxylate into oxalate in the cytosol of hepatocytes, the final step in the oxalate synthesis pathway14. People with LDH deficiency do not display any pathological phenotype, which supports the potential tolerability of therapeutic inhibition54. Stiripentol (Diacomit), an anti-epileptic drug initially used for Dravet syndrome55, has been shown to be a potent inhibitor of LDH, and was demonstrated to lower urine oxalate in cell cultures, animal models and a patient with PH1 who had preserved kidney function56. A phase II clinical trial (NCT03819647) investigating stiripentol for the treatment of PH is ongoing57. Of note, a case report showed that stiripentol was unable to significantly reduce plasma oxalate levels in a patient with PH1 and ESRD, suggesting difficulty in treating patients with PH1 showing a severe phenotype58. Nedosiran is an RNAi drug targeting LDH that had preclinical success in decreasing plasma oxalate in animal models59, and was granted Rare Paediatric Disease Designation by the FDA in June 2020 (ref.60). The results from the phase I PHYOX1 trial (NCT03392896) demonstrated tolerability of nedosiran and a mean maximal reduction of urinary oxalate levels of 66% (range of 35–100%) in 25 healthy volunteers and 18 patients with PH1 (ref.61). PHYOX2, a phase II double-blind, placebo-controlled, randomized trial (NCT03847909) assessing the safety and efficacy of nedosiran in patients aged ≥6 years achieved the primary end point, demonstrating a statistically significant reduction from baseline in urinary oxalate excretion compared with placebo (P < 0.0001)62. A case report published in 2021 demonstrated an exceptional response to nedosiran treatment in a patient with PH1 and ESRD who was awaiting CLKT; after 6 months of treatment, the patient had a drastic reduction in plasma oxalate (from 55.5 µmol/l to 13.9 µmol/l), leading to decreasing weekly dialysis appointments from six to three, and deferral of CLKT in favour of continued nedosiran treatment63. Finally, a small-molecule inhibitor of LDH, CHK-336, was granted Rare Pediatric Disease Designation by the FDA based on demonstration of dose-dependent reduction and normalization to wild-type levels of urinary oxalate in PH1 mouse models64. CHK-336 will be tested in a phase I clinical trial planned to initiate at the end of 2021 (ref.64).
Intestinal oxalate degradation
Promoting intestinal degradation of oxalate can lead to decreased oxalate absorption, thereby reducing plasma and urine oxalate levels and ameliorating the PH phenotype65. Methods of decreasing absorption of intestinal oxalate trialled in PH include probiotic administration and oral enzyme administration.
Natural intestinal bacterial flora, consisting of bacteria such as Oxalobacter formigenes, thrive on consumption of oxalate, which is broken down as an energy source66. In animal models, oral administration of O. formigenes has been shown to reduce levels of oxalate in urine and plasma through intestinal excretion of endogenous oxalate67. A phase I clinical trial showed that oral O. formigenes is safe and well-tolerated, leading to a reduction in urinary oxalate levels in 16 patients68. Oxabact, a lyophilized formulation of O. formigenes, was granted FDA Rare Pediatric Disease Designation in June 2020 (ref.69). Randomized phase II/III studies to date have confirmed the tolerability of Oxabact, but have shown no changes in urinary oxalate concentrations after 8 or 24 weeks of treatment; authors from these studies cited low bacteria viability and insufficient treatment time as possible reasons for drug failure70,71. Another phase III clinical trial for Oxabact (ePHex; NCT03116685) completed enrolment in April 2020, with results expected at the end of 2021 (ref.72). Compassionate use has been reported in two case reports that showed the potential of Oxabact in combination with intensive dialysis in infantile oxalosis, which is an early-onset severe presentation of PH1 in children under 6 months of age, resulting in reduced plasma oxalate and halting of disease progression73,74.
An alternative method of intestinal oxalate removal is enzyme administration therapy, in which oxalate-degrading enzymes, typically purified from non-human sources, can be orally administered to degrade intestinal oxalate in a safe and tolerable manner75. The enzyme that is primarily used is oxalate decarboxylase (OxDC), typically purified from fungi and bacteria, which converts oxalate into formate and carbon dioxide76. OxDC is available in multiple formulations that have been clinically tested, including Nephure, Oxazyme and reloxaliase. Nephure is purified OxDC from Synechococcus elongatus77. To date, enzyme administration therapy with OxDC formulations have only been clinically tested in healthy individuals and patients with secondary hyperoxaluria, which, unlike PH, is not caused by genetic errors of metabolism, but by increased dietary ingestion of oxalate, precursors of oxalate, or alterations in intestinal microflora78. A completed prospective, randomized study of Nephure in healthy adult volunteers on a 4-day controlled high-oxalate, low-calcium diet demonstrated a 24% reduction (P < 0.001) in 24-h oxalate excretion compared with placebo79. Oxazyme is purified from Bacillus subtilis and was found to substantially reduce oxalate content in vitro80. Results of an unpublished phase I clinical trial of oxazyme in 2012 demonstrated a significant reduction of urinary oxalate in 8 patients with secondary hyperoxaluria after Roux-en-Y gastric bypass (P = 0.018; NCT01127087)81, but additional clinical studies have not been initiated or performed to date. Reloxaliase, formerly ALLN-177, is an encapsulated crystalline form of OxDC from B. subtilis82. In a phase I clinical trial, reloxaliase demonstrated an ability to reduce urinary oxalate by 11.6 ± 2.7 mg/day in 30 healthy volunteers with hyperoxaluria induced by ingestion of a high-oxalate diet (P = 0.0002)82. In a subsequent study, treatment with reloxaliase in 5 patients with nephrolithiasis with enteric hyperoxaluria and 11 with idiopathic hyperoxaluria led to a mean reduction in urine oxalate excretion of 14 mg/24 h, and was well tolerated83. Reloxaliase has received orphan drug designation for PH, and a phase II clinical trial including patients with PH is ongoing (NCT03391804)84.
Chaperone therapy
The most common AGT mutations in PH1 result in a conformational change of the protein that can lead to increased aggregation or degradation, decreased or abolished enzyme function, and/or overall instability of the protein85,86,87. Chaperone therapy uses small-molecule therapeutics capable of restoring a functional enzyme conformation, and has been successfully employed in other disorders, such as lysosomal storage disease and cystic fibrosis88.
Pyridoxine has been shown to be effective in specific AGT variants at least in part because of its action on protein folding, determined by immunoprecipitation and thermal denaturation studies in cell culture and in vitro86. Specific mutations in AGT that lead to protein misfolding include the two most common mutations in PH1, Gly170Arg and Phe152Ile, which affect 30–40% and 20% of patients, respectively89,90,91. Pyridoxine treatment in patients with PH1 with sensitive mutations have the potential to return urine oxalate levels to normal and prevent development of ESRD92. In cellular models of PH1, pyridoxal 5′-phosphate (PLP), the active component of pyridoxine, was shown to both shift conformational equilibrium towards a more stable conformation of AGT, and promote acquirement and maintenance of a dimeric AGT structure, which is crucial for functionality86. Interestingly, combined bioinformatic and molecular studies have shown an inverse correlation between the degree of destabilization and misfolding induced by an AGT mutation and the extent of pyridoxine responsiveness, suggesting that pyridoxine function as a chaperone is a relatively late event in AGT folding and, therefore, a threshold beyond which pyridoxine can rescue the effects of destabilizing AGT mutations could exist93.
Betaine, also known as trimethylglycine, is a modified amino acid that is involved in methylation reactions, detoxification of homocysteine and anti-inflammatory functions94. In cell culture models of PH, betaine has been shown to exert a stabilizing effect on specific pathogenic AGT variants, including Phe152Ile, Gly170Arg and Ile244Thr95,96,97. Besides pyridoxine, betaine is the only chaperone therapeutic that has been clinically tested in an unpublished randomized phase II crossover clinical trial (NCT00283387)98 in which patients with PH1 were treated with betaine (n = 10) or placebo (n = 10); treatment was well tolerated without severe adverse effects, but no decrease was found in urinary oxalate excretion in the betaine treatment cohort98. Lack of efficacy might be a result of limited sample size or betaine formulation or dose, and further studies are needed to determine the utility of betaine treatment in PH.
Misfolding of proteins affects their function and also their proper subcellular localization99. For example, the common Gly170Arg mutation has been shown to lead to mislocalization of AGT within the mitochondria without affecting its enzymatic function in mammalian cell cultures100. Mitochondrial transport inhibitor dequalinium chloride (DECA), FDA-approved for oral and vaginal antibacterial treatment101, is a chaperone protein that has been shown to reduce oxalate secretion by correction of Gly170Arg-mediated mislocalization of AGT in vitro102. Similarly, emetine, a medicinal alkaloid, has been shown to decrease oxalate excretion in vitro through its action as a chemical chaperone, which rescues mislocalization of Gly170Arg-AGT103. To date, these medications have not been tried in a clinical setting.
In summary, chaperone therapy and SRT-enhanced intestinal oxalate degradation are clinically tested therapeutic strategies to combat PH that have shown varying degrees of clinical success. These therapeutics have begun transforming the lives of patients with PH1, although determining long-term efficacy and patient outcomes is ongoing.
Preclinical strategies in development
Multiple therapeutics for PH have shown some evidence of efficacy in preclinical studies, but have yet to be translated into clinical application. These include enzyme restoration therapy (ERT), CRISPR–Cas9 SRT and ERT, and inhibition of the inflammasome (Fig. 2).
Enzyme restoration therapy
PHs are caused by enzyme deficit or dysfunction; thus, direct administration of the deficient enzyme, known as ERT, is an obvious therapeutic strategy. To date, therapeutic efforts have focused on the enzyme deficient in PH1, AGT, with promising results obtained in preclinical models. ERT can be achieved through a direct or an indirect approach.
Direct ERT in PH1 involves AGT restoration in host hepatocytes. The first engineered form of the AGT enzyme (AGT-RHEAM) developed for ERT reported high catalytic activity and stability104, but had no avenue for delivery into cells. A fusion protein of AGT with a N-terminal cell-penetrating Tat peptide was then developed and successfully internalized in a cell culture model of PH1, but it was limited in therapeutic potential because of the strong immunogenicity of the Tat peptide105. Lastly, a subsequently developed form of AGT cross-linked to moieties of polyethylene glycol (PEG) and polyglutamic acid (PGA) (AGT-PGA-AGT) has been shown to reach the peroxisome and metabolize glyoxylate in cell culture models of PH1, and achieved stability and non-immunogenicity in plasma106. Another method of direct ERT involves the delivery of lipid nanoparticle-encapsulated mRNA. Successful delivery of AGT mRNA was demonstrated in Agxt-knockout mice, with a resultant 40% reduction in urinary oxalate107. Finally, direct ERT can also be performed through viral delivery of AGT complementary DNA (cDNA), which has been shown to significantly reduce urine oxalate by 2.7–3.6-fold (P < 0.05) in Agxt-knockout mice in two studies108,109.
Indirect ERT strategies include ex vivo restoration of AGT in the patient’s own stem cells before differentiation into hepatocytes and reimplantation into the liver, or liver cell transplantation from a healthy donor. Successful restoration of AGT expression was demonstrated in induced pluripotent stem cells (iPSCs) from fibroblasts of patients diagnosed with PH1, but cells failed to retain edited AGT upon differentiation into hepatocyte-like cells110; a subsequent study addressed the AGT-retention problem with the introduction of a liver-specific transthyretin promoter, which successfully provided hepatocyte-like cells showing rescued AGT expression after differentiation111. Alternatively, indirect ERT can be performed through liver cell transplantation from healthy donors, which has been shown to substantially reduce plasma oxalate in a case report of a 15-month-old patient with severe systemic oxalosis112. However, biopsy specimens taken from many areas of the explanted liver did not show any donor cells 5 months after transplant, highlighting that liver repopulation is a major challenge for indirect AGT replacement, because corrected hepatocytes will only represent a small percentage of total hepatocytes. Strategies to address liver repopulation after indirect ERT include decreasing the relative fitness of host liver cells using radiotherapy and increasing the relative fitness of corrected hepatocytes using hepatocyte growth factor, which was successful in a mouse model of PH1 (ref.113).
CRISPR–Cas9 therapy
CRISPR–Cas9 gene editing has the advantage of being permanent compared with the transient gene silencing technology of RNAi (such as lumasiran and nedosiran). Approximately 20 phase I/II clinical trials involving CRISPR–Cas9 delivery for therapeutic use in single-gene diseases are ongoing, including sickle cell anaemia, β-thalassaemia, leukaemia, non-small-cell lung cancer and more, but long-term follow-up data are limited114. CRISPR–Cas9 technology remains a clinical challenge owing to important limitations, including off-target effects, toxic effects and delivery challenges115.
Off-target effects, defined as induction of mutations at sites other than the intended on-target site, have been shown to occur at a frequency of at least 50%116. Double-stranded break repair by non-homologous end joining (NHEJ) can lead to unexpected mutations or genomic rearrangements117. Methods of decreasing NHEJ in favour of the less common but higher fidelity homology directed repair (HDR) include chemical or genomic silencing of NHEJ118,119, or the introduction of Cas9 nickase (Cas9n), which induces specific single-stranded breaks (SSBs) to promote HDR116. Decreasing DNA binding affinity of Cas9 and optimizing single guide RNA (sgRNA) targeting are two strategies to prevent Cas9 from binding to incorrect sites and causing unwanted alterations115. Cas9 mutants with decreased binding affinity of Cas9 have been created, including SpCas9, evoCas9 and HiFiCas9 (refs120,121). Similarly, platforms have been designed to optimize sgRNA sequences against target genes based on computational algorithms, such as E-Crisp, CRISPR-design, CasOFFinder and sgDesigner116,122,123.
Toxic effects caused by CRISPR–Cas9 delivery and gene editing can have multiple causes. DSBs can trigger apoptosis instead of gene editing, often in a p53-mediated manner124,125. Immunotoxicity as a result of delivery of CRISPR–Cas9 was described in a 2019 study showing that >50% of healthy donors have pre-existing anti-Cas9 antibodies against the most commonly used Cas9 orthologues, SaCas9 and SpCas9 (ref.126).
Finally, delivery of CRISPR–Cas9 cargo into patients’ tissues remains a barrier to its widespread clinical use. The most commonly used delivery method involves viral vectors, typically adenovirus and lentivirus, but these methods have some limitations, including a toxic effect at high doses in animal models127, limited carrying capacity128 and the potential for host immune activation129. Non-viral delivery systems include lipid-based delivery (such as lipofectamine)130 and cell-penetrating peptides131, but current limitations are relatively poor uptake and translocation efficiency into cells compared with viral approaches. Other delivery mechanisms remain largely untested in vivo132. These limitations mean that successful application of CRISPR–Cas9 in PH has been restricted to preclinical models, including cell culture and animal models, and has not yet been attempted clinically133. The preclinical data are especially promising for SRT, primarily for inhibition of glycolate oxidase and LDH. Liver-specific delivery of a sgRNA targeting mouse Hao1 led to the prevention of oxalate over-production and kidney damage in Agxt-knockout mice134, and another study showed similar efficacy using sgRNA to target Hao1 in AgxtD205N-mutant rats135. The same group showed that sgRNA knockdown of Ldha, decreasing LDH expression by 50% compared with untreated controls, led to a significant reduction in urinary oxalate levels at 1, 3 and 6 months in AGXTD205N-mutant rats (P ≤ 0.003)136. Using CRISPR–Cas9 for ERT in PH has not currently been attempted, but is a feasible future application of the technology.
Targeting the inflammasome
One of the primary mechanisms of renal failure in PH is oxalate-induced inflammation and activation of downstream inflammatory response pathways137,138,139. One crucial inflammatory pathway mediator in PH is NOD-, LRR- and pyrin domain-containing protein 3 (NLRP3), which is an intracellular sensor that detects cellular insults and activates downstream release of the pro-inflammatory cytokines IL-1β and IL-18 and subsequent cell death140. NLRP3-null mice were shown to be protected against progressive kidney failure and cell death compared with NLRP3 wild-type mice141. Other crucial inflammatory mediators are the tumour necrosis factor receptors 1 and 2 (TNFR1 and TNFR2), which lead to the release of pro-inflammatory cytokines IL-6 and TNF and, subsequently, to apoptosis142. TNFR1-null or TNFR2-null mice fed a high oxalate diet did not develop nephrocalcinosis or chronic kidney disease compared with wild-type mice143.
Rationally, targeting of NLRP3, TNFR1 and TNFR2, and pro-inflammatory cytokines could result in improved renal outcomes in patients with PH. Many therapeutics are being developed for other diseases that could be translatable to PH; however, these therapies have not yet been clinically tested in patients with PH, and models of hyperoxaluria (such as high oxalate diet mouse models) used in the corresponding studies might not completely capture the situation in PH. NLRP3 transcriptional inhibition with the microRNA miR-223 has been shown to prevent inflammasome activation and cytokine release in mouse models of intestinal inflammation144. The small-molecule NLRP3 inhibitor CP-456773, also known as CRID3 and MCC950, decreases renal inflammation and fibrosis in mouse models145. OLT1177 (dapansutrile) is another small-molecule NLRP3 inhibitor that has been shown to reduce inflammation induced by IL-1β and IL-18 production in preclinical models of chronic inflammatory diseases, including cultures of lipopolysaccharide (LPS)-stimulated macrophages, neutrophils and monocytes and LPS-challenged mice146. Results from completed phase II trials for gout147 and knee osteoarthritis (NCT01768975)148 demonstrated that OLT1177 is safe in humans and efficient for NLRP3 inhibition, and warrants further exploration in PH1. IL-1β receptor antagonist anakinra (Kineret) has been shown to protect from CaOx nephropathy in mice, reducing inflammation and kidney damage5. Anakinra is already FDA-approved for rheumatoid arthritis, cryopyrin-associated periodic syndromes (CAPS), and deficiency of the interleukin-1-receptor antagonist (DIRA)149, and has shown promising results in multiple clinical trials for hidradenitis suppurativa150, pericarditis151, COVID-19 (ref.152) and other inflammatory conditions, although it has not been clinically tested in PH. Anakinra might, therefore, be a therapeutic opportunity in PH1 and warrants testing in clinical trials. Finally, TNFR blockade by R-7050, a cell-permeable compound, decreased the inflammatory and fibrotic response to intrarenal CaOx crystallization in wild-type mice fed an oxalate-rich diet, a model of hyperoxaluria143.
In summary, ERT, CRISPR–Cas9 for SRT and ERT, and inhibition of the inflammasome, although clinically untested in PH thus far, are promising therapeutic strategies that warrant testing for tolerability and efficacy in clinical trials for PH.
Conclusions
PH is an extremely exciting area of preclinical and clinical research, in which some of the newest therapeutics are drastically influencing the lives of patients today. The FDA approval of lumasiran, and the FDA Rare Pediatric Disease Designation of nedosiran, CHK-336, and Oxabact are exciting, but still much more work is needed. For the newest clinically successful drugs, long-term safety and outcomes data are lacking, and the rate at which patients develop resistance to these therapeutics is unknown. Furthermore, many of the published clinical trials for these new therapeutics have been restricted to patients without ESRD, and case studies suggesting utility in these cohorts of patients with more severe disease are just emerging. Heterogeneity of response to these therapeutics is also a challenge, which could strongly affect the need for transplantation in patients. Identifying biomarkers of patients’ response is crucial for successful clinical implementation of PH therapies, and might also invite novel strategies or combination therapies in the future. For developmental stage therapeutics, such as CRISPR–Cas9 and AGT fusion proteins, optimizing delivery strategies and minimizing off-target effects remain important challenges to address. Additional therapeutics that are already FDA-approved for other indications, such as anakinra, might also have potential in PH. Moreover, in vitro and in vivo screening of existing compound libraries could be a high-throughput way of identifying new therapeutic candidates for PH. Considering the rapid development of new sequencing, gene therapy and drug development technologies, the diagnosis and management of PH will continue to evolve and transform the lives of future generations of patients with PH.
References
Lepoutre, L. Calculs multiples chez un enfant: Infiltration du parenchyme rénal par des dépôts cristallins. J. Urol. 20, 424 (1925).
Lieske, J. C. et al. International registry for primary hyperoxaluria. Am. J. Nephrol. 25, 290–296 (2005).
Hopp, K. et al. Phenotype-genotype correlations and estimated carrier frequencies of primary hyperoxaluria. J. Am. Soc. Nephrol. 26, 2559–2570 (2015).
Asplin, J. R. Hyperoxaluric calcium nephrolithiasis. Endocrinol. Metab. Clin. North Am. 31, 927–949 (2002).
Mulay, S. R. et al. Calcium oxalate crystals induce renal inflammation by NLRP3-mediated IL-1beta secretion. J. Clin. Invest. 123, 236–246 (2013).
Beck, B. B., Hoyer-Kuhn, H., Gobel, H., Habbig, S. & Hoppe, B. Hyperoxaluria and systemic oxalosis: an update on current therapy and future directions. Expert Opin. Invest. Drugs 22, 117–129 (2013).
Holmes, R. P. & Assimos, D. G. Glyoxylate synthesis, and its modulation and influence on oxalate synthesis. J. Urol. 160, 1617–1624 (1998).
Monico, C. G. et al. Primary hyperoxaluria type III gene HOGA1 (formerly DHDPSL) as a possible risk factor for idiopathic calcium oxalate urolithiasis. Clin. J. Am. Soc. Nephrol. 6, 2289–2295 (2011).
Giafi, C. F. & Rumsby, G. Kinetic analysis and tissue distribution of human D-glycerate dehydrogenase/glyoxylate reductase and its relevance to the diagnosis of primary hyperoxaluria type 2. Ann. Clin. Biochem. 35, 104–109 (1998).
Booth, M. P., Conners, R., Rumsby, G. & Brady, R. L. Structural basis of substrate specificity in human glyoxylate reductase/hydroxypyruvate reductase. J. Mol. Biol. 360, 178–189 (2006).
Belostotsky, R., Pitt, J. J. & Frishberg, Y. Primary hyperoxaluria type III — a model for studying perturbations in glyoxylate metabolism. J. Mol. Med. 90, 1497–1504 (2012).
Cellini, B., Bertoldi, M., Montioli, R., Paiardini, A. & Borri Voltattorni, C. Human wild-type alanine:glyoxylate aminotransferase and its naturally occurring G82E variant: functional properties and physiological implications. Biochem. J. 408, 39–50 (2007).
Mdluli, K., Booth, M. P., Brady, R. L. & Rumsby, G. A preliminary account of the properties of recombinant human glyoxylate reductase (GRHPR), LDHA and LDHB with glyoxylate, and their potential roles in its metabolism. Biochim. Biophys. Acta 1753, 209–216 (2005).
Dindo, M. et al. Molecular basis of primary hyperoxaluria: clues to innovative treatments. Urolithiasis 47, 67–78 (2019).
Cochat, P. et al. Epidemiology of primary hyperoxaluria type 1. Société de Nephrologie and the Société de Néphrologie Pédiatrique. Nephrol. Dial. Transpl. 10 (Suppl 8), 3–7 (1995).
van Woerden, C. S., Groothoff, J. W., Wanders, R. J., Davin, J. C. & Wijburg, F. A. Primary hyperoxaluria type 1 in The Netherlands: prevalence and outcome. Nephrol. Dial. Transpl. 18, 273–279 (2003).
Hoppe, B., Beck, B. B. & Milliner, D. S. The primary hyperoxalurias. Kidney Int. 75, 1264–1271 (2009).
Bergstralh, E. J. et al. Transplantation outcomes in primary hyperoxaluria. Am. J. Transpl. 10, 2493–2501 (2010).
van der Hoeven, S. M., van Woerden, C. S. & Groothoff, J. W. Primary hyperoxaluria type 1, a too often missed diagnosis and potentially treatable cause of end-stage renal disease in adults: results of the Dutch cohort. Nephrol. Dial. Transpl. 27, 3855–3862 (2012).
Harambat, J. et al. Characteristics and outcomes of children with primary oxalosis requiring renal replacement therapy. Clin. J. Am. Soc. Nephrol. 7, 458–465 (2012).
Cregeen, D. P., Williams, E. L., Hulton, S. & Rumsby, G. Molecular analysis of the glyoxylate reductase (GRHPR) gene and description of mutations underlying primary hyperoxaluria type 2. Hum. Mutat. 22, 497 (2003).
Cramer, S. D., Ferree, P. M., Lin, K., Milliner, D. S. & Holmes, R. P. The gene encoding hydroxypyruvate reductase (GRHPR) is mutated in patients with primary hyperoxaluria type II. Hum. Mol. Genet. 8, 2063–2069 (1999).
Milliner, D. S., Wilson, D. M. & Smith, L. H. Phenotypic expression of primary hyperoxaluria: comparative features of types I and II. Kidney Int. 59, 31–36 (2001).
Kemper, M. J., Conrad, S. & Muller-Wiefel, D. E. Primary hyperoxaluria type 2. Eur. J. Pediatr. 156, 509–512 (1997).
Belostotsky, R. et al. Mutations in DHDPSL are responsible for primary hyperoxaluria type III. Am. J. Hum. Genet. 87, 392–399 (2010).
Martin-Higueras, C. et al. A report from the European Hyperoxaluria Consortium (OxalEurope) Registry on a large cohort of patients with primary hyperoxaluria type 3. Kidney Int. 100, 621–635 (2021).
Cochat, P. & Rumsby, G. Primary hyperoxaluria. N. Engl. J. Med. 369, 649–658 (2013).
Alnylam Pharmaceuticals. Alnylam receives approval for OXLUMO™ (lumasiran) in the European Union for the treatment of primary hyperoxaluria type 1 in all age groups. BioSpace https://www.biospace.com/article/releases/alnylam-receives-approval-for-oxlumo-lumasiran-in-the-european-union-for-the-treatment-of-primary-hyperoxaluria-type-1-in-all-age-groups/ (2020).
FDA News Release. FDA approves first drug to treat rare metabolic disorder. FDA https://www.fda.gov/news-events/press-announcements/fda-approves-first-drug-treat-rare-metabolic-disorder (2020).
Leumann, E. & Hoppe, B. The primary hyperoxalurias. J. Am. Soc. Nephrol. 12, 1986–1993 (2001).
Sikora, P. et al. [13C2]oxalate absorption in children with idiopathic calcium oxalate urolithiasis or primary hyperoxaluria. Kidney Int. 73, 1181–1186 (2008).
Siener, R., Hoppe, B., Lohr, P., Muller, S. C. & Latz, S. Metabolic profile and impact of diet in patients with primary hyperoxaluria. Int. Urol. Nephrol. 50, 1583–1589 (2018).
Hallson, P. C., Rose, G. A. & Sulaiman, S. Raising urinary citrate lowers calcium oxalate and calcium phosphate crystal formation in whole urine. Urol. Int. 38, 179–181 (1983).
Milliner, D. S., Eickholt, J. T., Bergstralh, E. J., Wilson, D. M. & Smith, L. H. Results of long-term treatment with orthophosphate and pyridoxine in patients with primary hyperoxaluria. N. Engl. J. Med. 331, 1553–1558 (1994).
Smith, L. H. Jr & Williams, H. E. Treatment of primary hyperoxaluria. Mod. Treat. 4, 522–530 (1967).
Hoyer-Kuhn, H. et al. Vitamin B6 in primary hyperoxaluria I: first prospective trial after 40 years of practice. Clin. J. Am. Soc. Nephrol. 9, 468–477 (2014).
Fargue, S., Rumsby, G. & Danpure, C. J. Multiple mechanisms of action of pyridoxine in primary hyperoxaluria type 1. Biochim. Biophys. Acta 1832, 1776–1783 (2013).
Hoppe, B. et al. Oxalate elimination via hemodialysis or peritoneal dialysis in children with chronic renal failure. Pediatr. Nephrol. 10, 488–492 (1996).
Illies, F., Bonzel, K. E., Wingen, A. M., Latta, K. & Hoyer, P. F. Clearance and removal of oxalate in children on intensified dialysis for primary hyperoxaluria type 1. Kidney Int. 70, 1642–1648 (2006).
Bunchman, T. E. & Swartz, R. D. Oxalate removal in type I hyperoxaluria or acquired oxalosis using HD and equilibration PD. Perit. Dial. Int. 14, 81–84 (1994).
Saborio, P. & Scheinman, J. I. Transplantation for primary hyperoxaluria in the United States. Kidney Int. 56, 1094–1100 (1999).
Broyer, M., Ehrich, J., Jones, E. & Selwood, N. Five year survival of kidney transplantation in children: data from the European (EDTA-ERA) registry. Kidney Int. Suppl. 43, S22–S25 (1993).
Devresse, A., Cochat, P., Godefroid, N. & Kanaan, N. Transplantation for primary hyperoxaluria type 1: designing new strategies in the era of promising therapeutic perspectives. Kidney Int. Rep. 5, 2136–2145 (2020).
Ruiz, R. et al. Long-term analysis of combined liver and kidney transplantation at a single center. Arch. Surg. 141, 735–741 (2006). discussion 741–732.
Asrani, S. K. et al. Recipient characteristics and morbidity and mortality after liver transplantation. J. Hepatol. 69, 43–50 (2018).
Lam, N. N. et al. Mortality and morbidity in kidney transplant recipients with a failing graft: a matched cohort study. Can. J. Kidney Health Dis. 7, 2054358120908677 (2020).
Martin-Higueras, C., Luis-Lima, S. & Salido, E. Glycolate oxidase is a safe and efficient target for substrate reduction therapy in a mouse model of primary hyperoxaluria type I. Mol. Ther. 24, 719–725 (2016).
Bourhis, J. M. et al. Structure of human glycolate oxidase in complex with the inhibitor 4-carboxy-5-[(4-chlorophenyl)sulfanyl]-1,2,3-thiadiazole. Acta Crystallogr. Sect. F. Struct. Biol. Cryst. Commun. 65, 1246–1253 (2009).
Liebow, A. et al. An Investigational RNAi therapeutic targeting glycolate oxidase reduces oxalate production in models of primary hyperoxaluria. J. Am. Soc. Nephrol. 28, 494–503 (2017).
Springer, A. D. & Dowdy, S. F. GalNAc-siRNA conjugates: leading the way for delivery of RNAi therapeutics. Nucleic Acid. Ther. 28, 109–118 (2018).
Garrelfs, S. F. et al. ILLUMINATE-A, a phase 3 study of lumasiran, an investigational RNAi therapeutic, in children and adults with primary hyperoxaluria type 1 (PH1). Nephrol. Dial. Transplant. 35, 1–14 (2020).
Michael, M. et al. PO1624: ILLUMINATE-B, a Phase 3 Open-Label Study to Evaluate Lumasiran, an RNAi Therapeutic, in Young Children with Primary Hyperoxaluria Type 1 (PH1) (American Society of Nephrology, 2020).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/show/NCT04152200 (2021).
Takahashi, Y., Miyajima, H. & Kaneko, E. Genetic analysis of a family of lactate dehydrogenase A subunit deficiency. Intern. Med. 34, 326–329 (1995).
Frampton, J. E. Stiripentol: a review in Dravet syndrome. Drugs 79, 1785–1796 (2019).
Le Dudal, M. et al. Stiripentol protects against calcium oxalate nephrolithiasis and ethylene glycol poisoning. J. Clin. Invest. 129, 2571–2577 (2019).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/show/NCT03819647 (2021).
Kempf, C. et al. Stiripentol fails to lower plasma oxalate in a dialysis-dependent PH1 patient. Pediatr. Nephrol. 35, 1787–1789 (2020).
Lai, C. et al. Specific inhibition of hepatic lactate dehydrogenase reduces oxalate production in mouse models of primary hyperoxaluria. Mol. Ther. 26, 1983–1995 (2018).
Dicerna Pharmaceuticals. Dicerna receives rare pediatric disease designation from U.S. Food and Drug Administration for Nedosiran for treatment of primary hyperoxaluria. Business Wire https://www.businesswire.com/news/home/20200618005175/en (2020).
Hoppe, B. et al TH-PO449: PHYOX: A Safety and Tolerability Study of DCR-PHXC in Primary Hyperoxaluria Types 1 and 2 (American Society of Nephrology, 2019).
Dicerna Pharmaceuticals. Dicerna receives rare pediatric disease designation from U.S. Food and Drug Administration for Nedosiran for treatment of primary hyperoxaluria. Business Wire https://www.businesswire.com/news/home/20210805006062/en (2021).
Shee, K. et al. Nedosiran dramatically reduces serum oxalate in dialysis-dependent primary hyperoxaluria 1: a compassionate use case report. Urology 156, e147–e149 (2021).
Chinook Therapeutics. Dicerna receives rare pediatric disease designation from U.S. Food and Drug Administration for nedosiran for treatment of primary hyperoxaluria. GlobeNewswire https://www.businesswire.com/news/home/20200618005175/en (2021).
Hatch, M. Gut microbiota and oxalate homeostasis. Ann. Transl. Med. 5, 36 (2017).
Allison, M. J., Dawson, K. A., Mayberry, W. R. & Foss, J. G. Oxalobacter formigenes gen. nov., sp. nov.: oxalate-degrading anaerobes that inhabit the gastrointestinal tract. Arch. Microbiol. 141, 1–7 (1985).
Hatch, M. & Freel, R. W. A human strain of Oxalobacter (HC-1) promotes enteric oxalate secretion in the small intestine of mice and reduces urinary oxalate excretion. Urolithiasis 41, 379–384 (2013).
Hoppe, B. et al. Oxalobacter formigenes: a potential tool for the treatment of primary hyperoxaluria type 1. Kidney Int. 70, 1305–1311 (2006).
OxThera. OxThera receives rare pediatric disease designation from U.S. FDA for oxabact treatment of primary hyperoxaluria. OxThera https://news.cision.com/oxthera/r/oxthera-receives-rare-pediatric-disease-designation-from-u-s-fda-for-oxabact-treatment-of-primary-h (2020).
Hoppe, B. et al. A randomised phase I/II trial to evaluate the efficacy and safety of orally administered Oxalobacter formigenes to treat primary hyperoxaluria. Pediatr. Nephrol. 32, 781–790 (2017).
Milliner, D., Hoppe, B. & Groothoff, J. A randomised phase II/III study to evaluate the efficacy and safety of orally administered Oxalobacter formigenes to treat primary hyperoxaluria. Urolithiasis 46, 313–323 (2018).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/show/NCT03116685 (2021).
Hoppe, B. et al. Efficacy and safety of Oxalobacter formigenes to reduce urinary oxalate in primary hyperoxaluria. Nephrol. Dial. Transpl. 26, 3609–3615 (2011).
Pape, L., Ahlenstiel-Grunow, T., Birtel, J., Krohne, T. U. & Hoppe, B. Oxalobacter formigenes treatment combined with intensive dialysis lowers plasma oxalate and halts disease progression in a patient with severe infantile oxalosis. Pediatr. Nephrol. 35, 1121–1124 (2020).
Peck, A. B., Canales, B. K. & Nguyen, C. Q. Oxalate-degrading microorganisms or oxalate-degrading enzymes: which is the future therapy for enzymatic dissolution of calcium-oxalate uroliths in recurrent stone disease? Urolithiasis 44, 45–50 (2016).
Lee, E., Jeong, B. C., Park, Y. H. & Kim, H. H. Expression of the gene encoding oxalate decarboxylase from Bacillus subtilis and characterization of the recombinant enzyme. BMC Res. Notes 7, 598 (2014).
Cowley, H. et al. In vitro and in vivo safety evaluation of Nephure. Regul. Toxicol. Pharmacol. 86, 241–252 (2017).
Bhasin, B., Urekli, H. M. & Atta, M. G. Primary and secondary hyperoxaluria: understanding the enigma. World J. Nephrol. 4, 235–244 (2015).
Quintero, E. et al. A prospective, double-blind, randomized, placebo-controlled, crossover study using an orally administered oxalate decarboxylase (OxDC). Kidney360 1, 1284–1290 (2020).
Mufarrij, P. W., Lange, J. N., Knight, J., Assimos, D. G. & Holmes, R. P. The effects of Oxazyme on oxalate degradation: results and implications of in vitro experiments. J. Endourol. 27, 284–287 (2013).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/show/NCT01127087 (2012).
Langman, C. B. et al. A double-blind, placebo controlled, randomized phase 1 cross-over study with ALLN-177, an orally administered oxalate degrading enzyme. Am. J. Nephrol. 44, 150–158 (2016).
Lingeman, J. E. et al. ALLN-177, oral enzyme therapy for hyperoxaluria. Int. Urol. Nephrol. 51, 601–608 (2019).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/show/NCT03391804 (2020).
Hopper, E. D., Pittman, A. M., Fitzgerald, M. C. & Tucker, C. L. In vivo and in vitro examination of stability of primary hyperoxaluria-associated human alanine:glyoxylate aminotransferase. J. Biol. Chem. 283, 30493–30502 (2008).
Mesa-Torres, N. et al. The role of protein denaturation energetics and molecular chaperones in the aggregation and mistargeting of mutants causing primary hyperoxaluria type I. PLoS ONE 8, e71963 (2013).
Pey, A. L., Albert, A. & Salido, E. Protein homeostasis defects of alanine-glyoxylate aminotransferase: new therapeutic strategies in primary hyperoxaluria type I. Biomed. Res. Int. 2013, 687658 (2013).
Powers, E. T., Morimoto, R. I., Dillin, A., Kelly, J. W. & Balch, W. E. Biological and chemical approaches to diseases of proteostasis deficiency. Annu. Rev. Biochem. 78, 959–991 (2009).
Monico, C. G., Olson, J. B. & Milliner, D. S. Implications of genotype and enzyme phenotype in pyridoxine response of patients with type I primary hyperoxaluria. Am. J. Nephrol. 25, 183–188 (2005).
van Woerden, C. S. et al. Clinical implications of mutation analysis in primary hyperoxaluria type 1. Kidney Int. 66, 746–752 (2004).
Singh, P. et al. Pyridoxine responsiveness in a type 1 primary hyperoxaluria patient with a rare (Atypical) AGXT gene mutation. Kidney Int. Rep. 5, 955–958 (2020).
Monico, C. G., Rossetti, S., Olson, J. B. & Milliner, D. S. Pyridoxine effect in type I primary hyperoxaluria is associated with the most common mutant allele. Kidney Int. 67, 1704–1709 (2005).
Dindo, M., Oppici, E., Dell’Orco, D., Montone, R. & Cellini, B. Correlation between the molecular effects of mutations at the dimer interface of alanine-glyoxylate aminotransferase leading to primary hyperoxaluria type I and the cellular response to vitamin B6. J. Inherit. Metab. Dis. 41, 263–275 (2018).
Zhao, G. et al. Betaine in inflammation: mechanistic aspects and applications. Front. Immunol. 9, 1070 (2018).
Santana, A., Salido, E., Torres, A. & Shapiro, L. J. Primary hyperoxaluria type 1 in the Canary Islands: a conformational disease due to I244T mutation in the P11L-containing alanine:glyoxylate aminotransferase. Proc. Natl Acad. Sci. USA 100, 7277–7282 (2003).
Coulter-Mackie, M. B. & Lian, Q. Partial trypsin digestion as an indicator of mis-folding of mutant alanine:glyoxylate aminotransferase and chaperone effects of specific ligands. Study of a spectrum of missense mutants. Mol. Genet. Metab. 94, 368–374 (2008).
Lumb, M. J., Birdsey, G. M. & Danpure, C. J. Correction of an enzyme trafficking defect in hereditary kidney stone disease in vitro. Biochem. J. 374, 79–87 (2003).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/show/NCT00283387 (2013).
Valastyan, J. S. & Lindquist, S. Mechanisms of protein-folding diseases at a glance. Dis. Model. Mech. 7, 9–14 (2014).
Oppici, E., Roncador, A., Montioli, R., Bianconi, S. & Cellini, B. Gly161 mutations associated with primary hyperoxaluria type I induce the cytosolic aggregation and the intracellular degradation of the apo-form of alanine:glyoxylate aminotransferase. Biochim. Biophys. Acta 1832, 2277–2288 (2013).
Weissenbacher, E. R. et al. A comparison of dequalinium chloride vaginal tablets (Fluomizin(R)) and clindamycin vaginal cream in the treatment of bacterial vaginosis: a single-blind, randomized clinical trial of efficacy and safety. Gynecol. Obstet. Invest. 73, 8–15 (2012).
Miyata, N. et al. Pharmacologic rescue of an enzyme-trafficking defect in primary hyperoxaluria 1. Proc. Natl Acad. Sci. USA 111, 14406–14411 (2014).
Belostotsky, R. et al. Translation inhibition corrects aberrant localization of mutant alanine-glyoxylate aminotransferase: possible therapeutic approach for hyperoxaluria. J. Mol. Med. 96, 621–630 (2018).
Mesa-Torres, N. et al. The consensus-based approach for gene/enzyme replacement therapies and crystallization strategies: the case of human alanine-glyoxylate aminotransferase. Biochem. J. 462, 453–463 (2014).
Roncador, A., Oppici, E., Montioli, R., Maset, F. & Cellini, B. TAT-mediated delivery of human alanine:glyoxylate aminotransferase in a cellular model of primary hyperoxaluria type I. Int. J. Pept. Res. Ther. 19, 175–184 (2013).
Roncador, A. et al. Use of polymer conjugates for the intraperoxisomal delivery of engineered human alanine:glyoxylate aminotransferase as a protein therapy for primary hyperoxaluria type I. Nanomedicine 13, 897–907 (2017).
Kukreja, A. et al. Systemic alanine glyoxylate aminotransferase mRNA improves glyoxylate metabolism in a mouse model of primary hyperoxaluria type 1. Nucleic Acid. Ther. 29, 104–113 (2019).
Castello, R. et al. Helper-dependent adenoviral vectors for liver-directed gene therapy of primary hyperoxaluria type 1. Gene Ther. 23, 129–134 (2016).
Salido, E. et al. Phenotypic correction of a mouse model for primary hyperoxaluria with adeno-associated virus gene transfer. Mol. Ther. 19, 870–875 (2011).
Esteve, J. et al. Targeted gene therapy in human-induced pluripotent stem cells from a patient with primary hyperoxaluria type 1 using CRISPR/Cas9 technology. Biochem. Biophys. Res. Commun. 517, 677–683 (2019).
Esteve, J. et al. Generation of induced pluripotent stem cells-derived hepatocyte-like cells for ex vivo gene therapy of primary hyperoxaluria type 1. Stem Cell Res. 38, 101467 (2019).
Beck, B. B. et al. Liver cell transplantation in severe infantile oxalosis — a potential bridging procedure to orthotopic liver transplantation? Nephrol. Dial. Transpl. 27, 2984–2989 (2012).
Jiang, J. et al. Correction of hyperoxaluria by liver repopulation with hepatocytes in a mouse model of primary hyperoxaluria type-1. Transplantation 85, 1253–1260 (2008).
Sharma, G., Sharma, A. R., Bhattacharya, M., Lee, S. S. & Chakraborty, C. CRISPR-Cas9: a preclinical and clinical perspective for the treatment of human diseases. Mol. Ther. 29, 571–586 (2021).
Uddin, F., Rudin, C. M. & Sen, T. CRISPR gene therapy: applications, limitations, and implications for the future. Front. Oncol. 10, 1387 (2020).
Zhang, X. H., Tee, L. Y., Wang, X. G., Huang, Q. S. & Yang, S. H. Off-target effects in CRISPR/Cas9-mediated genome engineering. Mol. Ther. Nucleic Acids 4, e264 (2015).
Bunting, S. F. & Nussenzweig, A. End-joining, translocations and cancer. Nat. Rev. Cancer 13, 443–454 (2013).
Chu, V. T. et al. Increasing the efficiency of homology-directed repair for CRISPR-Cas9-induced precise gene editing in mammalian cells. Nat. Biotechnol. 33, 543–548 (2015).
Vartak, S. V. & Raghavan, S. C. Inhibition of nonhomologous end joining to increase the specificity of CRISPR/Cas9 genome editing. FEBS J. 282, 4289–4294 (2015).
Slaymaker, I. M. et al. Rationally engineered Cas9 nucleases with improved specificity. Science 351, 84–88 (2016).
Kleinstiver, B. P. et al. High-fidelity CRISPR-Cas9 nucleases with no detectable genome-wide off-target effects. Nature 529, 490–495 (2016).
Cui, Y., Xu, J., Cheng, M., Liao, X. & Peng, S. Review of CRISPR/Cas9 sgRNA Design Tools. Interdiscip. Sci. 10, 455–465 (2018).
Hiranniramol, K., Chen, Y., Liu, W. & Wang, X. Generalizable sgRNA design for improved CRISPR/Cas9 editing efficiency. Bioinformatics 36, 2684–2689 (2020).
Ihry, R. J. et al. p53 inhibits CRISPR-Cas9 engineering in human pluripotent stem cells. Nat. Med. 24, 939–946 (2018).
Hu, Z. et al. Disruption of HPV16-E7 by CRISPR/Cas system induces apoptosis and growth inhibition in HPV16 positive human cervical cancer cells. Biomed. Res. Int. 2014, 612823 (2014).
Charlesworth, C. T. et al. Identification of preexisting adaptive immunity to Cas9 proteins in humans. Nat. Med. 25, 249–254 (2019).
Lau, C. H. & Suh, Y. In vivo genome editing in animals using AAV-CRISPR system: applications to translational research of human disease. F1000Res 6, 2153 (2017).
Gaj, T., Epstein, B. E. & Schaffer, D. V. Genome engineering using adeno-associated virus: basic and clinical research applications. Mol. Ther. 24, 458–464 (2016).
Kim, S. et al. CRISPR RNAs trigger innate immune responses in human cells. Genome Res. https://doi.org/10.1101/gr.231936.117 (2018).
Berardo, C. et al. Comparison between lipofectamine RNAiMAX and GenMute transfection agents in two cellular models of human hepatoma. Eur. J. Histochem. 63, 3048 (2019).
Ramakrishna, S. et al. Gene disruption by cell-penetrating peptide-mediated delivery of Cas9 protein and guide RNA. Genome Res. 24, 1020–1027 (2014).
Yip, B. H. Recent advances in CRISPR/Cas9 delivery strategies. Biomolecules 10, 839 (2020).
Belostotsky, R. & Frishberg, Y. Novel therapeutic approaches for the primary hyperoxalurias. Pediatr. Nephrol. 36, 2593–2606 (2021).
Zabaleta, N. et al. CRISPR/Cas9-mediated glycolate oxidase disruption is an efficacious and safe treatment for primary hyperoxaluria type I. Nat. Commun. 9, 5454 (2018).
Zheng, R. et al. CRISPR/Cas9-mediated metabolic pathway reprogramming in a novel humanized rat model ameliorates primary hyperoxaluria type 1. Kidney Int. 98, 947–957 (2020).
Zheng, R. et al. Knockdown of lactate dehydrogenase by adeno-associated virus-delivered CRISPR/Cas9 system alleviates primary hyperoxaluria type 1. Clin. Transl. Med. 10, e261 (2020).
Komada, T. & Muruve, D. A. The role of inflammasomes in kidney disease. Nat. Rev. Nephrol. 15, 501–520 (2019).
Anders, H. J. et al. The macrophage phenotype and inflammasome component NLRP3 contributes to nephrocalcinosis-related chronic kidney disease independent from IL-1-mediated tissue injury. Kidney Int. 93, 656–669 (2018).
Ermer, T., Eckardt, K. U., Aronson, P. S. & Knauf, F. Oxalate, inflammasome, and progression of kidney disease. Curr. Opin. Nephrol. Hypertens. 25, 363–371 (2016).
Swanson, K. V., Deng, M. & Ting, J. P. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 19, 477–489 (2019).
Knauf, F. et al. NALP3-mediated inflammation is a principal cause of progressive renal failure in oxalate nephropathy. Kidney Int. 84, 895–901 (2013).
Wajant, H. & Siegmund, D. TNFR1 and TNFR2 in the control of the life and death balance of macrophages. Front. Cell Dev. Biol. 7, 91 (2019).
Mulay, S. R. et al. Hyperoxaluria requires TNF receptors to initiate crystal adhesion and kidney stone disease. J. Am. Soc. Nephrol. 28, 761–768 (2017).
Neudecker, V. et al. Myeloid-derived miR-223 regulates intestinal inflammation via repression of the NLRP3 inflammasome. J. Exp. Med. 214, 1737–1752 (2017).
Ludwig-Portugall, I. et al. An NLRP3-specific inflammasome inhibitor attenuates crystal-induced kidney fibrosis in mice. Kidney Int. 90, 525–539 (2016).
Marchetti, C. et al. OLT1177, a beta-sulfonyl nitrile compound, safe in humans, inhibits the NLRP3 inflammasome and reverses the metabolic cost of inflammation. Proc. Natl Acad. Sci. USA 115, E1530–E1539 (2018).
Kluck, V. et al. Dapansutrile, an oral selective NLRP3 inflammasome inhibitor, for treatment of gout flares: an open-label, dose-adaptive, proof-of-concept, phase 2a trial. Lancet Rheumatol. 2, e270–e280 (2020).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/show/NCT01768975 (2014).
Sobi North America. FDA Approves KINERET® (anakinra) for the treatment of deficiency of IL-1 receptor antagonist (DIRA). GlobeNewswire https://www.globenewswire.com/news-release/2020/12/22/2149573/0/en/FDA-Approves-KINERET-anakinra-for-the-Treatment-of-Deficiency-of-IL-1-Receptor-Antagonist-DIRA.html (2020).
Tzanetakou, V. et al. Safety and efficacy of anakinra in severe hidradenitis suppurativa: a randomized clinical trial. JAMA Dermatol. 152, 52–59 (2016).
Brucato, A. et al. Effect of anakinra on recurrent pericarditis among patients with colchicine resistance and corticosteroid dependence: the AIRTRIP randomized clinical trial. JAMA 316, 1906–1912 (2016).
King, A. et al. Anakinra in COVID-19: important considerations for clinical trials. Lancet Rheumatol. 2, e379–e381 (2020).
Yendt, E. R. & Cohanim, M. Response to a physiologic dose of pyridoxine in type I primary hyperoxaluria. N. Engl. J. Med. 312, 953–957 (1985).
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Urology thanks the anonymous reviewers for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Human Gene Mutation Database: http://www.hgmd.cf.ac.uk
Rights and permissions
About this article
Cite this article
Shee, K., Stoller, M.L. Perspectives in primary hyperoxaluria — historical, current and future clinical interventions. Nat Rev Urol 19, 137–146 (2022). https://doi.org/10.1038/s41585-021-00543-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41585-021-00543-4
This article is cited by
-
Qualitative assessment of the patient experience of primary hyperoxaluria type 1: an observational study
BMC Nephrology (2023)
-
Oxalate homeostasis
Nature Reviews Nephrology (2023)
-
Primary hyperoxaluria type 1 in children: clinical and laboratory manifestations and outcome
Pediatric Nephrology (2023)
-
Nedosiran in primary hyperoxaluria subtype 3: results from a phase I, single-dose study (PHYOX4)
Urolithiasis (2023)
-
Healthcare utilization, quality of life, and work productivity associated with primary hyperoxaluria: a cross-sectional web-based US survey
Urolithiasis (2023)