Abstract
GeoBioMed — a new transdisciplinary approach that integrates the fields of geology, biology and medicine — reveals that kidney stones composed of calcium-rich minerals precipitate from a continuum of repeated events of crystallization, dissolution and recrystallization that result from the same fundamental natural processes that have governed billions of years of biomineralization on Earth. This contextual change in our understanding of renal stone formation opens fundamentally new avenues of human kidney stone investigation that include analyses of crystalline structure and stratigraphy, diagenetic phase transitions, and paragenetic sequences across broad length scales from hundreds of nanometres to centimetres (five Powers of 10). This paradigm shift has also enabled the development of a new kidney stone classification scheme according to thermodynamic energetics and crystalline architecture. Evidence suggests that ≥50% of the total volume of individual stones have undergone repeated in vivo dissolution and recrystallization. Amorphous calcium phosphate and hydroxyapatite spherules coalesce to form planar concentric zoning and sector zones that indicate disequilibrium precipitation. In addition, calcium oxalate dihydrate and calcium oxalate monohydrate crystal aggregates exhibit high-frequency organic-matter-rich and mineral-rich nanolayering that is orders of magnitude higher than layering observed in analogous coral reef, Roman aqueduct, cave, deep subsurface and hot-spring deposits. This higher frequency nanolayering represents the unique microenvironment of the kidney in which potent crystallization promoters and inhibitors are working in opposition. These GeoBioMed insights identify previously unexplored strategies for development and testing of new clinical therapies for the prevention and treatment of kidney stones.
Key points
-
Data from an emerging field, GeoBioMed, show that human kidney stone formation is controlled by the same fundamental sequence of processes that governs phosphate, carbonate and silicate deposition in other natural and engineered environments on Earth, which are known as universal biomineralization and diagenetic phase transitions.
-
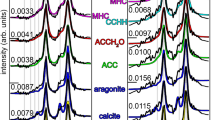
Human kidney stones classified as ‘apatite’, ‘COD’ (calcium oxalate dihydrate), ‘COM’ (calcium oxalate monohydrate), or simply ‘CaOx’ (calcium oxalate) are actually composed of multiple mineralogical components (none is 100% purely one mineral) that comprise the continuum of diagenetic phase transitions from which they formed, which include amorphous calcium phosphate (ACP), hydroxyapatite (HAP), COD and COM.
-
ACP and HAP spherules grow, cluster and coalesce to form euhedral COD crystals with planar concentric zoning and sector zones, indicating disequilibrium precipitation from supersaturated urine.
-
ACP, HAP, COD and COM nanolayering and cross-cutting crystalline relationships (for example, dissolution voids, fracturing and faulting) record a complete stratigraphic record and paragenetic sequence that is analogous to natural and engineered biomineralization and diagenetic phase transition systems, the only difference being time and scale.
-
At least 50% of the total volume of whole and fragmented kidney stones has naturally undergone repeated events of in vivo dissolution and recrystallization.
-
This approach has revealed multiple testing targets for the development of new clinical therapies, which includes growth of kidney stones within GeoBioCell microfluidic testbeds during control of key parameters, such as gradients and fluctuations in urine solution chemistry, changing flow, protein and small-molecule concentrations, and microbiome composition.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout










Similar content being viewed by others
References
Von Humboldt, A. Personal Narrative of a Journey to the Equinoctial Regions of the New Continent (Penguin Books Ltd., 1995).
Ball, P. Life’s matrix: water in the cell. Cell Mol. Biol. 47, 717–720 (2001).
Lane, N. The vital question: energy, evolution and the origins of complex life (Norton and Company, 2015).
NASA. Water in the Solar System and Beyond (2020).
NASA. Lifting the Veil of Star Formation in the Orion Nebula (2019).
Stumm, W. & Morgan, J. J. Aquatic Chemistry vol. 1040 (John Wiley & Sons, 2012).
Folk, R. L. & Assereto, R. Comparative fabrics of length-slow and length-fast calcite and calcitized aragonite in a Holocene speleothem, Carlsbad caverns, New-Mexico. J. Sediment. Petrol. 46, 486–496 (1976).
Assereto, R. & Folk, R. L. Brick-like texture and radial rays in triassic pisolites of Lombardy, Italy — clue to distinguish ancient aragonitic pisolites. Sediment. Geol. 16, 205–222 (1976).
Perrin, C. et al. Aragonite-calcite speleothems: identifying original and diagenetic features. J. Sediment. Res. 84, 245–269 (2014).
NASA. Astrobiology Strategy (2015).
Russell, M. J. & Nitschke, W. Methane: fuel or exhaust at the emergence of life? Astrobiology 17, 1053–1066 (2017).
Price, R. et al. Alkaline vents and steep Na+ gradients from ridge-flank basalts — Implications for the origin and evolution of life. Geology 45, 1135–1138 (2017).
Meunier, A., Petit, S., Cockell, C. S., El Albani, A. & Beaufort, D. The Fe-rich clay microsystems in Basalt-Komatiite lavas: importance of Fe-smectites for pre-biotic molecule catalysis during the Hadean Eon. Orig. Life Evol. B 40, 253–272 (2010).
Woese, C. R., Kandler, O. & Wheelis, M. L. Towards a natural system of organisms - proposal for the domains Archaea, Bacteria, and Eucarya. Proc. Natl Acad. Sci. USA 87, 4576–4579 (1990).
Knoll, T. Epidemiology, pathogenesis, and pathophysiology of urolithiasis. Eur. Urol. Suppl. 9, 802–806 (2010).
Fouke, B. W. & Murphy, T. The Art of Yellowstone Science: Mammoth Hot Springs as a Window on the Universe (Crystal Creek Press, 2016). A comprehensive systems-level understanding is reviewed of the life–water–mineral interactions intrinsic to universal biomineralization.
Addadi, L. & Weiner, S. Control and design principles in biological mineralization. Angew. Chem. Int. Ed. 31, 153–169 (1992). A review of the mechanisms by which organisms control the structure and mineralogy of crystallization.
Weiner, S. & Addadi, L. Crystallization pathways in biomineralization. Annu. Rev. Mater. Res. 41, 21–40 (2011). An evaluation of the mechanisms underlying the four most common biomineralization pathways observed in nature.
Lowenstam, H. A. Goethite in radular teeth of recent marine gastropods. Science 137, 279–280 (1962).
Lowenstam, H. A. & Weiner, S. Transformation of amorphous calcium phosphate to crystalline dahillite in the radular teeth of chitons. Science 227, 51–53 (1985).
Lowenstam, H. A. Lepidocrocite, an apatite mineral, and magnetic in teeth of chitons (Polyplacophora). Science 156, 1373–1375 (1967).
Towe, K. M. & Lowenstam, H. A. Ultrastructure and development of iron mineralization in the radular teeth of Cryptochiton stelleri (Mollusca). J. Ultrastruct. Res. 17, 1–13 (1967).
Beniash, E., Aizenberg, J., Addadi, L. & Weiner, S. Amorphous calcium carbonate transforms into calcite during sea urchin larval spicule growth. Proc. R. Soc. B Biol. Sci. 264, 461–465 (1997).
Addadi, L., Aizenberg, J., Beniash, E. & Weiner, S. On the concept of a single crystal in biomineralization. in Crystal Engineering: From Molecules and Crystals to Materials vol. 538 (eds Braga, D., Grepioni, F. & Orpen, A. G.) 1–22 (Springer, 1999).
Weiner, S., Sagi, I. & Addadi, L. Structural biology. Choosing the crystallization path less traveled. Science 309, 1027–1028 (2005).
Mass, T. et al. Amorphous calcium carbonate particles form coral skeletons. Proc. Natl Acad. Sci. USA 114, E7670–E7678 (2017).
Rimer, J. D., Kolbach-Mandel, A. M., Ward, M. D. & Wesson, J. A. The role of macromolecules in the formation of kidney stones. Urolithiasis 45, 57–74 (2017).
Ryall, R. L. The future of stone research: rummagings in the attic, Randallís plaque, nanobacteria, and lessons from phylogeny. Urol. Res. 36, 77–97 (2008). A thorough evaluation of the differing schools of thought that have developed since the 1930s regarding kidney stone formation, which highlights the importance of early renal biomineralization.
Lowenstam, H. A. Minerals formed by organisms. Science 211, 1126–1131 (1981).
Mann, S. Biomineralization: Principles and Concepts in Bioinorganic Materials Chemistry (Oxford University Press, 2001).
Mann, S. in Skeletal biomineralization — patterns, processes and evolutionary trends Vol. 1–2 (ed. Carter, J. G.) (Springer, 1991).
Dove, P. M., De Yoreo, J. J. & Weiner, S. in Reviews in Mineralogy and Geochemistry Vol. 54 (Mineralogical Society of America, 2003).
Seymour, V. The human-nature relationship and its impact on health: a critical review. Front. Public Health 4, 260 (2016).
Humboldt, A. V. Cosmos: A Sketch of a Physical Description of the Universe Vol. I (Henry G. Bohn, 1849).
Heriche, J. K., Alexander, S. & Ellenberg, J. Integrating imaging and omics: computational methods and challenges. Annu. Rev. Biomed. Data Sci. 2, 175–197 (2019).
National Research Council. Convergence: facilitating transdiciplinary integration of life sciences, physical sciences, engineering, and beyond (The National Academies Press, 2014).
Sivaguru, M., Lieske, J. C., Krambeck, A. E. & Fouke, B. W. GeoBioMed sheds new light on human kidney stone crystallization and dissolution. Nat. Rev. Urol. 17, 1–2 (2020).
Sivaguru, M. et al. Geobiology reveals how human kidney stones dissolve in vivo. Sci. Rep. 8, 13731 (2018). Interdisciplinary geobiology analyses are presented that indicate calcium oxalate kidney stones undergo multiple repeated in vivo events of crystallization, dissolution and recrystallization during their formation.
Moran, M. E. Urolithiasis — A Comprehensive History (Springer, 2014).
Modlin, M. A history of urinary stone. S. Afr. Med. J. 58, 652–655 (1980).
Geller, M. J. & Cohen, S. L. Kidney and urinary tract disease in ancient Babylonia, with translations of the cuneiform sources. Kidney Int. 47, 1811–1815 (1995).
Wang, W. et al. Prevalence of kidney stones in mainland China: a systematic review. Sci. Rep. 7, 41630 (2017).
Evan, A. P. et al. Mechanism of formation of human calcium oxalate renal stones on Randall’s plaque. Anat. Rec. 290, 1315–1323 (2007). A detailed review of the mechanisms proposed for Randall’s plaque calcium oxalate kidney stone formation.
Khan, S. R. et al. Kidney stones. Nat. Rev. Dis. Prim. 2, 1–22 (2016). A comprehensive review of the mechanisms proposed for Randall’s plaque and Randall’s plug calcium oxalate kidney stone formation.
Alelign, T. & Petros, B. Kidney stone disease: an update on current concepts. Adv. Urol. 2018, 3068365 (2018).
Tanikawa, C. et al. Novel risk loci identified in a genome-wide association study of urolithiasis in a Japanese population. J. Am. Soc. Nephrol. 30, 855–864 (2019).
Khan, S. R., Canales, B. K. & Dominguez-Gutierrez, P. R. Randall’s plaque and calcium oxalate stone formation: role for immunity and inflammation. Nat. Rev. Nephrol. https://doi.org/10.1038/s41581-020-00392-1 (2021).
Stamatelou, K. K., Francis, M. E., Jones, C. A., L., M. N. Jr & Curhan, G. C. Time trends in reported prevalence of kidney stones in the United States: 1976–1994. Kidney Int. 63, 1817–1823 (2003).
Scales, C. D., Smith, A. C., Hanley, J. M., Saigal, C. S. & Urologic Diseases in America Project. Prevalence of Kidney Stones in the United States. Eur. Urol. 62, 160–165 (2012).
Scales, C. D. et al. Changing gender prevalence of stone disease. J. Urol. 177, 979–982 (2007).
Dwyer, M. E. et al. Temporal trends in incidence of kidney stones among children: a 25-year population based study. J. Urol. 188, 247–252 (2012).
Finlayson, B. Physicochemical aspects of urolithiasis. Kidney Int. 13, 344–360 (1978). A review summarizing the biochemical mechanisms behind kidney stone crystallization and dissolution from a thermodynamic perspective.
Finlayson, B. & Reid, F. The expectation of free and fixed particles in urinary stone disease. Invest. Urol. 15, 442–448 (1978).
Finlayson, B., Roth, R. & DuBois, L. in Urinary Calculi. Recent Advances in Aetiology, Stone Structure and Treatment (eds Cifuentes Delatte, L., Rapado, A., & Hodgkinson, A.) 1–7 (S. Karger, 1973).
Finlayson, B., Vermeulen, C. W. & Stewart, E. J. Stone matrix and mucoprotein from urine. J. Urol. 86, 355–363 (1961).
Fleisch, H., Robertson, W. G., Smith, L. H. & Vahlensieck, W. Urolithiasis Research (Plenum Press, 1976).
Hesse, A., Berg, W., Schneider, H. J. & Hienzsch, E. A contribution to the formation mechanism of calcium oxalate urinary calculi. II. In vitro experiments concerning the theory of the formation of Whewellite and Weddellite urinary calculi. Urol. Res. 4, 157–160 (1976).
Hesse, A., Berg, W., Schneider, H. J. & Hienzsch, E. A contribution to the formation mechanism of calcium oxalate urinary calculi. I. Stabilising urinary constituents in the formation of Weddellite. Urol. Res. 4, 125–128 (1976).
Hesse, A., Lange, P., Berg, W., Bothor, C. & Hienzsch, E. Scanning electron microscope and microprobe investigation of phosphate phases in uroliths. Urol. Int. 34, 81–94 (1979).
Hesse, A. & Miersch, W. Special aspects of stone composition and aetiology of different types of urinary calculi. Int. Urol. Nephrol. 21, 257–267 (1989).
Hesse, A. & Siener, R. Current aspects of epidemiology and nutrition in urinary stone disease. World J. Urol. 15, 165–171 (1997).
Murphy, B. T. & Pyrah, L. N. The composition, structure, and mechanisms of the formation of urinary calculi. Br. J. Urol. 34, 129–159 (1962). The earliest microscopy study of kidney stones in thin section, which described diagenetic phase transitions as “transformations”.
Pyrah, L. N. Renal calculus. Br. J. Clin. Pract. 11, 649–656 (1957).
Pyrah, L. N. Renal calculus (Springer Verlag, 1979).
Wesson, J. A. & Ward, M. D. Role of crystal surface adhesion in kidney stone disease. Curr. Opin. Nephrol. Hypertens. 15, 386–393 (2006).
Evan, A. P. et al. Mechanism by which shock wave lithotripsy can promote formation of human calcium phosphate stones. Am. J. Physiol. Renal Physiol. 308, F938–F949 (2015).
Khan, S. R. & Canales, B. K. Unified theory on the pathogenesis of Randall’s plaques and plugs. Urolithiasis 43, 109–123 (2015). A comprehensive review of the proposed mechanisms of kidney stone formation from in vivo and in vitro studies of both human and animal models.
Daudon, M. et al. Comprehensive morpho-constitutional analysis of urinary stones improves etiological diagnosis and therapeutic strategy of nephrolithiasis. C. R. Chim. 19, 1470–1491 (2016).
Saw, J. J. et al. In vivo entombment of bacteria and fungi during calcium oxalate, brushite, and struvite urolithiasis. Kidney360 2, 298–311 (2021). Provides direct evidence from both microscopy and molecular tools for entombment of a microbiome during in vivo kidney stone growth.
Beniash, E., Addadi, L. & Weiner, S. Cellular control over spicule formation in sea urchin embryos: a structural approach. J. Struct. Biol. 125, 50–62 (1999).
Addadi, L., Aizenberg, J., Albeck, S., Falini, G. & Weiner, S. Structural control over the formation of calcium carbonate mineral phases in biomineralization. Supramol. Stereochem. 473, 127–139 (1995).
Agency for Healthcare Research and Quality. Effective Health Care Program (Agency for Healthcare Research and Quality, 2016).
Pearle, M. S. et al. Medical management of kidney stones: AUA Guideline. J. Urol. 192, 316 (2014).
Hughes, P. Kidney stones epidemiology. Nephrology 12, 26–30 (2007).
Reveillaud, R. J., Daudon, M., Protat, M. F. & Ayrole, G. Analysis of urinary calculi in adults. Attempt of correlations between morphology and composition. Eur. Urol. 6, 161–165 (1980).
Daudon, M., Bader, C. A. & Jungers, P. Urinary calculi: review of classification methods and correlations with etiology. Scanning Microsc. 7, 1081–1106 (1993).
Tovberg Jensen, A. On concrements from the urinary tract II. Acta Chir. Scand. 84, 207–225 (1941).
Sohnel, O. & Grases, F. Fine structure of calcium oxalate monohydrate renal calculi. Nephron 63, 176–182 (1993).
Grases, F., Costabauza, A. & Conte, A. Studies on structure of calcium-oxalate monohydrate renal papillary calculi - mechanism of formation. Scanning Microsc. 7, 1067–1074 (1993).
Cloutier, J., Villa, L., Traxer, O. & Daudon, M. Kidney stone analysis: “Give me your stone, I will tell you who you are!”. World J. Urol. 33, 157–169 (2015). A detailed review that characterizes and compares detailed kidney stone analyses with patient history.
Evan, A. P., Worcester, E. M., Coe, F. L., Williams, J. Jr. & Lingeman, J. E. Mechanisms of human kidney stone formation. Urolithiasis 43, 19–32 (2015).
Daudon, M., Marfisi, C., Lacour, B. & Bader, C. Investigation of urinary crystals by Fourier transform infrared microscopy. Clin. Chem. 37, 83–87 (1991).
Williams Jr, J. C., Lingeman, J. E., Coe, F. L., Worcester, E. M. & Evan, A. P. Micro‑CT imaging of Randall’s plaques. Urolithiasis 43, 13–17 (2015).
Grases, F. et al. On the origin of calcium oxalate monohydrate papillary renal stones. Urolithiasis 43, S33–S39 (2015).
Berg, W., Schnapp, J. D., Schneider, H. J., Hesse, A. & Hienzsch, E. Crystaloptical and spectroscopical findings with calcium oxalate crystals in the urine sediment: a contribution to the genesis of oxalate stones. Eur. Urol. 2, 92–97 (1976).
Hosli, P. O. Uber Genese und Aufbau von Harnsteinen; Inaug. Diss. Zurich (1957).
Cifuentes Delatte, L., Hidalgo, A., Bellanato, J. & Santos, M. in Urinary Calculi. Recent Advances in Aetiology, Stone Structure and Treatment (eds Cifuentes Delatte, L., Rapado, A., & Hodgkinson, A.) 220–230 (S. Karger, 1973).
Prien, E. L. & Frondel, C. Studies in urolithiasis: I. The composition of urinary calculi. J. Urol. 57, 949–994 (1947).
Hesse, A., Berg, W. & Bothor, C. Scanning electron microscopic investigations on the morphology and phase conversions of uroliths. Int. Urol. Nephrol. 11, 11–20 (1979).
Grases, F., Costa-Bauza, A. & Garcia-Ferragut, L. Biopathological crystallization: a general view about the mechanisms of renal stone formation. Adv. Colloid Interfac. 74, 169–194 (1998). A comprehensive review of the biological and pathological conditions that lead to each major type of kidney stone formation.
Kolossov, V. L. et al. Airyscan super-resolution microscopy of mitochondrial morphology and dynamics in living tumor cells. Microsc. Res. Tech. 81, 115–128 (2018).
Sivaguru, M. et al. Imaging horse tendons using multimodal 2-photon microscopy. Methods 66, 256–267 (2014).
Sivaguru, M. et al. Correction factors for delta O-18-derived global sea surface temperature reconstructions from diagenetically altered intervals of coral skeletal density banding. Front. Mar. Sci. https://doi.org/10.3389/fmars.2019.00306 (2019).
Sivaguru, M. et al. Application of an advanced maximum likelihood estimation restoration method for enhanced-resolution and contrast in second-harmonic generation microscopy. J. Microsc. 267, 397–408 (2017).
Sivaguru, M., Mander, L., Fried, G. & Punyasena, S. W. Capturing the surface texture and shape of pollen: a comparison of microscopy techniques. PLoS ONE 7, e39129 (2012).
Sivaguru, M. et al. Comparative performance of airyscan and structured illumination superresolution microscopy in the study of the surface texture and 3D shape of pollen. Microsc. Res. Tech. 81, 101–114 (2016). A detailed description of the super-resolution autofluorescence (SRAF) technique, which has been applied to characterize kidney stone paragenetic sequences and diagenetic phase transitions.
Stoller, M. L., Low, R. K., Shami, G. S., McCormick, V. D. & Kerschmann, R. L. High resolution radiography of cadaveric kidneys: unraveling the mystery of Randall’s plaque formation. J. Urol. 156, 1263–1266 (1996).
Ho, S. P. et al. Architecture-guided fluid flow directs renal biomineralization. Sci. Rep. 8, 14157 (2018). A description and proposed interpretation of the continuum of biomineralization events within the renal pyramid as detected with high-resolution micro-computed tomography (micro-CT).
Anderson, P. W. More is different — broken symmetry and nature of hierarchical structure of science. Science 177, 393 (1972).
Goldenfeld, N. & Kadanoff, L. P. Simple lessons from complexity. Science 284, 87–89 (1999).
Fouke, B. W. Hot-spring Systems Geobiology: abiotic and biotic influences on travertine formation at Mammoth Hot Springs, Yellowstone National Park, USA. Sedimentology 58, 170–219 (2011). A comprehensive review of the physical, chemical and biological factors and mechanisms that control biomineralization in hot springs.
Taylor, J. E. A Descriptive and Illustrated Catalogue of the Calculi and Other Animal Concretions Contained in the Museum of the Royal College of Surgeons in London (Richard and John E. Taylor, 1842).
Beale, L. S. Illustrations of the Constituents of Urine Urinary Deposits, and Calculi (John Churchill, 1858).
Matlaga, B. R. et al. Endoscopic evidence of calculus attachment to Randall’s plaque. J. Urol. 175, 1720–1724 (2006).
Fasano, J. M. & Khan, S. R. Intratubular crystalization of calcium oxalate in the presence of membrane vesicles: an in vitro study. Kidney Int. 59, 169–178 (2001).
Bamberger, J. N. et al. Clinical and metabolic correlates of calcium oxalate stone subtypes: implications for etiology and management. J. Endourol. 33, 755–760 (2019). Correlations are evaluated between kidney stone former patient histories as a means of predicting calcium oxalate urolithiasis.
Coe, F. L., Evan, A. P., Lingeman, J. E. & Worcester, E. M. Plaque and deposits in nine human stone diseases. Urol. Res. 38, 239–247 (2010).
Schepers, M. S. et al. Internalization of calcium oxalate crystals by renal tubular cells: a nephron segment-specific process? Kidney Int. 64, 493–500 (2003).
Thamilselvan, S. & Menon, M. Vitamin E therapy prevents hyperoxaluria-induced calcium oxalate crystal deposition in the kidney by improving renal tissue antioxidant status. BJU Int. 96, 117–126 (2005).
Vervaet, B. A., Verhulst, A., Broe, M. E. D. & D’Haese, P. C. The tubular epithelium in the initiation and course of intratubular nephrocalcinosis. Urol. Res. 38, 249–256 (2010).
Evan, A. P. et al. Renal intratubular crystals and hyaluronan staining occur in stone formers with bypass surgery but not with idiopathic calcium oxalate stones. Anat. Rec. 291, 325–334 (2008).
Sethmann, I. et al. Microstructures of Randall’s plaques and their interfaces with calcium oxalate monohydrate kidney stones reflect underlying mineral precipitation mechanisms. Urolithiasis 45, 235–248 (2017).
Daudon, M. & Jungers, P. in Urolithiasis, Basic Science and Clinical Practice Ch. 15 (eds Talati, J., Tiselius, H.-G., Albala D. M. & Ye, Z.) 113–140 (Springer, 2012).
Daudon, M., Frochot, V., Bazin, D. & Jungers, P. Drug-induced kidney stones and crystalline nephropathy: pathophysiology, prevention and treatment. Drugs 78, 163–201 (2018).
Evan, A. P. Physiopathology and etiology of stone formation in the kidney and the urinary tract. Pediatr. Nephrol. 25, 831–841 (2010).
Coe, F. L., Evan, A. P., Worcester, E. M. & Lingeman, J. E. Three pathways for human kidney stone formation. Urol. Res. 38, 147–160 (2010).
Randall, A. The origin and growth of renal calculi. Ann. Surg. 105, 1009–1027 (1937).
Anderson, L. & McDonald, J. R. The origin, frequency, and significance of microscopic calculi in kidney. Surg. Gynecol. Obstet. 82, 275–282 (1946). The first study to conclusively demonstrate that early calcium phosphate mineralization occurs in stone-former and non-stone-former cadaver kidneys (found in 246 out of 246 kidneys analysed).
Bruwer, A. Primary renal calculi: Anderson-Carr-Randall progression? AJR 132, 751–758 (1979). A detailed review of literature from the 1930s/1940s, combined with histology analyses of 61 kidneys, are used to demonstrate that early renal tissue mineralization takes place in stone formers and non-stone formers.
Haggitt, R. C. & Pitcock, J. A. Renal medullary calcifications: a light and electron microscopic study. J. Urol. 106, 342–347 (1971).
Carr, R. J. A new theory on the formation of renal calculi. Br. J. Urol. 26, 105–117 (1954).
Sherer, B. A. et al. A continuum of mineralization from human renal pyramid to stones on stems. Acta Biomater. 71, 72–85 (2018).
Paterlini, M. There shall be order. The legacy of Linnaeus in the age of molecular biology. EMBO Rep. 8, 814–816 (2007).
SEPM STRATA-SEPM Stratigraphy Web. Carbonate classification. SEPM STRATA http://www.sepmstrata.org/page.aspx?pageid=89 (2013).
Dunham, R. J. in Classification of Carbonate Rocks (ed. Ham, W. E.) 108–121 (American Association of Petroleum Geologists Memoir, 1962).
Mann, S. & Weiner, S. Biomineralization: structural questions at all length scales. J. Struct. Biol. 126, 179–181 (1999).
Lowenstam, H. A. Similarities of and differences between biogenic and inorganic mineralization products. Abstr. Pap. Am. Chem. Soc. 196, 115 (1988).
Lowenstam, H. A., Traub, W. & Weiner, S. Nautilus hard parts — a study of the mineral and organic constitutents. Paleobiology 10, 268–279 (1984).
Lowenstam, H. A. & Weiner, S. Phosphatic shell plate of the barnacle Ibla (Cirripedia): a bone-like structure. Proc. Natl Acad. Sci. USA 89, 10573–10577 (1992).
Lowenstam, H. A., Weiner, S. & Newman, W. A. Carbonate apatite-containing shell plates of a Barnacle (Cirripedia). Int. Congr. Ser. 1002, 73–84 (1992).
Lowenstam, H. A. & Weiner, S. On Biomineralization (Oxford University Press, 1989).
Keith, H. D. & Padden, F. J. A phenomenological theory of spherulitic crystallization. J. Appl. Phys. 34, 2409 (1963).
Frondel, C. & Prien, E. L. Carbonate-apatite and hydroxyl-apatite in urinary calculi. Science 95, 431 (1942). One of the first studies to document that carbonate minerals, in combination with apatite, can be mineralogical constituents of human kidney stone formation.
Watkins, J., Manga, M., Huber, C. & Martin, M. Diffusion-controlled spherulite growth in obsidian inferred from H2O concentration profiles. Contrib. Mineral. Petrol. 157, 163–172 (2009).
Addadi, L., Politi, Y., Nudelman, F. & Weiner, S. in Engineering of Crystalline Materials Properties (eds Novoa, J. J., Braga, D. & Addadi, L.) 1–15 (Springer, 2008).
Aizenberg, J. et al. Design strategies in mineralized biological materials. Abstr. Pap. Am. Chem. Soc. 213, 768 (1997).
Weiner, S. Biomineralization: a structural perspective. J. Struct. Biol. 163, 229–234 (2008).
Weiner, S., Mahamid, J. & Addadi, L. Biomineralization processes in vertebrates. Bone 50, S19–S20 (2012).
Weiner, S., Sagi, I. & Addadi, L. Choosing the crystallization path less traveled. Science 309, 1027–1028 (2005).
Gower, L. B. Biomimetic model systems for investigating the amorphous precursor pathway and its role in biomineralization. Chem. Rev. 108, 4551–4627 (2008). A comprehensive biomineralization review of the early amorphous chemical precursors to calcium oxalate kidney stone formation.
Combes, C., Cazalbou, S. & Rey, C. Apatite biominerals. Minerals 6, 34 (2016). A comprehensive review of diagenetic phase transitions of amorphous calcium phosphate to apatite minerals in natural systems.
Rimer, J. D. Inorganic ions regulate amorphous-to-crystal shape preservation in biomineralization. Proc. Natl Acad. Sci. USA 117, 3360–3362 (2020).
Nancollas, G. H. & Johnsson, M. A. Calculus formation and inhibition. Adv. Dent. Res. 8, 307–311 (1994).
Ruiz-Agudo, E. et al. A non-classical view on calcium oxalate precipitation and the role of citrate. Nat. Commun. 8, 768 (2017).
Wang, L. J. & Nancollas, G. H. Calcium orthophosphates: crystallization and dissolution. Chem. Rev. 108, 4628–4669 (2008).
Ibsen, C. J. S., Chernyshov, D. & Birkedal, H. Apatite formation from amorphous calcium phosphate and mixed amorphous calcium phosphate/amorphous calcium carbonate. Chem. Eur. J. 22, 12347–12357 (2016). This study experimentally demonstrates the mineralization pathway from amorphous calcium phosphate and mixed amorphous calcium carbonate to apatite kidney stone formation.
Addadi, L. Biomineralization: the mechanism of crystal formation in bone and other mineralized tissues. Considerations on the relevance to gout. Ann. Rheum. Dis. 73, 5–5 (2014).
Johnsson, M. S.-A. & Nancollas, G. H. The role of brushite and octacalcium phosphate in apatite formation. Crit. Rev. Oral Biol. Med. 3, 61–82 (1992). A detailed theoretical and experimental summary of the diagenetic phase transitions is presented, from the unstable polymorphs of calcium phosphate to tricalcium phosphate and octacalcium phosphate.
Hill, M. G., Konigsberger, E. & May, P. M. Mineral precipitation and dissolution in the kidney. Am. Miner. 102, 701–710 (2017).
Konigsberger, E., Konigsberger, L. Biomineralization — Medical Aspects of Solubility vol. 277 (John Wiley and Sons Ltd., 2006).
Tucker, M. E., Bathurst, R. G. C. Carbonate Diagenesis Vol. 320 (John Wiley & Sons, 2009).
Bathurst, R. G. C. Carbonate Sediments and Their Diagenesis (Elsevier, 1971).
Mercedes-Martin, R. et al. A depositional model for spherulitic carbonates associated with alkaline, volcanic lakes. Mar. Petrol. Geol. 86, 168–191 (2017).
Weiner, S., Lowenstam, H. A. & Hood, L. Characterization of 80-million-year-old mollusk shell proteins. Proc. Natl Acad. Sci. USA 73, 2541–2545 (1976).
Dai, L. J., Cheng, X. G. & Gower, L. B. Transition bars during transformation of an amorphous calcium carbonate precursor. Chem. Mater. 20, 6917–6928 (2008).
Amos, F. F., Dai, L., Kumar, R., Khan, S. R. & Gower, L. B. Mechanism of formation of concentrically laminated spherules: implication to Randall’s plaque and stone formation. Urol. Res. 37, 11–17 (2009). A detailed experimental study of how individual concentrically laminated spherules form and the implications for plaque formation.
Chidambaram, A., Rodriguez, D., Khan, S. & Gower, L. Biomimetic Randall’s plaque as an in vitro model system for studying the role of acidic biopolymers in idiopathic stone formation. Urolithiasis 43, S77–S92 (2015).
Zhang, J., Wang, L. J. & Putnis, C. V. Underlying role of brushite in pathological mineralization of hydroxyapatite. J. Phys. Chem. B 123, 2874–2881 (2019).
Baghel, S., Cathcart, H. & O’Reilly, N. J. Understanding the generation and maintenance of supersaturation during the dissolution of amorphous solid dispersions using modulated DSC and H-1 NMR. Int. J. Pharm. 536, 414–425 (2018).
Hajir, M., Graf, R. & Tremel, W. Stable amorphous calcium oxalate: synthesis and potential intermediate in biomineralization. Chem. Commun. 50, 6534–6536 (2014).
Berg, W., Lange, P., Rossler, D. & Bothor, C. [Pseudomorphoses in calcium oxalate urinary calculi]. Z. Urol. Nephrol. 72, 351–357 (1979).
Cifuentes Delatte, L., Rapado, A. & Hodgkinson, A. Urinary Calculi. Recent Advances in Aetiology, Stone Structure and Treatment (S. Karger, 1973).
Schubert, G. & Brien, G. Crystallographic investigations of urinary calcium oxalate calculi. Int. Urol. Nephrol. 13, 249–260 (1981).
Castiglione, V. et al. Raman chemical imaging, a new tool in kidney stone structure analysis: case-study and comparison to fourier transform infrared spectroscopy. PLoS ONE 13, e0201460 (2018).
Cui, X. Y. et al. Analysis and classification of kidney stones based on Raman spectroscopy. Biomed. Opt. Express 9, 4175–4183 (2018).
Langdon, A. & Grohe, B. The osteopontin-controlled switching of calcium oxalate monohydrate morphologies in artificial urine provides insights into the formation of papillary kidney stones. Colloids Surf. B Biointerfaces 146, 296–306 (2016).
Ryall, R. L. et al. The hole truth: intracrystalline proteins and calcium oxalate kidney stones. Mol. Urol. 4, 391–402 (2000).
Lieske, J. C., Norris, R., Swift, H. & Toback, F. G. Adhesion, internalization and metabolism of calcium oxalate monohydrate crystals by renal epithelial cells. Kidney Int. 52, 1291–1301 (1997). A detailed experimental study providing direct evidence that initial crystallization in kidney is intratubular mineralization and the crystals could be aggregated and become endocytosed (called “internalization”).
Pierce, L. W. & Bloom, B. Observations on urolithiasis among American troops in a desert area. J. Urol. 54, 466–470 (1945).
Evans, K. & Costabile, R. A. Time to development of symptomatic urinary calculi in a high risk environment. J. Urol. 173, 858–861 (2005).
Sivaguru, M. et al. Quantitative analysis of collagen fiber organization in injured tendons using Fourier transform-second harmonic generation imaging. Opt. Express 18, 24983–24993 (2010).
Sivaguru, M., Fried, G. A., Miller, C. A. H. & Fouke, B. W. Multimodal optical microscopy methods reveal polyp tissue morphology and structure in Caribbean Reef building corals. J. Vis. Exp. 3791, e51824 (2014).
Sivaguru, M., Khaw, Y. M. & Inoue, M. A confocal reflection super-resolution technique to image Golgi-Cox stained neurons. J. Microsc. 275, 115–130 (2019).
Miller, N. L. The origin and significance of Randall’s plaque in nephrolithiasis. J. Urol. 186, 783–784 (2011).
Jones, F. T. Iris agate. Am. Miner. 37, 578–587 (1952).
Akhavan, A. C. The Quartz Page. The Quartz Page http://www.quartzpage.de/index.html (2005).
Doyle, L. J., Blake, N. J., Woo, C. C. & Yevich, P. Recent biogenic phosphorite - concretions in mollusk kidneys. Science 199, 1431–1433 (1978).
Schmidt-Nielsen, B. August Krogh Lecture. The renal concentrating mechanism in insects and mammals: a new hypothesis involving hydrostatic pressures. Am. J. Physiol. 268, R1087–R1100 (1995).
Boron, W. F. & Boulpaep, E. L. The electrogenic Na/HCO3 cotransporter. Kidney Int. 36, 392–402 (1989).
Tombs, M. P. & Peacocke, A. R. The Osmotic Pressure of Biological Macromolecules (Clarendon Press, 1974).
Thomas, S. R. & Wexler, A. S. Inner medullary external osmotic driving force in a 3-D model of the renal concentrating mechanism. Am. J. Physiol. Renal Fluid Electrolyte Physiol. 269, F159–F171 (1995).
Thain, J. F. Principles of Osmotic Phenomena (The Royal Institute of Chemistry, 1967).
Siga, E. & Horster, M. F. Regulation of osmotic water permeability during differentiation of inner medullary collecting duct. Am. J. Physiol. 260, F710–F716 (1991).
Randall, A. The initiating lesions of renal calculus. Surg. Gynecol. Obstet. 64, 201–208 (1937).
Bird, V. Y. & Khan, S. R. How do stones form? Is unification of theories on stone formation possible? Arch. Esp. Urol. 70, 12–27 (2017).
Ciftcioglu, N. et al. Association between Randall’s plaque and calcifying nanoparticles. Int. J. Nanomed. 3, 105–115 (2008).
Reid, D. G., Jackson, G. J., Duer, M. J. & Rodgers, A. L. Apatite in kidney stones is a molecular composite with glycosaminoglycans and proteins: evidence from nuclear magnetic resonance spectroscopy, and relevance to Randall’s plaque, pathogenesis and prophylaxis. J. Urol. 185, 725–730 (2011).
Verkoelen, C. F. Crystal retention in renal stone disease: a crucial role for the glycosaminoglycan hyaluronan? J. Am. Soc. Nephrol. 17, 1673–1687 (2006). A detailed review of early biomineralization within renal tubule epithelial cells and how mucus and extracelluar biomolecules such as polysaccharides play a central role in crystal adhesion, retention, growth, aggregation and tubule plugging.
Kajander, E. O. Nanobacteria-propagating calcifying nanoparticles. Lett. Appl. Microbiol. 42, 549–552 (2006).
Miller, V. M. et al. Evidence of nanobacterial-like structures in calcified human arteries and cardiac valves. Am. J. Physiol. Heart C. 287, H1115–H1124 (2004).
Folk, R. L. Interaction between bacteria, nannobacteria, and mineral precipitation in hot-springs of central Italy. Geogr. Phys. Quatern. 48, 233–246 (1994).
Kaufman, D. P., Basit, H., Knohl, S. J. in StatPearls (StatPearls Publishing, 2019).
Castillo, C. G., Lo, W.-K., Kuck, J. F. R. & Yu, N.-T. Nature and localization of avian lens glycogen by electron microscopy and Raman spectroscopy. Biophys. J. 61, 839–844 (1992).
Sauer, F. in Handbook of Physiology, Renal Physiology (eds Orloff, J. & Berliner, R. W.) 399–414 (American Physiological Society, 1972).
Shin, J. C. et al. Mechanism underlying renal failure caused by pathogenic Candida albicans infection. Biomed. Rep. 3, 179–182 (2015).
Fouke, B. W. & Reeder, R. J. Surface structural controls on dolomite composition — evidence from sectoral zoning. Geochim. Cosmochim. Acta 56, 4015–4024 (1992). Cathodoluminescence microscopy and geochemical analyses of how sector zones are formed within dolomite crystals precipitated in ancient coral reef limestones.
Chien, Y. C. et al. Modulation of calcium oxalate dihydrate growth by selective crystal-face binding of phosphorylated osteopontin and polyaspartate peptide showing occlusion by sectoral (compositional) zoning. J. Biol. Chem. 284, 23491–23501 (2009). An experimental study of the precipitation of calcium oxalate dihydrate crystals and how inhibitors such as osteopontin impact sector zone development.
Evan, A. P., Lingeman, J. E., Coe, F. L. & Worcester, E. M. Role of interstitial apatite plaque in the pathogenesis of the common calcium oxalate stone. Semin. Nephrol. 28, 111–119 (2008).
Coe, F. L., Evan, A. P. & Worcester, E. M. Kidney stone disease. J. Clin. Invest. 115, 2598–2608 (2005).
Khan, S. R. Calcium phosphate/calcium oxalate crystal association in urinary stones: Implications for heterogeneous nucleation of calcium oxalate. J. Urol. 157, 376–383 (1997).
Sokol, E., Nigmatulina, E., Maksimova, N. & Chiglintsev, A. CaC2O4•H2O spherulites in human kidney stones: morphology, chemical composition, and growth regime. Eur. J. Mineral. 17, 285–295 (2005).
Oehler, J. H. Hydrothermal crystallization of Silica-gel. Geol. Soc. Am. Bull. 87, 1143–1152 (1976).
Frondel, C. Characters of quartz fibers. Am. Miner. 63, 17–27 (1978).
Kastner, M., Keene, J. B. & Gieskes, J. M. Diagenesis of siliceous oozes. 1. Chemical controls on rate of Opal-A to Opal-CT transformation — experimental-study. Geochim. Cosmochim. Acta 41, 1041–151 (1977). 1053-1059.
Ostwald, W. Studien über die Bildung und Umwandlung fester Körper. Zeitschr. Phys. Chem. 22, 280–330 (1897).
Threlfall, T. Structural and thermodynamic explanations of Ostwald’s rule. Org. Process. Res. Dev. 7, 1017–1027 (2003). A translation of Ostwald’s original work, explaining how spherules at different thermodynamic energy levels can either coalesce to form planar structures or increase in size without changing shape.
Zhang, C. et al. Pore-scale study of transverse mixing induced CaCO3 precipitation and permeability reduction in a model subsurface sedimentary system. Environ. Sci. Technol. 44, 7833–7838 (2010).
Saffo, M. B. & Lowenstam, H. A. Calcareous deposits in the renal sac of a molgulid tunicate. Science 200, 1166–1168 (1978).
Assereto, R. & Folk, R. L. Diagenetic fabrics of aragonite, calcite, and dolomite in an ancient peritidal-spelean environment — Triassic Calcare Rosso, Lombardia, Italy. J. Sediment. Res. 50, 371–394 (1980).
Knoll, A. H. Life on a Young Planet (Princeton University Press, 2015).
Hendry, J. P. & Marshall, J. D. Disequilibrium trace-element partitioning in Jurassic Sparry Calcite Cements — implications for crystal-growth mechanisms during diagenesis. J. Geol. Soc. Lond. 148, 835–848 (1991).
Basavaraj, D. R., Biyani, C. S., Browning, A. J. & Cartledge, J. J. The role of urinary kidney stone inhibitors and promoters in the pathogenesis of calcium containing renal stones. EAU-EBU Update Ser. 5, 126–136 (2007). A comprehensive review of the inhibitors and promoters of kidney stone mineralization and their relationship to the urine supersaturation state and crystal dissolution.
Kolossov, V. L. et al. Airyscan super-resolution microscopy of mitochondrial morphology and dynamics in living tumor cells. Microsc. Res. Tech. https://doi.org/10.1002/jemt.22968 (2017).
Sivaguru, M. Special issue: intact organs: super resolution multimodal optical 4D imaging preface. Microsc. Res. Tech. 81, 99–100 (2018).
Urban, M. A., Barclay, R. S., Sivaguru, M. & Punyasena, S. W. Cuticle and subsurface ornamentation of intact plant leaf epidermis under confocal and superresolution microscopy. Microsc. Res. Tech. 81, 129–140 (2018).
Miall, A. D. The Geology of Stratigraphic Sequences (Springer, 2010).
Craig, J. R., and Vaughan, D. J. Ore Microscopy and Ore Petrography 434 (John Wiley & Sons, 1994).
Fouke, B. W., Meyers, W. J., Hanson, G. N. & Beets, C. J. Chronostratigraphy and dolomitization of the Seroe Domi Formation, Curaçao, Netherlands Antilles. Facies 35, 293–320 (1996).
Claes, H. et al. Sedimentology, three-dimensional geobody reconstruction and carbon dioxide origin of Pleistocene travertine deposits in the Ballk area (south-west Turkey). Sedimentology 62, 1408–1445 (2015).
Laudan, R. From Mineralogy to Geology The Foundations of a Science, 1650−1830 (University of Chicago Press, Chicago, 1987).
Rosenberg, G. D. Nicholas Steno’s Chaos and the shaping of evolutionary thought in the scientific revolution. Geology 34, 793–796 (2006).
Boggs, J. S. Principles of Sedimentology and Stratigraphy 608 (Pearson, 2011).
Schlager, W. Carbonate sedimentology and sequence stratigraphy. Soc. Sediment. Geol. https://doi.org/10.2110/csp.05.08 (2005).
Catuneanu, O. et al. Towards the standardization of sequence stratigraphy. Earth Sci. Rev. 92, 1–33 (2009).
Dyer, R. & Nordin, B. E. Urinary crystals and their relation to stone formation. Nature 215, 751–752 (1967).
Cerini, C. et al. Nucleation of calcium oxalate crystals by albumin: involvement in the prevention of stone formation. Kidney Int. 55, 1776–1786 (1999).
Daudon, M., Jungers, P. & Bazin, D. Peculiar morphology of stones in primary hyperoxaluria. N. Engl. J. Med. 359, 100–102 (2008).
Lewis, R. D., Lowenstam, H. A. & Rossman, G. R. Oxalate nephrosis and crystalline myocarditis. Case report with postmortem and crystallographic studies. Arch. Pathol. 98, 149–155 (1974).
Chung, J. et al. Molecular modifiers reveal a mechanism of pathological crystal growth inhibition. Nature 536, 446–450 (2016).
De Coninck, V., Keller, E. X., Daudon, M. & Traxer, O. RE: Geobiology reveals how human kidney stones dissolve in vivo (by: Sivaguru et al. 2018). World J. Urol. 37, 2543–2543 (2019).
Grases, F., Rodriguez, A. & Costa-Bauza, A. Efficacy of mixtures of magnesium, citrate and phytate as calcium oxalate crystallization inhibitors in urine. J. Urol. 194, 812–819 (2015).
Grases, F., Söhnel, O., Costa-Bauza, A., Servera, A. & Benejam, J. A case of Randall’s plugs associated to calcium oxalate dihydrate calculi. Urol. Case Rep. 7, 37–38 (2016).
Grases, F., Sohnel, O., Garcia-Ferragut, L. & Costa-Bauza, A. A study on calcium oxalate monohydrate renal uroliths. II. Fine inner structure. Scand. J. Urol. Nephrol. 29, 421–428 (1995).
Fouke, K. W. Crystalline Architecture and Stratigraphy of Coral Skeletal Density Banding: A Geobiological Record of Changing Coral Reef Ecology. Thesis, Bucknell University (2020).
Sivaguru, M. et al. Corals regulate the distribution and abundance of Symbiodiniaceae and biomolecules in response to changing water depth and sea surface temperature. Sci. Rep. 11, 2230 (2021).
Cohen, A. L. & McConnaughey, T. A. Geochemical perspectives on coral mineralization. Rev. Miner. Geochem. 54, 151–187 (2003).
Madin, E. M. P. et al. Multi-trophic species interactions shape seascape-scale coral reef vegetation patterns. Front. Ecol. Evol. https://doi.org/10.3389/fevo.2019.00102 (2019).
Jones, D. K., Motta, D., Garcia, M. H. & Fouke, B. W. Travertine-based estimates of the amount of water supplied by ancient Rome’s Anio Novus aqueduct. J. Archaeol. Sci. Rep. 3, 1–10 (2015).
Deng, J. et al. Adaptive evolution of Escherichia coli to ciprofloxacin in controlled stress environments: contrasting patterns of resistance in spatially varying versus uniformly mixed concentration conditions. Env. Sci. Technol. 53, 7996–8005 (2019).
Zhou, J. Z. et al. Geochemistry of speleothem records from southern Illinois: development of (U-234)/(U-238) as a proxy for paleoprecipitation. Chem. Geol. 221, 1–20 (2005).
Dhami, N. K., Mukherjee, A. & Watkin, E. L. J. Microbial diversity and mineralogical-mechanical properties of calcitic cave speleothems in natural and in vitro biomineralization conditions. Front. Microbiol. 9, 40 (2018).
He, H. H., Veneklaas, E. J., Kuo, J. & Lambers, H. Physiological and ecological significance of biomineralization in plants. Trends Plant. Sci. 19, 166–174 (2014).
Taylor, M. G., Simkiss, K., Greaves, G. N., Okazaki, M. & Mann, S. An X-ray-absorption spectroscopy study of the structure and transformation of amorphous calcium-carbonate from plant cystoliths. Proc. Roy. Soc. B Biol. Sci. 252, 75–80 (1993).
Pierantoni, M. et al. Mineral deposits in ficus leaves: morphologies and locations in relation to function. Plant Physiol. 176, 1751–1763 (2018).
Hartl, W. P. et al. Diversity of calcium oxalate crystals in Cactaceae. Can. J. Bot. 85, 501–517 (2007).
Ensikat, H. J., Geisler, T. & Weigend, M. A first report of hydroxylated apatite as structural biomineral in Loasaceae — plants’ teeth against herbivores. Sci. Rep. 6, 26073 (2016).
Ryall, R. L. Urinary inhibitors of calcium oxalate crystallization and their potential role in stone formation. World J. Urol. 15, 155–164 (1997).
Tooulakou, G. et al. Alarm photosynthesis: calcium oxalate crystals as an internal CO2 source in plants. Plant Physiol. 171, 2577–2585 (2016).
Bo, M. et al. Isolation and identification of chitin in the black coral Parantipathes larix (Anthozoa: Cnidaria). Int. J. Biol. Macromol. 51, 129–137 (2012).
Chen, J. K., Shen, C. R. & Liu, C. L. N-Acetylglucosamine: production and applications. Mar. Drugs 8, 2493–2516 (2010).
Tang, W. J., Fernandez, J. G., Sohn, J. J. & Amemiya, C. T. Chitin is endogenously produced in vertebrates. Curr. Biol. 25, 897–900 (2015).
Konopka, J. B. N-acetylglucosamine (GlcNAc) functions in cell signaling. Scientifica 2012, 489208 (2012).
Nesse, A., Garbossa, G., Romero, M. C., Bogado, C. E. & Zanchetta, J. R. Glycosaminoglycans in Urolithiasis. Nephron 62, 36–39 (1992).
Baggio, B., Gambaro, G., Oliva, O., Favaro, S. & Borsatti, A. Calcium-oxalate nephrolithiasis — an easy way to detect an imbalance between promoting and inhibiting factors. Clin. Chim. Acta 124, 149–155 (1982).
Kim, D., Rimer, J. D. & Asplin, J. R. Hydroxycitrate: a potential new therapy for calcium urolithiasis. Urolithiasis 47, 311–320 (2019).
Nirumand, M. C. et al. Dietary plants for the prevention and management of kidney stones: preclinical and clinical evidence and molecular mechanisms. Int. J. Mol. Sci. 19, 765 (2018). This review provides mechanistic insights into plant-based therapies for kidney stone pathogenesis, including the impact of diet on stone recurrence.
Micali, S. et al. Can phyllanthus niruri affect the efficacy of extracorporeal shock wave lithotripsy for renal stones? A randomized, prospective, long-term study. J. Urol. 176, 1020–1022 (2006).
Franceschi, V. R. Calcium-oxalate formation is a rapid and reversible process in Lemna-Minor-L. Protoplasma 148, 130–137 (1989).
Franceschi, V. R. & Horner, H. T. Calcium-oxalate crystals in plants. Bot. Rev. 46, 361–427 (1980).
Curry, J. N. et al. Claudin-2 deficiency associates with hypercalciuria in mice and human kidney stone disease. J. Clin. Invest. 130, 1948–1960 (2020).
Fenner, A. Claudin 2 and hypercalciuria — of mice and men. Nat. Rev. Urol. 17, 255 (2020).
Kandianis, M. T., Fouke, B. W., Johnson, R. W. II, J., V. & Inskeep, W. P. Microbial biomass: a catalyst for CaCO3 precipitation in advection-dominated transport regimes. GSA Bull. 120, 442–450 (2007).
Dong, Y. et al. Physiology, metabolism, and fossilization of hot-spring filamentous microbial mats. Astrobiology 19, 1442–1458 (2019).
Group, N. H. W. et al. The NIH human microbiome project. Genome Res. 19, 2317–2323 (2009).
Schwaderer, A. L. & Wolfe, A. J. The association between bacteria and urinary stones. Ann. Transl Med. 5, 32 (2017).
Amimanan, P. et al. Elongation factor Tu on Escherichia coli isolated from urine of kidney stone patients promotes calcium oxalate crystal growth and aggregation. Sci. Rep. 7, 2953 (2017).
Chutipongtanate, S., Sutthimethakorn, S., Chiangjong, W. & Thongboonkerd, V. Bacteria Fcan promote calcium oxalate crystal growth and aggregation. J. Biol. Inorg. Chem. 18, 299–308 (2013).
Flannigan, R., Choi, W. H., Chew, B. & Lange, D. Renal struvite stones — pathogenesis, microbiology, and management strategies. Nat. Rev. Urol. 11, 333–341 (2014).
salvadori, O. & Municchia, A. C. The role of fungi and lichens in the biodeterioration of stone monuments. Open Conf. Proc. J. 7, 39–54 (2016).
de Cógáin, M. R., Lieske, J. C., Vrtiska, T. J., Tosh, P. K. & Krambeck, A. E. Secondarily infected nonstruvite urolithiasis: a prospective evaluation. Urology 84, 1295–1300 (2014).
Cockerill, P. A., Rivera, M. E. & Krambeck, A. E. Analysis of the utility of stone gram stain in urolithiasis treated with percutaneous nephrolithotomy. Urology 83, 1254–1257 (2014).
Hilt, E. E. et al. Urine is not sterile: use of enhanced urine culture techniques to detect resident bacterial flora in the adult female bladder. J. Clin. Microbiol. 52, 871–876 (2014).
Ackerman, A. L. & Underhill, D. M. The mycobiome of the human urinary track: potential roles for fungi in urology. Ann. Transl Med. 5, 31 (2017).
Miller, A. W., Choy, D., Penniston, K. L. & Lange, D. Inhibition of urinary stone disease by a multi-species bacterial network ensures healthy oxalate homeostasis. Kidney Int. 96, 180–188 (2019).
Shore, M. & Fowler, A. D. Oscillatory zoning in minerals: a common phenomenon. Can. Miner. 34, 1111–1126 (1996).
Kuliasha, C. A., Rodriguez, D., Lovett, A. & Gower, L. B. In situ flow cell platform for examining calcium oxalate and calcium phosphate crystallization on films of basement membrane extract in the presence of urinary ‘inhibitors’. Crystengcomm 22, 1448–1458 (2020). A microfluidic in vitro study of calcium phosphate and calcium oxalate crystal formation on the basement membrane and the impact of crystallization inhibitors.
Gorski, J. P. Biomineralization of bone: a fresh view of the roles of non-collagenous proteins. Front. Biosci-Landmrk 16, 2598–2621 (2011).
Singh, R. et al. Real rock-microfluidic flow cell: a test bed for real-time in situ analysis of flow, transport, and reaction in a subsurface reactive transport environment. J. Contam. Hydrol. 204, 28–39 (2017).
Balandrin, M. F., Klocke, J. A., Wurtele, E. S. & Bollinger, W. H. Natural plant-chemicals – sources of industrial and medicinal materials. Science 228, 1154–1160 (1985).
Balandrin, M. F. Commercial utilization of plant-derived saponins: an overview of medicinal, pharmaceutical, and industrial applications. Adv. Exp. Med. Biol. 404, 1–14 (1996).
Borghi, L. et al. Urinary volume, water and recurrences in idiopathic calcium nephrolithiasis: a 5-year randomized prospective study. J. Urol. 155, 839–843 (1996).
Muldowney, F. P., Freaney, R. & Moloney, M. F. Importance of dietary-sodium in the hypercalciuria syndrome. Kidney Int. 22, 292–296 (1982).
Johansson, G. et al. Effects of magnesium-hydroxide in renal stone disease. J. Am. Coll. Nutr. 1, 179–185 (1982).
Curhan, G. C. & Taylor, E. N. 24-h uric acid excretion and the risk of kidney stones. Kidney Int. 73, 489–496 (2007).
Sorensen, M. D. et al. Dietary intake of fiber, fruit and vegetables decreases the risk of incident kidney stones in women: a women’s health initiative report. J. Urol. 192, 1694–1699 (2014).
Sakhaee, K., Maalouf, N. M. & Sinnott, B. Kidney stones 2012: pathogenesis, diagnosis, and management. J. Clin. Endocr. Metab. 97, 1847–1860 (2012).
Fink, H. A. et al. Medical management to prevent recurrent nephrolithiasis in adults: a systematic review for an American College of Physicians Clinical Guideline (vol 158, pg 535, 2013). Ann. Intern. Med. 159, 230–230 (2013).
El-Salam, M. A. et al. The syntheized plant metabolite 3,4,5-Tri-O-Galloylquinic acid methyl ester inhibits calcium oxalate crystal growth in a Drosophila model, downregulates renal cell surface annexin A1 expression, and decreases crystal adhesion to cells. J. Med. Chem. 61, 1609–1621 (2018).
Erickson, S. B., Vrtiska, T. J., Canzanello, V. J. & Lieske, J. C. Cystone® for 1 year did not change urine chemistry or decrease stone burden in cystine stone formers. Urol. Res. 39, 197–203 (2011).
Alcalde, R. E. et al. Motility of Shewanella oneidensis MR-1 allows for nitrate reduction in the toxic region of a ciprofloxacin concentration gradient in a microfluidic reactor. Environ. Sci. Technol. 53, 2778–2787 (2019).
Lin, N. Y. C. et al. Renal reabsorption in 3D vascularized proximal tubule models. Proc. Natl Acad. Sci. USA 116, 5399–5404 (2019).
Lieske, J. C., Leonard, R. & Toback, F. G. Adhesion of calcium oxalate monohydrate crystals to renal epithelial cells is inhibited by specific anions. Am. J. Physiol. Renal Fluid Electrolyte Physiol. 268, F604–F612 (1995).
Cvetkovic, C., Rich, M. H., Raman, R., Kong, H. & Bashir, R. A 3D-printed platform for modular neuromuscular motor units. Microsyst. Nanoeng. 3, 17015 (2017).
Lonsdale, K. Epitaxy as a growth factor in urinary calculi and gallstones. Nature 217, 56–58 (1968).
Daudon, M. & Bazin, D. When the Synchrotron radiations highlight the Randall’s plaques and kidney concretions. J. Phys. Conf. Ser. 425, 022006 (2013).
Sethmann, I., Grohe, B. & Kleebe, H.-J. Replacement of hydroxylapatite by whewellite: implications for kidney-stone formation. Mineral. Mag. 78, 91–100 (2014).
Takacs-Novak, K. et al. Equilibrium solubility measurement of compounds with low dissolution rate by Higuchi’s facilitated dissolution method. A validation study. Eur. J. Pharm. Sci. 106, 133–141 (2017).
Moreno, A. Advanced Methods of Protein Crystallization 51–76 (Springer Science+Business Media, 2017).
Lowenstam, H. A. Opal precipitation by marine gastropods (Mollusca). Science 171, 487–490 (1971).
Call, H. Chalcedone pseudomorph after coral or agatized coral. Rocks Miner. 49, 561–562 (1974).
Fouke, B. W. & Sivaguru, M. Calcium kidney stones naturally undergo 50% by volume in vivo dissolution and recrystallization via universal biomineralization. AUA News 26, 16–18 (2021).
Acknowledgements
This research was supported by the Mayo Clinic and University of Illinois Strategic Alliance for Technology-Based Healthcare, the Mayo Clinic Center for Individualized Medicine, the Mayo Clinic O’Brien Urology Research Center (no. DK100227), the Mayo Nephrology/Urology Summer Undergraduate Research Fellowship (nuSURF; no. DK101405), and the National Aeronautics and Space Administration (NASA) Astrobiology Institute (Cooperative Agreement no. NNA13AA91A) issued through the Science Mission Directorate. The authors thank Charles J. Werth for ongoing invaluable scientific discussions and detailed editing, as well as Tom Shearer for providing high-resolution imaging and sample collection information on agate geodes.
Author information
Authors and Affiliations
Contributions
M.S., J.J.S., E.M.W., J.C.L., A.E.K., J.C.W., M.F.R., K.W.F., M.W.C., J.L.K.-S. and B.W.F. researched data for the article. M.S. and B.W.F. made substantial contributions to discussions of content and wrote the manuscript. All authors reviewed and edited the manuscript before submission.
Corresponding authors
Ethics declarations
Competing interests
The Microscopy Facility at the Carl R. Woese Institute for Genomic Biology, University of Urbana-Champaign, is a Carl Zeiss Labs @ Location Partner and has a priority access “before market” agreement for Carl Zeiss Microscope systems for testing, evaluation and reporting. The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Urology thanks L. Gower and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
High resolution main figures and supplementary figures link: https://figshare.com/s/9277572cd07d0be5bc9d
Supplementary information
Glossary
- Biomineralization
-
The processes and products of mineral precipitation that are influenced by the ThreeDomains of Life.
- Diagenetic phase transitions
-
Sequential direct and step-wise events of crystal precipitation, dissolution and reprecipitation.
- GeoBioMed
-
A new transdisciplinary convergence approach that integrates cutting-edge technologies and fundamental governing principles in geology and biology (geobiology) with those in medicine.
- Fourier transform infrared spectroscopy
-
Used to determine the mineralogical composition of a crystal or stone.
- Super-resolution autofluorescence microscopy
-
(SRAF). Blue (405 nm), green (488 nm) and red (561 nm) laser excitation wavelengths, which generate blue (410–480 nm), green (500–550 nm) and red (575–615 nm) light emission wavelengths that can be detected at an average spatial resolution of 140 nm.
- Powers of 10
-
A contextualization system with which to make comparisons over small to large spatial and temporal scales, with relative dimensions described using exponential notations of the number 10.
- Gibbs free energy
-
Originally described as “available energy” by Josiah Willard Gibbs in 1873, this parameter characterizes the thermodynamic nature of any given system. It is the total energy available to execute a given reaction under constant temperature and pressure.
- Solubility product
-
(Ksp). A quantitative thermodynamic evaluation of whether minerals will precipitate or dissolve in solution based primarily on the relative concentration of dissolved ions and temperature.
- Bipyramid
-
A structural category of crystalline solids that is composed of two pyramid-shaped structures that are flipped mirror images of each other joined at the base (for example, calcium oxalate dihydrate).
- Monoclinic
-
A structural category of crystalline solids that has three axes of unequal lengths, one of which is perpendicular to the other two (for example, calcium oxalate dihydrate).
- Anhedral crystals
-
Crystals that have grown to form irregular-shaped faces.
- Euhedral crystals
-
Crystals that have grown to form perfect geometrically shaped faces.
- Sector zones
-
Individual age-equivalent crystal faces exhibit differences in the rate of incorporation of ions and biomolecules, including organic matter, independent of the bulk urine chemistry at the time of crystallization.
- Ostwald’s rule
-
Crystals evolve through a series of phase transitions that progress towards the smallest loss of free energy, transitioning from less thermodynamically stable to more stable polymorphs. The first type is Ostwald ripening, where spherules merge, coalesce and increase in size but maintain their original shape. The second type is Ostwald coalescence, where spherules of similar sizes laterally aggregate and eventually form a planar surface.
- Paragenetic sequence
-
The entire history of diagenetic phase transitions that have formed crystalline stone deposits. These result from the physical, chemical and biological processes active within the environment of deposition.
- Mold
-
An original crystal that was entombed by another crystal or crystal aggregate, which was then fully dissolved, leaving a void space in the shape of the original crystal (also called moldic porosity).
- Calvin cycle
-
A process that plants use to transform carbon dioxide into sugar.
- GeoBioCell
-
A custom designed and constructed microfluidic bioreactor in which the interaction between urine at variable states of saturation, renal microorganisms, stone fragments and many other relevant kidney parameters can be tracked under highly controlled environmental conditions.
Rights and permissions
About this article
Cite this article
Sivaguru, M., Saw, J.J., Wilson, E.M. et al. Human kidney stones: a natural record of universal biomineralization. Nat Rev Urol 18, 404–432 (2021). https://doi.org/10.1038/s41585-021-00469-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41585-021-00469-x
This article is cited by
-
The impact of crystal phase transition on the hardness and structure of kidney stones
Urolithiasis (2024)
-
Strontium isotope ratios in kidney stones reveal the environmental implications for humans in Beijing, China
Environmental Geochemistry and Health (2023)
-
Performance of brushite plaster as kidney stone phantoms for laser lithotripsy
Urolithiasis (2023)
-
Microbiota manipulation to prevent oxalate kidney stone formation
Nature Reviews Urology (2022)
-
An apatite for kidney stones
Nature Geoscience (2022)