Abstract
In the past 10–15 years, paediatric transgender care has emerged at the forefront of several general practice and subspecialty guidelines and is the topic of continuing medical education for various medical disciplines. Providers in specialties ranging from family medicine, paediatrics and adolescent medicine to endocrinology, gynaecology and urology are caring for transgender patients in increasing numbers. Current and evolving national and international best practice guidelines recommend offering a halt of endogenous puberty for patients with early gender dysphoria, in whom impending puberty is unacceptable for their psychosocial health and wellness. Pubertal blockade has implications for fertility preservation, transgender surgical care and psychosocial health, all of which must be considered and discussed with the patient and their family and/or legal guardian before initiation.
Key points
-
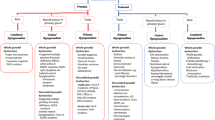
Puberty blockade is the earliest point of medical intervention indicated in paediatric patients expressing gender dysphoria and should be initiated as soon as signs of puberty are observed on physical examination.
-
Puberty blockade has been shown to positively affect mental health outcomes in adolescent patients with gender incongruence.
-
Blockade of puberty does affect future fertility options for transgender adults treated early in adolescence and patients should be counselled regarding the options available to them for future fertility.
-
Blockade of puberty prevents the development of secondary sexual characteristics and, therefore, reduces the number of gender-affirming therapies required to transition and also reduces the cost of surgical care.
-
A number of questions remain surrounding early intervention and pubertal blockade, including the future effects on surgical outcomes, cancer risk and economic outcomes.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Bonifacio, H. J. & Rosenthal, S. M. Gender variance and dysphoria in children and adolescents. Pediatr. Clin. North. Am. 62, 1001–1016 (2015).
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. (American Psychiatric Association, 2013).
Lindmeier, C. WHO releases new International Classification of Diseases (ICD 11) (WHO, 2018).
Olson, K. R., Durwood, L., DeMeules, M. & McLaughlin, K. A. Mental health of transgender children who are supported in their identities. Pediatrics 137, e20153223 (2016).
Rafferty, J. & Committee on psychosocial aspects of child and family health, Committee on adolescence & section on lesbian, gay, bisexual, and transgender health and wellness. Ensuring comprehensive care and support for transgender and gender-diverse children and adolescents. Pediatrics 142, e20182162 (2018).
Malpas, J. Between pink and blue: a multi-dimensional family approach to gender nonconforming children and their families. Fam. Process 50, 453–470 (2011).
Yeung, H., Luk, K. M., Chen, S. C., Ginsberg, B. A. & Katz, K. A. Dermatologic care for lesbian, gay, bisexual, and transgender persons: terminology, demographics, health disparities, and approaches to care. J. Am. Acad. Dermatol. 80, 581–589 (2019).
Meerwijk, E. L. & Sevelius, J. M. Transgender population size in the United States: a meta-regression of population-based probability samples. Am. J. Public. Health 107, e1–e8 (2017).
Herman, J. L., Flores, A. R., Brown, T. N. T., Wilson, B. D. M. & Conron, K. J. Age of Individuals Who Identify as Transgender in the United States (The Williams Institute, UCLA School of Law, 2017)
Johns, M. M. et al. Transgender identity and experiences of violence victimization, substance use, suicide risk, and sexual risk behaviors among high school students — 19 states and large urban school districts, 2017. MMWR Morb. Mortal. Wkly Rep. 68, 67–71 (2019).
Spack, N. P. et al. Children and adolescents with gender identity disorder referred to a pediatric medical center. Pediatrics 129, 418–425 (2012).
Marshall, W. A. & Tanner, J. M. Variations in pattern of pubertal changes in girls. Arch. Dis. Child. 44, 291–303 (1969).
Marshall, W. A. & Tanner, J. M. Variations in the pattern of pubertal changes in boys. Arch. Dis. Child. 45, 13–23 (1970).
Ellis, B. J., Shirtcliff, E. A., Boyce, W. T., Deardorff, J. & Essex, M. J. Quality of early family relationships and the timing and tempo of puberty: effects depend on biological sensitivity to context. Dev. Psychopathol. 23, 85–99 (2011).
Özen, S. & Darcan, Ş. Effects of environmental endocrine disruptors on pubertal development. J. Clin. Res. Pediatr. Endocrinol. 3, 1–6 (2011).
Howard, S. R. Genes underlying delayed puberty. Mol. Cell. Endocrinol. 476, 119–128 (2018).
Panagiotakopoulos, L. Transgender medicine — puberty suppression. Rev. Endocr. Metab. Disord. 19, 221–225 (2018).
Roger, M. et al. Treatment of precocious puberty with LH-RH agonists. Multicenter study using D-Trp-6-LH-RH in a programmed-release form [French]. Rev. Fr. Gynecol. Obstet. 81, 297–305 (1986).
Kreukels, B. P. C. & Cohen-Kettenis, P. T. Puberty suppression in gender identity disorder: the Amsterdam experience. Nat. Rev. Endocrinol. 7, 466–472 (2011).
de Vries, A. L. C. et al. Young adult psychological outcome after puberty suppression and gender reassignment. Pediatrics 134, 696–704 (2014).
Turban, J. L., King, D., Carswell, J. M. & Keuroghlian, A. S. Pubertal suppression for transgender youth and risk of suicidal ideation. Pediatrics 145, e20191725 (2020).
Edwards-Leeper, L. & Spack, N. P. Psychological evaluation and medical treatment of transgender youth in an interdisciplinary ‘Gender Management Service’ (GeMS) in a major pediatric center. J. Homosex. 59, 321–336 (2012).
Martinerie, L., Le Heuzey, M.-F., Delorme, R., Carel, J.-C. & Bargiacchi, A. Assessment and management of gender dysphoria in children and adolescents [French]. Arch. Pediatr. 23, 668–673 (2016).
Ladjouze, A. & Donaldson, M. Primary gonadal failure. Best. Pract. Res. Clin. Endocrinol. Metab. 33, 101295 (2019).
Chen, D., Simons, L., Johnson, E. K., Lockart, B. A. & Finlayson, C. Fertility preservation for transgender adolescents. J. Adolesc. Health 61, 120–123 (2017).
Nahata, L., Tishelman, A. C., Caltabellotta, N. M. & Quinn, G. P. Low fertility preservation utilization among transgender youth. J. Adolesc. Health 61, 40–44 (2017).
Chiniara, L. N., Viner, C., Palmert, M. & Bonifacio, H. Perspectives on fertility preservation and parenthood among transgender youth and their parents. Arch. Dis. Child. 104, 739–744 (2019).
Joshi, S. et al. Clinical guide to fertility preservation in hematopoietic cell transplant recipients. Bone Marrow Transpl. 49, 477–484 (2014).
Sonnenburg, D. W., Brames, M. J., Case-Eads, S. & Einhorn, L. H. Utilization of sperm banking and barriers to its use in testicular cancer patients. Support. Care Cancer 23, 2763–2768 (2015).
Baram, S., Myers, S. A., Yee, S. & Librach, C. L. Fertility preservation for transgender adolescents and young adults: a systematic review. Hum. Reprod. Update 25, 694–716 (2019).
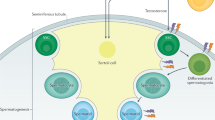
Stukenborg, J.-B. et al. Spermatogonial quantity in human prepubertal testicular tissue collected for fertility preservation prior to potentially sterilizing therapy. Hum. Reprod. 33, 1677–1683 (2018).
Onofre, J., Baert, Y., Faes, K. & Goossens, E. Cryopreservation of testicular tissue or testicular cell suspensions: a pivotal step in fertility preservation. Hum. Reprod. Update 22, 744–761 (2016).
Fayomi, A. P. et al. Autologous grafting of cryopreserved prepubertal rhesus testis produces sperm and offspring. Science 363, 1314–1319 (2019).
Schaefer, F., Marr, J., Seidel, C., Tilgen, W. & Schärer, K. Assessment of gonadal maturation by evaluation of spermaturia. Arch. Dis. Child. 65, 1205–1207 (1990).
Cheng, P. J., Pastuszak, A. W., Myers, J. B., Goodwin, I. A. & Hotaling, J. M. Fertility concerns of the transgender patient. Transl. Androl. Urol. 8, 209–218 (2019).
Martinez, F. & International Society for Fertility Preservation–ESHRE–ASRM Expert Working Group. Update on fertility preservation from the Barcelona International Society for Fertility Preservation-ESHRE-ASRM 2015 expert meeting: indications, results and future perspectives. Fertil. Steril. 108, 407–415.e11 (2017).
Dinikina, Y. et al. Ovarian tissue cryopreservation in prepubertal patients with oncological diseases: multidisciplinary approach and outcomes. J. Matern. Fetal Neonatal Med. https://doi.org/10.1080/14767058.2019.1666364 (2019).
Algarroba, G. N., Sanfilippo, J. S. & Valli-Pulaski, H. Female fertility preservation in the pediatric and adolescent cancer patient population. Best. Pract. Res. Clin. Obstet. Gynaecol. 48, 147–157 (2018).
Light, A. D., Obedin-Maliver, J., Sevelius, J. M. & Kerns, J. L. Transgender men who experienced pregnancy after female-to-male gender transitioning. Obstet. Gynecol. 124, 1120–1127 (2014).
Lee, P. A. et al. Efficacy and safety of leuprolide acetate 3-month depot 11.25 milligrams or 30 milligrams for the treatment of central precocious puberty. J. Clin. Endocrinol. Metab. 97, 1572–1580 (2012).
Fuld, K., Chi, C. & Neely, E. K. A randomized trial of 1- and 3-month depot leuprolide doses in the treatment of central precocious puberty. J. Pediatr. 159, 982–987.e1 (2011).
Carel, J.-C. et al. Three-month sustained-release triptorelin (11.25 mg) in the treatment of central precocious puberty. Eur. J. Endocrinol. 154, 119–124 (2006).
Hewitt, J. K. et al. Hormone treatment of gender identity disorder in a cohort of children and adolescents. Med. J. Aust. 196, 578–581 (2012).
Evans, M. B. et al. Evaluation of the cost-effectiveness of ovulation suppression with progestins compared with GnRH analogs in assisted reproduction cycles. Reprod. Biomed. Online 38, 691–698 (2019).
Grimstad, F. W. et al. Uterine pathology in transmasculine persons on testosterone: a retrospective multicenter case series. Am. J. Obstet. Gynecol. 220, 257.e1–257.e7 (2019).
Carswell, J. M. & Roberts, S. A. Induction and maintenance of amenorrhea in transmasculine and nonbinary adolescents. Transgend. Health 2, 195–201 (2017).
Spinder, T. et al. The effects of long term testosterone administration on pulsatile luteinizing hormone secretion and on ovarian histology in eugonadal female to male transsexual subjects. J. Clin. Endocrinol. Metab. 69, 151–157 (1989).
Nakamura, A. et al. Dose-response analysis of testosterone replacement therapy in patients with female to male gender identity disorder. Endocr. J. 60, 275–281 (2013).
Pelusi, C. et al. Effects of three different testosterone formulations in female-to-male transsexual persons. J. Sex. Med. 11, 3002–3011 (2014).
Taub, R. L. et al. The effect of testosterone on ovulatory function in transmasculine individuals. Am. J. Obstet. Gynecol. 223, 229.e1–229.e8 (2020).
Hakim, C., Padmanabhan, V. & Vyas, A. K. Gestational hyperandrogenism in developmental programming. Endocrinology 158, 199–212 (2017).
Kim, H. J., Lee, D.-Y., Yoon, B.-K. & Choi, D. Uterine development after estrogen replacement therapy in women with different etiologies of primary hypogonadism. J. Pediatr. Adolesc. Gynecol. 29, 344–347 (2016).
Gomez, A., Walters, P. C. & Dao, L. T. “Testosterone in a way is birth control”: contraceptive attitudes and experiences among transmasculine and genderqueer young adults. Contraception 94, 422–423 (2016).
Committee on Adolescent Health Care. Committee Opinion No. 685: care for transgender adolescents. Obstet. Gynecol. 129, e11–e16 (2017).
Uysal, G. et al. The efficacy of dienogest in the treatment of simple endometrial hyperplasia without atypia. Gynecol. Obstet. Invest. 83, 151–155 (2018).
Trussell, J. Contraceptive failure in the United States. Contraception 83, 397–404 (2011).
Sundaram, A. et al. Contraceptive failure in the United States: estimates from the 2006–2010 National Survey of Family Growth. Perspect. Sex. Reprod. Health 49, 7–16 (2017).
Cohen-Kettenis, P. T. & Klink, D. Adolescents with gender dysphoria. Best Pract. Res. Clin. Endocrinol. Metab. 29, 485–495 (2015).
Deshmukh, P., Antell, K. & Brown, E. J. Contraception update: progestin-only implants and injections. FP Essent. 462, 25–29 (2017).
Belsey, E. M. Vaginal bleeding patterns among women using one natural and eight hormonal methods of contraception. Contraception 38, 181–206 (1988).
Berenson, A. B., Odom, S. D., Breitkopf, C. R. & Rahman, M. Physiologic and psychologic symptoms associated with use of injectable contraception and 20 microg oral contraceptive pills. Am. J. Obstet. Gynecol. 199, 351.e1–12 (2008).
Zheng, S. R., Zheng, H. M., Qian, S. Z., Sang, G. W. & Kaper, R. F. A randomized multicenter study comparing the efficacy and bleeding pattern of a single-rod (Implanon) and a six-capsule (Norplant) hormonal contraceptive implant. Contraception 60, 1–8 (1999).
Mansour, D., Korver, T., Marintcheva-Petrova, M. & Fraser, I. S. The effects of Implanon on menstrual bleeding patterns. Eur. J. Contracept. Reprod. Health Care 13, 13–28 (2008).
Darney, P., Patel, A., Rosen, K., Shapiro, L. S. & Kaunitz, A. M. Safety and efficacy of a single-rod etonogestrel implant (Implanon): results from 11 international clinical trials. Fertil. Steril. 91, 1646–1653 (2009).
Freeman, S. & Shulman, L. P. Considerations for the use of progestin-only contraceptives. J. Am. Acad. Nurse Pract. 22, 81–91 (2010).
Schwartz, A. R., Russell, K. & Gray, B. A. Approaches to vaginal bleeding and contraceptive counseling in transgender and gender nonbinary patients. Obstet. Gynecol. 134, 81–90 (2019).
Zigler, R. E. & McNicholas, C. Unscheduled vaginal bleeding with progestin-only contraceptive use. Am. J. Obstet. Gynecol. 216, 443–450 (2017).
Guss, C. E. Intrauterine devices in gender minority youth: an option to decrease dysphoria and unintended pregnancies. J. Adolesc. Health 65, 3–4 (2019).
Nolan, I. T., Dy, G. W. & Levitt, N. Considerations in gender-affirming surgery: demographic trends. Urol. Clin. North. Am. 46, 459–465 (2019).
James, S. E. et al. The report of the 2015 U.S. Transgender Survey. National Center for Transgender Equality https://www.transequality.org/sites/default/files/docs/USTS-Full-Report-FINAL.PDF (2016).
Zurada, A. et al. The evolution of transgender surgery. Clin. Anat. 31, 878–886 (2018).
Morrison, S. D. et al. Facial feminization: systematic review of the literature. Plast. Reconstr. Surg. 137, 1759–1770 (2016).
Horbach, S. E. R. et al. Outcome of vaginoplasty in male-to-female transgenders: a systematic review of surgical techniques. J. Sex. Med. 12, 1499–1512 (2015).
Safa, B., Lin, W. C., Salim, A. M., Deschamps-Braly, J. C. & Poh, M. M. Current concepts in feminizing gender surgery. Plast. Reconstr. Surg. 143, 1081e–1091e (2019).
Berli, J. U. et al. What surgeons need to know about gender confirmation surgery when providing care for transgender individuals: a review. JAMA Surg. 152, 394–400 (2017).
van der Sluis, W. B., Tuynman, J. B., Meijerink, W. J. H. J. & Bouman, M.-B. Laparoscopic intestinal vaginoplasty in transgender women: an update on surgical indications, operative technique, perioperative care, and short- and long-term postoperative issues. Urol. Clin. North. Am. 46, 527–539 (2019).
Cristofari, S. et al. Postoperative complications of male to female sex reassignment surgery: a 10-year French retrospective study. Ann. Chir. Plast. Esthet. 64, 24–32 (2019).
van der Sluis, W. B., Bouman, M., Meijerink, W., Neefjes-Borst, E. A. & van Bodegraven, A. A. Refractory diversion neovaginitis in a sigmoid-colon-derived neovagina: clinical and histopathological considerations. Frontline Gastroenterol. 7, 227–230 (2016).
van der Sluis, W. B. et al. Diversion neovaginitis after sigmoid vaginoplasty: endoscopic and clinical characteristics. Fertil. Steril. 105, 834–839.e1 (2016).
Bouman, M.-B. et al. Patient-reported esthetic and functional outcomes of primary total laparoscopic intestinal vaginoplasty in transgender women with penoscrotal hypoplasia. J. Sex. Med. 13, 1438–1444 (2016).
Manrique, O. J. et al. Complications and patient-reported outcomes in male-to-female vaginoplasty-where we are today: a systematic review and meta-analysis. Ann. Plast. Surg. 80, 684–691 (2018).
Garcia, M. M. Sexual function after shallow and full-depth vaginoplasty: challenges, clinical findings, and treatment strategies — urologic perspectives. Clin. Plast. Surg. 45, 437–446 (2018).
Hadj-Moussa, M., Ohl, D. A. & Kuzon, W. M. Feminizing genital gender-confirmation surgery. Sex. Med. Rev. 6, 457–468.e2 (2018).
LeBreton, M. et al. Genital sensory detection thresholds and patient satisfaction with vaginoplasty in male-to-female transgender women. J. Sex. Med. 14, 274–281 (2017).
Buvat, J. & Bou Jaoudé, G. Significance of hypogonadism in erectile dysfunction. World J. Urol. 24, 657–667 (2006).
Cornelisse, V. J., Jones, R. A., Fairley, C. K. & Grover, S. R. The medical care of the neovagina of transgender women: a review. Sex. Health 14, 442–450 (2017).
Oelschlager, A.-M. A., Debiec, K. & Appelbaum, H. Primary vaginal dilation for vaginal agenesis: strategies to anticipate challenges and optimize outcomes. Curr. Opin. Obstet. Gynecol. 28, 345–349 (2016).
Coleman, E. et al. Standards of care for the health of transsexual, transgender, and gender-nonconforming people, version 7. Int. J. Transgend. 13, 165–232 (2012).
Massie, J. P., Morrison, S. D., Van Maasdam, J. & Satterwhite, T. Predictors of patient satisfaction and postoperative complications in penile inversion vaginoplasty. Plast. Reconstr. Surg. 141, 911e–921e (2018).
Wierckx, K. et al. Sexual desire in trans persons: associations with sex reassignment treatment. J. Sex. Med. 11, 107–118 (2014).
Lawrence, A. A. Factors associated with satisfaction or regret following male-to-female sex reassignment surgery. Arch. Sex. Behav. 32, 299–315 (2003).
Holmberg, M., Arver, S. & Dhejne, C. Supporting sexuality and improving sexual function in transgender persons. Nat. Rev. Urol. 16, 121–139 (2019).
Coleman, E. et al. Standards of care for the health of transsexual, transgender, and gender-nonconforming people. WPATH https://wpath.org/publications/soc (2012).
Deutsch, M. Guidelines for the Primary and Gender-Affirming Care of Transgender and Gender Nonbinary People (UCSF Transgender Care, 2016).
Chandhoke, G., Shayegan, B. & Hotte, S. J. Exogenous estrogen therapy, testicular cancer, and the male to female transgender population: a case report. J. Med. Case Rep. 12, 373 (2018).
Ingham, M. D., Lee, R. J., MacDermed, D. & Olumi, A. F. Prostate cancer in transgender women. Urol. Oncol. 36, 518–525 (2018).
Deebel, N. A. et al. Prostate cancer in transgender women: incidence, etiopathogenesis, and management challenges. Urology 110, 166–171 (2017).
Tollinche, L. E. et al. The perioperative care of the transgender patient. Anesth. Analg. 127, 359–366 (2018).
Mahfouda, S. et al. Gender-affirming hormones and surgery in transgender children and adolescents. Lancet Diabetes Endocrinol. 7, 484–498 (2019).
Safa, B., Lin, W. C., Salim, A. M., Deschamps-Braly, J. C. & Poh, M. M. Current concepts in masculinizing gender surgery. Plast. Reconstr. Surg. 143, 857e–871e (2019).
Cuccolo, N. G. et al. Masculinizing chest reconstruction in transgender and nonbinary individuals: an analysis of epidemiology, surgical technique, and postoperative outcomes. Aesthetic Plast. Surg. 7 (Suppl 8), 38 (2019).
Kühn, S. et al. Mastectomy in female-to-male transgender patients: a single-center 24-year retrospective analysis. Arch. Plast. Surg. 46, 433–440 (2019).
Top, H. & Balta, S. Transsexual mastectomy: selection of appropriate technique according to breast characteristics. Balk. Med. J. 34, 147–155 (2017).
Cuccolo, N. G. et al. Mastectomy in transgender and cisgender patients: a comparative analysis of epidemiology and postoperative outcomes. Plast. Reconstr. Surg. Glob. Open 7, e2316 (2019).
Taniguchi, F. et al. Gonadotropin-releasing hormone analogues reduce the proliferation of endometrial stromal cells but not endometriotic cells. Gynecol. Obstet. Invest. 75, 9–15 (2013).
Stone, J. P., Hartley, R. L. & Temple-Oberle, C. Breast cancer in transgender patients: a systematic review. Part 2: female to male. Eur. J. Surg. Oncol. 44, 1463–1468 (2018).
Joint, R., Chen, Z. E. & Cameron, S. Breast and reproductive cancers in the transgender population: a systematic review. BJOG 125, 1505–1512 (2018).
Kiran, T. et al. Cancer screening rates among transgender adults: cross-sectional analysis of primary care data. Can. Fam. Physician 65, e30–e37 (2019).
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (American Psychiatric Association, 2013).
Hembree, W. C. et al. Endocrine treatment of gender-dysphoric/gender-incongruent persons: an Endocrine Society clinical practice guideline. Endocr. Pract. 23, 1437 (2017).
Olson-Kennedy, J. et al. Impact of early medical treatment for transgender youth: protocol for the longitudinal, observational trans youth care study. JMIR Res. Protoc. 8, e14434 (2019).
Chen, D. et al. Multidisciplinary care for gender-diverse youth: a narrative review and unique model of gender-affirming care. Transgend. Health 1, 117–123 (2016).
Winter, S. et al. Transgender people: health at the margins of society. Lancet 388, 390–400 (2016).
Hatzenbuehler, M. L. & Pachankis, J. E. Stigma and minority stress as social determinants of health among lesbian, gay, bisexual, and transgender youth: research evidence and clinical implications. Pediatr. Clin. North. Am. 63, 985–997 (2016).
Drescher, J., Haller, E. & Yarbrough, E. Position Statement on Discrimination Against Transgender and Gender Diverse Individuals (APA, 2018).
Kosciw, J. G. et al. The 2017 National School Climate Survey: The Experiences of Lesbian, Gay, Bisexual, Transgender, and Queer Youth in Our Nation’s Schools (Gay, Lesbian and Straight Education Network (GLSEN), 2018).
Becerra-Culqui, T. A. et al. Mental health of transgender and gender nonconforming youth compared with their peers. Pediatrics 141, e20173845 (2018).
Newcomb, M. E. et al. High burden of mental health problems, substance use, violence, and related psychosocial factors in transgender, non-binary, and gender diverse youth and young adults. Arch. Sex. Behav. 49, 645–659 (2019).
Travers, R., Bauer, G. & Pyne, J. Impacts of Strong Parental Support for Trans Youth: a Report Prepared for Children’s Aid Society of Toronto and Delisle Youth Services (Trans PULSE, 2012).
GLSEN 2015 National School Climate Survey (NSCS) — Executive Summary.pdf.
Safer, J. D. & Tangpricha, V. Care transgender persons. N. Engl. J. Med. 381, 2451–2460 (2019).
Kaltiala-Heino, R., Bergman, H., Työläjärvi, M. & Frisén, L. Gender dysphoria in adolescence: current perspectives. Adolesc. Health Med. Ther. 9, 31–41 (2018).
Marks, D. H., Hagigeorges, D., Manatis-Lornell, A. J., Dommasch, E. & Senna, M. M. Excess hair, hair removal methods, and barriers to care in gender minority patients: a survey study. J. Cosmet. Dermatol. 19, 1494–1498 (2019).
Hardy, T. L. D., Rieger, J. M., Wells, K. & Boliek, C. A. Acoustic predictors of gender attribution, masculinity-femininity, and vocal naturalness ratings amongst transgender and cisgender speakers. J. Voice 34, 300.e11–300.e26 (2018).
Ngaage, L. M. et al. Gender surgery beyond chest and genitals: current insurance landscape. Aesthet. Surg. J. https://doi.org/10.1093/asj/sjz262 (2019).
Ngaage, L. M. et al. Health insurance coverage of gender-affirming top surgery in the United States. Plast. Reconstr. Surg. 144, 824–833 (2019).
Ngaage, L. M. et al. A review of insurance coverage of gender affirming genital surgery. Plast. Reconstr. Surg. 145, 803–812 (2019).
Canner, J. K. et al. Temporal trends in gender-affirming surgery among transgender patients in the United States. JAMA Surg. 153, 609–616 (2018).
Defreyne, J., Motmans, J. & T’sjoen, G. Healthcare costs and quality of life outcomes following gender affirming surgery in trans men: a review. Expert. Rev. Pharmacoecon. Outcomes Res. 17, 543–556 (2017).
Dowshen, N. L., Christensen, J. & Gruschow, S. M. Health insurance coverage of recommended gender-affirming health care services for transgender youth: shopping online for coverage information. Transgend. Health 4, 131–135 (2019).
Littman, L. Parent reports of adolescents and young adults perceived to show signs of a rapid onset of gender dysphoria. PLoS ONE 13, e0202330 (2018).
Kimberly, L. L. et al. Ethical issues in gender-affirming care for youth. Pediatrics 142, e20181537 (2018).
Pediatric Endocrine Society. PES 2019 transgender statement. PES https://pedsendo.org/clinical-resource/pes-2019-transgender-statement/ (2019).
Author information
Authors and Affiliations
Contributions
All authors researched data for the article and wrote the manuscript. L.P. made substantial contributions to discussion of content and reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Urology thanks G. Quinn, J. Warus and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Panagiotakopoulos, L., Chulani, V., Koyama, A. et al. The effect of early puberty suppression on treatment options and outcomes in transgender patients. Nat Rev Urol 17, 626–636 (2020). https://doi.org/10.1038/s41585-020-0372-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41585-020-0372-2
This article is cited by
-
Update on bioethical, medical and fertility issues in gender incongruence during transition age
Journal of Endocrinological Investigation (2023)