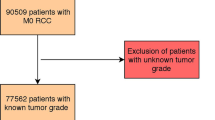
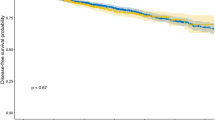
Abstract
Renal cell carcinoma (RCC) incidence is increasing worldwide. A high proportion of individuals are asymptomatic at diagnosis, but RCC has a high mortality rate. These facts suggest that RCC meets some of the criteria for screening, and a new analysis shows that screening for RCC could potentially be cost-effective. Targeted screening of high-risk individuals is likely to be the most cost-effective strategy to maximize the benefits and reduce the harms of screening. However, the size of the benefit of earlier initiation of treatment and the overall cost-effectiveness of screening remains uncertain. The optimal screening modality and target population is also unclear, and uncertainties exist regarding the specification and implementation of a screening programme. Before moving to a fully powered trial of screening, future work should focus on the following: developing and validating accurate risk prediction models; developing non-invasive methods of early RCC detection; establishing the feasibility, public acceptability and potential uptake of screening; establishing the prevalence of RCC and stage distribution of RCC detected by screening; and evaluating the potential harms of screening, including the impact on quality of life, overdiagnosis and over-treatment.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Jones, J. et al. The kidney cancer research priority-setting partnership: identifying the top 10 research priorities as defined by patients, caregivers, and expert clinicians. Can. Urol. Assoc. J. 11, 379–387 (2017).
Motzer, R. J. Perspective: what next for treatment? Nature 537, S111 (2016).
Rossi, S. H. et al. Essential research priorities in renal cancer: a modified Delphi consensus statement. Eur. Urol. Focus 6, 991–998 (2019).
Rossi, S. H. et al. Setting research priorities in partnership with patients to provide patient-centred urological cancer care. Eur. Urol. 75, 891–893 (2019).
Znaor, A., Lortet-Tieulent, J., Laversanne, M., Jemal, A. & Bray, F. International variations and trends in renal cell carcinoma incidence and mortality. Eur. Urol. 67, 519–530 (2015).
Rossi, S. H., Klatte, T., Usher-Smith, J. & Stewart, G. D. Epidemiology and screening for renal cancer. World J. Urol. 36, 1341–1353 (2018).
Hock, L. M., Lynch, J. & Balaji, K. C. Increasing incidence of all stages of kidney cancer in the last 2 decades in the United States: an analysis of surveillance, epidemiology and end results program data. J. Urol. 167, 57–60 (2002).
Lightfoot, N. et al. Impact of noninvasive imaging on increased incidental detection of renal cell carcinoma. Eur. Urol. 37, 521–527 (2000).
Selby, P. J. et al. Methods for the evaluation of biomarkers in patients with kidney and liver diseases: multicentre research programme including ELUCIDATE RCT (NIHR Journals Library, 2018).
Cancer Research UK. Kidney Cancer Statistics http://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/kidney-cancer (2020).
Office for National Statistics. Cancer Survival by Stage at Diagnosis for England https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/cancersurvivalbystageatdiagnosisforenglandexperimentalstatistics/adultsdiagnosed20122013and2014andfollowedupto2015 (2016).
Spouge, A. R., Wilson, S. R. & Wooley, B. Abdominal sonography in asymptomatic executives: prevalence of pathologic findings, potential benefits, and problems. J Ultrasound Med. 15, 763–767 (1996).
Fujii, Y., Ajima, J., Oka, K., Tosaka, A. & Takehara, Y. Benign renal tumors detected among healthy adults by abdominal ultrasonography. Eur. Urol 27, 124–127 (1995).
Mihara, S., Kuroda, K., Yoshioka, R. & Koyama, W. Early detection of renal cell carcinoma by ultrasonographic screening — based on the results of 13 years screening in Japan. Ultrasound Med. Biol. 25, 1033–1039 (1999).
National Screening Committee. Criteria for Appraising the Viability, Effectiveness and Appropriateness of a Screening Programme https://www.gov.uk/government/publications/evidence-review-criteria-national-screening-programmes/criteria-for-appraising-the-viability-effectiveness-and-appropriateness-of-a-screening-programme (2015).
Wilson, J. M. G., Jungner, G. Principles and practice of screening for disease. Public Health Paper Number 34. (WHO, 1968).
Fenton, J. J. & Weiss, N. S. Screening computed tomography: will it result in overdiagnosis of renal carcinoma? Cancer 100, 986–990 (2004).
Rossi, S. H. et al. Meta-analysis of the prevalence of renal cancer detected by abdominal ultrasonography. Br. J. Surg. 104, 648–659 (2017).
Saad, A. M. et al. Trends in renal-cell carcinoma incidence and mortality in the United States in the last 2 decades: a SEER-based study. Clin. Genitourin. Cancer 17, 46–57 (2019).
Turner, R. M. 2nd, Morgan, T. M. & Jacobs, B. L. Epidemiology of the small renal mass and the treatment disconnect phenomenon. Urol. Clin. North Am. 44, 147–154 (2017).
Hollingsworth, J. M., Miller, D. C., Daignault, S. & Hollenbeck, B. K. Rising incidence of small renal masses: a need to reassess treatment effect. J. Natl Cancer Inst. 98, 1331–1334 (2006).
Smaldone, M. C. et al. Understanding treatment disconnect and mortality trends in renal cell carcinoma using tumor registry data. Med Care 55, 398–404 (2017).
Welch, H. G., Skinner, J. S., Schroeck, F. R., Zhou, W. & Black, W. C. Regional variation of computed tomographic imaging in the United States and the risk of nephrectomy. JAMA Intern. Med. 178, 221–227 (2018).
Bangma, C. H. et al. Outcomes of a bladder cancer screening program using home hematuria testing and molecular markers. Eur. Urol. 64, 41–47 (2013).
Messing, E. M. et al. Long-term outcome of hematuria home screening for bladder cancer in men. Cancer 107, 2173–2179 (2006).
Messing, E. M. et al. Comparison of bladder cancer outcome in men undergoing hematuria home screening versus those with standard clinical presentations. Urology 45, 387–396 (1995).
Ljungberg, B. et al. European Association of Urology guidelines on renal cell carcinoma: the 2019 update. Eur. Urol. 75, 799–810 (2019).
Beinfeld, M. T., Wittenberg, E. & Gazelle, G. S. Cost-effectiveness of whole-body CT screening. Radiology 234, 415–422 (2005).
Ishikawa, S. et al. Mass screening of multiple abdominal solid organs using mobile helical computed tomography scanner — a preliminary report. Asian J. Surg. 30, 118–121 (2007).
Wernli, K. J., Rutter, C. M., Dachman, A. H. & Zafar, H. M. Suspected extracolonic neoplasms detected on CT colonography: literature review and possible outcomes. Acad. Radiol. 20, 667–674 (2013).
US Preventive Services Task Force et al. Screening for colorectal cancer: US preventive services task force recommendation statement. JAMA 315, 2564–2575 (2016).
ISRCTN Registry. The Yorkshire Lung Screening Trial http://www.isrctn.com/ISRCTN42704678 (2020).
Bobridge, A., Price, K., Gill, T. K. & Taylor, A. W. Influencing cancer screening participation rates-providing a combined cancer screening program (a ‘One Stop’ Shop) could be a potential answer. Front. Oncol. 7, 308 (2017).
Labeit, A. & Peinemann, F. Breast and cervical cancer screening in Great Britain: dynamic interrelated processes. Health Econ. Rev. 5, 32 (2015).
Riccabona, M. et al. Renal masses — evaluation by amplitude coded colour Doppler sonography and multiphasic contrast-enhanced CT. Acta Radiol. 40, 457–461 (1999).
Darwood, R. et al. Twenty-year review of abdominal aortic aneurysm screening in men in the county of Gloucestershire, United Kingdom. J. Vasc. Surg. 56, 8–13 (2012).
Wanhainen, A. et al. Outcome of the Swedish nationwide abdominal aortic aneurysm screening program. Circulation 134, 1141–1148 (2016).
Mizuma, Y., Watanabe, Y., Ozasa, K., Hayashi, K. & Kawai, K. Validity of sonographic screening for the detection of abdominal cancers. J. Clin. Ultrasound 30, 408–415 (2002).
Filipas, D. et al. Screening for renal cell carcinoma using ultrasonography: a feasibility study. BJU Int. 91, 595–599 (2003).
Uppot, R. N. Technical challenges of imaging & image-guided interventions in obese patients. Br. J. Radiol. 91, 20170931 (2018).
Kyrgiou, M. et al. Adiposity and cancer at major anatomical sites: umbrella review of the literature. BrMedJ 356, j477 (2017).
Warshauer, D. M. et al. Detection of renal masses: sensitivities and specificities of excretory urography/linear tomography, US, and CT. Radiology 169, 363–365 (1988).
Tosaka, A. et al. Incidence and properties of renal masses and asymptomatic renal cell carcinoma detected by abdominal ultrasonography. J. Urol. 144, 1097–1099 (1990).
Ficarra, V. et al. Incidental detection beyond pathological factors as prognostic predictor of renal cell carcinoma. Eur. Urol. 43, 663–669 (2003).
Patard, J. J., Rodriguez, A., Rioux-Leclercq, N., Guille, F. & Lobel, B. Prognostic significance of the mode of detection in renal tumours. BJU Int. 90, 358–363 (2002).
Stephenson, A. J., Kuritzky, L. & Campbell, S. C. Screening for urologic malignancies in primary care: pros, cons, and recommendations. Cleve. Clin. J. Med. 74 (Suppl. 3), S6–S14 (2007).
Golombos, D. M. et al. Minimally invasive vs open nephrectomy in the modern era: does approach matter? World J. Urol. 35, 1557–1568 (2017).
Rossi, S. H. et al. A decision analysis evaluating screening for kidney cancer using focused renal ultrasound. Eur. Urol. Focus https://doi.org/10.1016/j.euf.2019.09.002 (2019).
Lotan, Y. et al. Renal-cell carcinoma risk estimates based on participants in the prostate, lung, colorectal, and ovarian cancer screening trial and national lung screening trial. Urol. Oncol. 34, 167 e9–e116 (2016).
Malaeb, B. S. et al. The utility of screening renal ultrasonography: identifying renal cell carcinoma in an elderly asymptomatic population. BJU Int. 95, 977–981 (2005).
Shea, M. W. A proposal for a targeted screening program for renal cancer. Front. Oncol. 3, 207 (2013).
Usher-Smith, J. A., Sharp, S. J., Luben, R. & Griffin, S. J. Development and validation of lifestyle-based models to predict incidence of the most common potentially preventable cancers. Cancer Epidemiol. Biomarkers Prev. 28, 67–75 (2019).
Wu, Y. et al. Genetic scores based on risk-associated single nucleotide polymorphisms (SNPs) can reveal inherited risk of renal cell carcinoma. Oncotarget 7, 18631–18637 (2016).
Scelo, G. et al. KIM-1 as a blood-based marker for early detection of kidney cancer: a prospective nested case-control study. Clin. Cancer Res. 24, 5594–5601 (2018).
Frantzi, M. et al. Discovery and validation of urinary biomarkers for detection of renal cell carcinoma. J. Proteom. 98, 44–58 (2014).
Starke, N., Singla, N., Haddad, A. & Lotan, Y. Long-term outcomes in a high-risk bladder cancer screening cohort. BJU Int. 117, 611–617 (2016).
Mitchell, T. J. et al. Timing the landmark events in the evolution of clear cell renal cell cancer: TRACERx renal. Cell 173, 611–623 (2018).
Gerstung, M. et al. The evolutionary history of 2,658 cancers. Nature 578, 122–128 (2020).
Meisel, S. F. et al. Adjusting the frequency of mammography screening on the basis of genetic risk: attitudes among women in the UK. Breast 24, 237–241 (2015).
Meisel, S. F. et al. Population-based, risk-stratified genetic testing for ovarian cancer risk: a focus group study. Public Health Genomics 16, 184–191 (2013).
Grebe, S. K. & Erickson, L. A. Screening for kidney cancer: is there a role for aquaporin-1 and adipophilin? Mayo Clin. Proc. 85, 410–412 (2010).
Volpe, A. et al. The natural history of incidentally detected small renal masses. Cancer 100, 738–745 (2004).
Jewett, M. A. et al. Active surveillance of small renal masses: progression patterns of early stage kidney cancer. Eur. Urol. 60, 39–44 (2011).
Volpe, A. European Active SurveillancE of Renal Cell Carcinoma Study Facts and Figures https://uroweb.org/wp-content/uploads/EASE-Facts-figures-05-03-2020.pdf (2016).
Corcoran, A. T. et al. A review of contemporary data on surgically resected renal masses — benign or malignant? Urology 81, 707–713 (2013).
Borghesi, M. et al. Active surveillance for clinically localized renal tumors: an updated review of current indications and clinical outcomes. Int. J. Urol. 22, 432–438 (2015).
Frank, I. et al. Solid renal tumors: an analysis of pathological features related to tumor size. J. Urol. 170, 2217–2220 (2003).
Neves, J. B. et al. Protocol for a feasibility study of a cohort embedded randomised controlled trial comparing nephron sparing treatment (NEST) for small renal masses. BMJ Open. 9, e030965 (2019).
Blick, C., Ritchie, A. W. S., Eisen, T. & Stewart, G. D. Improving outcomes in high-risk, nonmetastatic renal cancer: new data and ongoing trials. Nat. Rev. Urol. 14, 753–759 (2017).
Kidney Cancer UK. Patient Survey Results Autumn 2018 https://www.kcuk.org.uk/patient-survey-autumn-2018/ (2018).
Klatte, T., Lam, J. S., Shuch, B., Belldegrun, A. S. & Pantuck, A. J. Surveillance for renal cell carcinoma: why and how? When and how often? Urol. Oncol. 26, 550–554 (2008).
Usher-Smith, J. A. et al. External validation of risk prediction models for incident colorectal cancer using UK Biobank. Br. J. Cancer 118, 750–759 (2018).
Lee, A. et al. BOADICEA: a comprehensive breast cancer risk prediction model incorporating genetic and nongenetic risk factors. Genet. Med. 21, 1708–1718 (2019).
Li, K. et al. Selecting high-risk individuals for lung cancer screening: a prospective evaluation of existing risk models and eligibility criteria in the German EPIC cohort. Cancer Prev. Res. 8, 777–785 (2015).
Usher-Smith, J. A., Emery, J., Kassianos, A. P. & Walter, F. M. Risk prediction models for melanoma: a systematic review. Cancer Epidemiol. Biomarkers Prev. 23, 1450–1463 (2014).
Usher-Smith, J. A., Walter, F. M., Emery, J. D., Win, A. K. & Griffin, S. J. Risk prediction models for colorectal cancer: a systematic review. Cancer Prev. Res. 9, 13–26 (2016).
Rabjerg, M., Mikkelsen, M. N., Walter, S. & Marcussen, N. Incidental renal neoplasms: is there a need for routine screening? A Danish single-center epidemiological study. APMIS 122, 708–714 (2014).
Luciani, L. G., Cestari, R. & Tallarigo, C. Incidental renal cell carcinoma-age and stage characterization and clinical implications: study of 1092 patients (1982–1997). Urology 56, 58–62 (2000).
Acknowledgements
The work of J.U.S., G.D.S. and S.H.R. on screening in RCC is funded by a research grant from Kidney Cancer UK. J.U.S. is supported by a Cancer Research UK Cancer Prevention Fellowship (C55650/A21464). G.D.S. is supported by Cancer Research UK Cambridge Cancer Centre (Major Centre Award A25117). S.H.R. is supported by The Urology Foundation, the Renal Cancer Research Fund and a Cancer Research UK Clinical Research Fellowship. We thank patient representatives Philip Dondi and Phil Alsop for their helpful comments on this paper.
Author information
Authors and Affiliations
Contributions
R.K.S. wrote the manuscript and all authors provided a substantial contribution to the discussion of content and reviewed and/or edited the article before submission.
Corresponding author
Ethics declarations
Competing interests
G.D.S. has received educational grants from Pfizer, AstraZeneca and Intuitive Surgical; consultancy fees from Pfizer, Merck, EUSA Pharma and CMR Surgical; travel expenses from Pfizer and speaker fees from Pfizer. R.K.S. is the Chair of the International Advisory Board for the Danish Diabetes Academy, which is funded by the Novo Nordisk Foundation. The other authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Urology thanks C. Szczylik and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Glossary
- Allocation bias
-
Overestimation of mortality rates in registry data owing to increasing numbers of individuals diagnosed with disease and therefore ‘eligible’ to die with the disease over time.
- Lead-time bias
-
Overestimation of survival duration owing to screening detecting disease earlier (during the asymptomatic phase), meaning individuals with screen-detected cancer appear to live longer simply owing to earlier diagnosis (rather than a true improvement in survival).
- Length-time bias
-
Overestimation of survival owing to screening detecting more slowly progressive and less aggressive disease in asymptomatic individuals whereas individuals with more aggressive disease are more likely to be diagnosed outside a screening programme owing to symptoms.
- Treatment disconnect
-
The paradoxical rise in overall and cancer-specific mortality despite increased detection and treatment of renal cell carcinoma.
- Value of information analysis
-
In health economic evaluations, decisions regarding whether to adopt an intervention are taken in the context of uncertainty; the value of information analysis assesses the value of additional evidence to reduce decision uncertainty, suggesting whether further research into this topic might be a good use of resources.
Rights and permissions
About this article
Cite this article
Usher-Smith, J., Simmons, R.K., Rossi, S.H. et al. Current evidence on screening for renal cancer. Nat Rev Urol 17, 637–642 (2020). https://doi.org/10.1038/s41585-020-0363-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41585-020-0363-3
This article is cited by
-
Effect of smoking, hypertension and lifestyle factors on kidney cancer — perspectives for prevention and screening programmes
Nature Reviews Urology (2023)
-
Serum extracellular vesicles derived hsa-miR-320d as an indicator for progression of clear cell renal cell carcinoma
Discover Oncology (2023)
-
Screening programs for renal cell carcinoma: a systematic review by the EAU young academic urologists renal cancer working group
World Journal of Urology (2022)
-
Uncovering the microbiota in renal cell carcinoma tissue using 16S rRNA gene sequencing
Journal of Cancer Research and Clinical Oncology (2021)
-
MiR-6838-5p facilitates the proliferation and invasion of renal cell carcinoma cells through inhibiting the DMTF1/ARF-p53 axis
Journal of Bioenergetics and Biomembranes (2021)