Abstract
The female reproductive tract (FRT), similar to other mucosal sites, harbours a site-specific microbiome, which has an essential role in maintaining health and homeostasis. In the majority of women of reproductive age, the microbiota of the lower FRT (vagina and cervix) microenvironment is dominated by Lactobacillus species, which benefit the host through symbiotic relationships. By contrast, the upper FRT (uterus, Fallopian tubes and ovaries) might be sterile in healthy individuals or contain a low-biomass microbiome with a diverse mixture of microorganisms. When dysbiosis occurs, altered immune and metabolic signalling can affect hallmarks of cancer, including chronic inflammation, epithelial barrier breach, changes in cellular proliferation and apoptosis, genome instability, angiogenesis and metabolic dysregulation. These pathophysiological changes might lead to gynaecological cancer. Emerging evidence shows that genital dysbiosis and/or specific bacteria might have an active role in the development and/or progression and metastasis of gynaecological malignancies, such as cervical, endometrial and ovarian cancers, through direct and indirect mechanisms, including modulation of oestrogen metabolism. Cancer therapies might also alter microbiota at sites throughout the body. Reciprocally, microbiota composition can influence the efficacy and toxic effects of cancer therapies, as well as quality of life following cancer treatment. Modulation of the microbiome via probiotics or microbiota transplant might prove useful in improving responsiveness to cancer treatment and quality of life. Elucidating these complex host–microbiome interactions, including the crosstalk between distal and local sites, will translate into interventions for prevention, therapeutic efficacy and toxic effects to enhance health outcomes for women with gynaecological cancers.
Key points
-
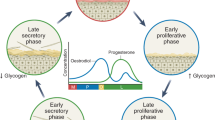
The majority of bacteria in the female reproductive tract (FRT) reside in the vagina and cervix; however, the upper FRT might have a distinct low-biomass microbiome and site-specific microenvironmental factors.
-
A vaginal microbiome dominated by Lactobacillus species benefits the host, whereas a dysbiotic vaginal microbiome consisting of anaerobic bacteria is linked to numerous gynaecological and obstetric conditions, including gynaecological cancer.
-
Multiple socioeconomic, behavioural, environmental, hormonal and genetic factors can affect the genital microbiome by disrupting homeostasis and promoting dysbiosis; the FRT microbiome is intimately interconnected with other mucosal sites.
-
Emerging evidence suggests that microbial communities within the FRT might contribute to aetiology, disease severity and/or treatment of gynaecological cancers; however, further well-designed, large-cohort and mechanistic studies are needed.
-
The gut microbiome can modulate oestrogen levels and thereby affect carcinogenesis of oestrogen-mediated cancers, might dictate therapeutic efficacy and toxicity for gynaecological cancer and, ultimately, influence quality of life.
-
Vaginal microbiome modulation via probiotics, novel antimicrobials and/or vaginal microbiota transplantation might be a novel approach to the prevention of gynaecological cancers and/or the reduction of vaginal toxicities related to cancer treatment.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Gilbert, J. A. et al. Current understanding of the human microbiome. Nat. Med. 24, 392–400 (2018).
Marchesi, J. R. & Ravel, J. The vocabulary of microbiome research: a proposal. Microbiome 3, 31 (2015).
Santiago-Rodriguez, T. M., Ly, M., Bonilla, N. & Pride, D. T. The human urine virome in association with urinary tract infections. Front. Microbiol. 6, 14 (2015).
Mukhopadhya, I., Segal, J. P., Carding, S. R., Hart, A. L. & Hold, G. L. The gut virome: the ‘missing link’ between gut bacteria and host immunity? Ther. Adv. Gastroenterol. 12, 1756284819836620 (2019).
Nash, A. K. et al. The gut mycobiome of the Human Microbiome Project healthy cohort. Microbiome 5, 153 (2017).
Bradford, L. L. & Ravel, J. The vaginal mycobiome: a contemporary perspective on fungi in women’s health and diseases. Virulence 8, 342–351 (2017).
Garretto, A., Miller-Ensminger, T., Wolfe, A. J. & Putonti, C. Bacteriophages of the lower urinary tract. Nat. Rev. Urol. 16, 422–432 (2019).
Sender, R., Fuchs, S. & Milo, R. Revised estimates for the number of human and bacteria cells in the body. PLoS Biol. 14, e1002533 (2016).
Gill, S. R. et al. Metagenomic analysis of the human distal gut microbiome. Science 312, 1355–1359 (2006).
Raskov, H., Burcharth, J. & Pommergaard, H. C. Linking gut microbiota to colorectal cancer. J. Cancer 8, 3378–3395 (2017).
Liu, H. X. et al. Difference of lower airway microbiome in bilateral protected specimen brush between lung cancer patients with unilateral lobar masses and control subjects. Int. J. Cancer 142, 769–778 (2018).
Baker, J. M., Chase, D. M. & Herbst-Kralovetz, M. M. Uterine microbiota: residents, tourists, or invaders? Front. Immunol. 9, 208 (2018).
Whiteside, S. A., Razvi, H., Dave, S., Reid, G. & Burton, J. P. The microbiome of the urinary tract — a role beyond infection. Nat. Rev. Urol. 12, 81–90 (2015).
Thomas-White, K. et al. Culturing of female bladder bacteria reveals an interconnected urogenital microbiota. Nat. Commun. 9, 1557 (2018).
Siddiqui, H., Nederbragt, A. J., Lagesen, K., Jeansson, S. L. & Jakobsen, K. S. Assessing diversity of the female urine microbiota by high throughput sequencing of 16S rDNA amplicons. BMC Microbiol. 11, 244 (2011).
Pearce, M. M. et al. The female urinary microbiome: a comparison of women with and without urgency urinary incontinence. MBio 5, e01283–01214 (2014).
Aragon, I. M. et al. The urinary tract microbiome in health and disease. Eur. Urol. Focus. 4, 128–138 (2018).
Sfanos, K. S., Yegnasubramanian, S., Nelson, W. G. & De Marzo, A. M. The inflammatory microenvironment and microbiome in prostate cancer development. Nat. Rev. Urol. 15, 11–24 (2018).
Walther-Antonio, M. R. et al. Potential contribution of the uterine microbiome in the development of endometrial cancer. Genome Med. 8, 122 (2016).
Verstraelen, H. et al. Characterisation of the human uterine microbiome in non-pregnant women through deep sequencing of the V1-2 region of the 16S rRNA gene. PeerJ 4, e1602 (2016).
Moreno, I. et al. Evidence that the endometrial microbiota has an effect on implantation success or failure. Am. J. Obstet. Gynecol. 215, 684–703 (2016).
Franasiak, J. M. et al. Endometrial microbiome at the time of embryo transfer: next-generation sequencing of the 16S ribosomal subunit. J. Assist. Reprod. Genet. 33, 129–136 (2016).
Chen, C. et al. The microbiota continuum along the female reproductive tract and its relation to uterine-related diseases. Nat. Commun. 8, 875 (2017).
Zozaya, M. et al. Bacterial communities in penile skin, male urethra, and vaginas of heterosexual couples with and without bacterial vaginosis. Microbiome 4, 16 (2016).
Altmae, S., Franasiak, J. M. & Mandar, R. The seminal microbiome in health and disease. Nat. Rev. Urol. 16, 703–721 (2019).
Ravel, J. et al. Vaginal microbiome of reproductive-age women. Proc. Natl Acad. Sci. USA 108, 4680–4687 (2011).
Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature 486, 207–214 (2012).
Martin, D. H. & Marrazzo, J. M. The vaginal microbiome: current understanding and future directions. J. Infect. Dis. 214, S36–S41 (2016).
Nunn, K. L. & Forney, L. J. Unraveling the dynamics of the human vaginal microbiome. Yale J. Biol. Med. 89, 331–337 (2016).
Beamer, M. A. et al. Bacterial species colonizing the vagina of healthy women are not associated with race. Anaerobe 45, 40–43 (2017).
Jespers, V. et al. A longitudinal analysis of the vaginal microbiota and vaginal immune mediators in women from sub-Saharan Africa. Sci. Rep. 7, 11974 (2017).
Kyongo, J. K. et al. Cross-sectional analysis of selected genital tract immunological markers and molecular vaginal microbiota in sub-Saharan African women, with relevance to HIV risk and prevention. Clin. Vaccine Immunol. 22, 526–538 (2015).
Antonio, M. A., Hawes, S. E. & Hillier, S. L. The identification of vaginal Lactobacillus species and the demographic and microbiologic characteristics of women colonized by these species. J. Infect. Dis. 180, 1950–1956 (1999).
Younes, J. A. et al. Women and their microbes: the unexpected friendship. Trends Microbiol. 26, 16–32 (2018).
Miller, E. A., Beasley, D. E., Dunn, R. R. & Archie, E. A. Lactobacilli dominance and vaginal pH: why is the human vaginal microbiome unique? Front. Microbiol. 7, 1936 (2016).
Hickey, R. J., Zhou, X., Pierson, J. D., Ravel, J. & Forney, L. J. Understanding vaginal microbiome complexity from an ecological perspective. Transl Res. 22, 267–282 (2012).
Łaniewski, P. & Herbst-Kralovetz, M. in Encyclopedia of Reproduction Vol. 2 (ed M. K. Skinner) 353-359 (Academic Press: Elsevier, 2018).
Graver, M. A. & Wade, J. J. The role of acidification in the inhibition of Neisseria gonorrhoeae by vaginal lactobacilli during anaerobic growth. Ann. Clin. Microbiol. Antimicrob. 10, 8 (2011).
Gong, Z., Luna, Y., Yu, P. & Fan, H. Lactobacilli inactivate Chlamydia trachomatis through lactic acid but not H2O2. PLoS One 9, e107758 (2014).
Conti, C., Malacrino, C. & Mastromarino, P. Inhibition of herpes simplex virus type 2 by vaginal lactobacilli. J. Physiol. Pharmacol. 60, 19–26 (2009).
Tyssen, D. et al. Anti-HIV-1 activity of lactic acid in human cervicovaginal fluid. mSphere 3, e00055-18 (2018).
Cadieux, P. A., Burton, J., Devillard, E. & Reid, G. Lactobacillus by-products inhibit the growth and virulence of uropathogenic Escherichia coli. J. Physiol. Pharmacol. 60, 13–18 (2009).
O’Hanlon, D. E., Moench, T. R. & Cone, R. A. Vaginal pH and microbicidal lactic acid when lactobacilli dominate the microbiota. PLoS One 8, e80074 (2013).
Tachedjian, G., O’Hanlon, D. E. & Ravel, J. The implausible “in vivo” role of hydrogen peroxide as an antimicrobial factor produced by vaginal microbiota. Microbiome 6, 29 (2018).
Maldonado-Barragan, A., Caballero-Guerrero, B., Martin, V., Ruiz-Barba, J. L. & Rodriguez, J. M. Purification and genetic characterization of gassericin E, a novel co-culture inducible bacteriocin from Lactobacillus gasseri EV1461 isolated from the vagina of a healthy woman. BMC Microbiol. 16, 37 (2016).
Mirmonsef, P. et al. Glycogen levels in undiluted genital fluid and their relationship to vaginal pH, estrogen, and progesterone. PLoS One 11, e0153553 (2016).
Zhou, X. et al. Differences in the composition of vaginal microbial communities found in healthy Caucasian and black women. ISME J. 1, 121–133 (2007).
Fettweis, J. M. et al. Differences in vaginal microbiome in African American women versus women of European ancestry. Microbiology 160, 2272–2282 (2014).
Peebles, K., Velloza, J., Balkus, J. E., McClelland, R. S. & Barnabas, R. V. High global burden and costs of bacterial vaginosis: a systematic review and meta-analysis. Sex. Transm. Dis. 46, 304–311 (2019).
Borgdorff, H. et al. The association between ethnicity and vaginal microbiota composition in Amsterdam, the Netherlands. PLoS One 12, e0181135 (2017).
Łaniewski, P. et al. Linking cervicovaginal immune signatures, HPV and microbiota composition in cervical carcinogenesis in non-Hispanic and Hispanic women. Sci. Rep. 8, 7593 (2018).
Peipert, J. F. et al. Bacterial vaginosis, race, and sexually transmitted infections: does race modify the association? Sex. Transm. Dis. 35, 363–367 (2008).
Cherpes, T. L., Hillier, S. L., Meyn, L. A., Busch, J. L. & Krohn, M. A. A delicate balance: risk factors for acquisition of bacterial vaginosis include sexual activity, absence of hydrogen peroxide-producing lactobacilli, black race, and positive herpes simplex virus type 2 serology. Sex. Transm. Dis. 35, 78–83 (2008).
Kenyon, C., Colebunders, R. & Crucitti, T. The global epidemiology of bacterial vaginosis: a systematic review. Am. J. Obstet. Gynecol. 209, 505–523 (2013).
Lewis, F. M., Bernstein, K. T. & Aral, S. O. Vaginal microbiome and its relationship to behavior, sexual health, and sexually transmitted diseases. Obstet. Gynecol. 129, 643–654 (2017).
Gajer, P. et al. Temporal dynamics of the human vaginal microbiota. Sci. Transl Med. 4, 132ra152 (2012).
Witkin, S. S. et al. Influence of vaginal bacteria and D- and L-lactic acid isomers on vaginal extracellular matrix metalloproteinase inducer: implications for protection against upper genital tract infections. MBio 4, e00460-13 (2013).
Serrano, M. G. et al. Racioethnic diversity in the dynamics of the vaginal microbiome during pregnancy. Nat. Med. 25, 1001–1011 (2019).
Nelson, T. M. et al. Cigarette smoking is associated with an altered vaginal tract metabolomic profile. Sci. Rep. 8, 852 (2018).
Brotman, R. M. et al. Association between cigarette smoking and the vaginal microbiota: a pilot study. BMC Infect. Dis. 14, 471 (2014).
Sabo, M. C. et al. Association between vaginal washing and vaginal bacterial concentrations. PLoS One 14, e0210825 (2019).
Brotman, R. M. et al. A longitudinal study of vaginal douching and bacterial vaginosis — a marginal structural modeling analysis. Am. J. Epidemiol. 168, 188–196 (2008).
Thoma, M. E. et al. Bacterial vaginosis is associated with variation in dietary indices. J. Nutr. 141, 1698–1704 (2011).
Neggers, Y. H. et al. Dietary intake of selected nutrients affects bacterial vaginosis in women. J. Nutr. 137, 2128–2133 (2007).
Wilkinson, E. M., Herbst-Kralovetz, M. M. & Brotman, R. M. Clinical and personal lubricants alter cell viability, cytotoxicity and mucin production in human vaginal epithelial cell models. Am. J. Obstet. Gynecol. 219, 638 (2018).
Muhleisen, A. L. & Herbst-Kralovetz, M. M. Menopause and the vaginal microbiome. Maturitas 91, 42–50 (2016).
Romero, R. et al. The composition and stability of the vaginal microbiota of normal pregnant women is different from that of non-pregnant women. Microbiome 2, 4 (2014).
Karstens, L. et al. Community profiling of the urinary microbiota: considerations for low-biomass samples. Nat. Rev. Urol. 15, 735–749 (2018).
Perez-Munoz, M. E., Arrieta, M. C., Ramer-Tait, A. E. & Walter, J. A critical assessment of the “sterile womb” and “in utero colonization” hypotheses: implications for research on the pioneer infant microbiome. Microbiome 5, 48 (2017).
Fang, R. L. et al. Barcoded sequencing reveals diverse intrauterine microbiomes in patients suffering with endometrial polyps. Am. J. Transl Res. 8, 1581–1592 (2016).
Mitchell, C. M. et al. Colonization of the upper genital tract by vaginal bacterial species in nonpregnant women. Am. J. Obstet. Gynecol. 212, 611.e1–611.e9 (2015).
Franasiak, J. M. & Scott, R. T. Jr. Reproductive tract microbiome in assisted reproductive technologies. Fertil. Steril. 104, 1364–1371 (2015).
Nelson, D. E. et al. Characteristic male urine microbiomes associate with asymptomatic sexually transmitted infection. PLoS One 5, e14116 (2010).
Nelson, D. E. et al. Bacterial communities of the coronal sulcus and distal urethra of adolescent males. PLoS One 7, e36298 (2012).
Dong, Q. et al. The microbial communities in male first catch urine are highly similar to those in paired urethral swab specimens. PLoS One 6, e19709 (2011).
Price, L. B. et al. The effects of circumcision on the penis microbiome. PLoS One 5, e8422 (2010).
Weng, S. L. et al. Bacterial communities in semen from men of infertile couples: metagenomic sequencing reveals relationships of seminal microbiota to semen quality. PLoS One 9, e110152 (2014).
Dawson, S. G., Ison, C. A., Csonka, G. & Easmon, C. S. Male carriage of Gardnerella vaginalis. Br. J. Vener. Dis. 58, 243–245 (1982).
Kinghorn, G. R., Jones, B. M., Chowdhury, F. H. & Geary, I. Balanoposthitis associated with Gardnerella vaginalis infection in men. Br. J. Vener. Dis. 58, 127–129 (1982).
Olson, K. M., Boohaker, L. J., Schwebke, J. R., Aslibekyan, S. & Muzny, C. A. Comparisons of vaginal flora patterns among sexual behaviour groups of women: implications for the pathogenesis of bacterial vaginosis. Sex. Health 15, 61–67 (2018).
Muzny, C. A., Lensing, S. Y., Aaron, K. J. & Schwebke, J. R. Incubation period and risk factors support sexual transmission of bacterial vaginosis in women who have sex with women. Sex. Transm. Infect. 95, 511–515 (2019).
Vodstrcil, L. A. et al. Incident bacterial vaginosis (BV) in women who have sex with women is associated with behaviors that suggest sexual transmission of BV. Clin. Infect. Dis. 60, 1042–1053 (2015).
Forcey, D. S. et al. Factors associated with bacterial vaginosis among women who have sex with women: a systematic review. PLoS One 10, e0141905 (2015).
Fouts, D. E. et al. Integrated next-generation sequencing of 16S rDNA and metaproteomics differentiate the healthy urine microbiome from asymptomatic bacteriuria in neuropathic bladder associated with spinal cord injury. J. Transl Med. 10, 174 (2012).
Hilt, E. E. et al. Urine is not sterile: use of enhanced urine culture techniques to detect resident bacterial flora in the adult female bladder. J. Clin. Microbiol. 52, 871–876 (2014).
Brubaker, L. & Wolfe, A. J. The female urinary microbiota, urinary health and common urinary disorders. Ann. Transl Med. 5, 34 (2017).
Carda-Dieguez, M. et al. Variations in vaginal, penile, and oral microbiota after sexual intercourse: a case report. Front. Med. 6, 178 (2019).
Antonio, M. A., Rabe, L. K. & Hillier, S. L. Colonization of the rectum by Lactobacillus species and decreased risk of bacterial vaginosis. J. Infect. Dis. 192, 394–398 (2005).
El Aila, N. A. et al. Identification and genotyping of bacteria from paired vaginal and rectal samples from pregnant women indicates similarity between vaginal and rectal microflora. BMC Infect. Dis. 9, 167 (2009).
Plottel, C. S. & Blaser, M. J. Microbiome and malignancy. Cell Host Microbe 10, 324–335 (2011).
Flores, R. et al. Fecal microbial determinants of fecal and systemic estrogens and estrogen metabolites: a cross-sectional study. J. Transl Med. 10, 253 (2012).
Baker, J. M., Al-Nakkash, L. & Herbst-Kralovetz, M. M. Estrogen-gut microbiome axis: physiological and clinical implications. Maturitas 103, 45–53 (2017).
Schistosomes, liver flukes and Helicobacter pylori. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Lyon, 7–14 June 1994. IARC Monogr. Eval. Carcinog. Risks Hum. 61, 1–241 (1994).
Wang, F., Meng, W., Wang, B. & Qiao, L. Helicobacter pylori-induced gastric inflammation and gastric cancer. Cancer Lett. 345, 196–202 (2014).
Welton, J. C., Marr, J. S. & Friedman, S. M. Association between hepatobiliary cancer and typhoid carrier state. Lancet 1, 791–794 (1979).
Lecuit, M. et al. Immunoproliferative small intestinal disease associated with Campylobacter jejuni. N. Engl. J. Med. 350, 239–248 (2004).
Cerroni, L., Zochling, N., Putz, B. & Kerl, H. Infection by Borrelia burgdorferi and cutaneous B-cell lymphoma. J. Cutan. Pathol. 24, 457–461 (1997).
Ferreri, A. J. et al. Chlamydophila psittaci eradication with doxycycline as first-line targeted therapy for ocular adnexae lymphoma: final results of an international phase II trial. J. Clin. Oncol. 30, 2988–2994 (2012).
Akram, N. et al. Oncogenic role of tumor viruses in humans. Viral Immunol. 30, 20–27 (2017).
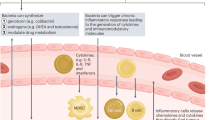
Garrett, W. S. Cancer and the microbiota. Science 348, 80–86 (2015).
Schwabe, R. F. & Jobin, C. The microbiome and cancer. Nat. Rev. Cancer 13, 800–812 (2013).
Kang, M. & Martin, A. Microbiome and colorectal cancer: unraveling host-microbiota interactions in colitis-associated colorectal cancer development. Semin. Immunol. 32, 3–13 (2017).
Sobhani, I. et al. Colorectal cancer-associated microbiota contributes to oncogenic epigenetic signatures. Proc. Natl Acad. Sci. USA 116, 24285–24295 (2019).
Chen, J., Domingue, J. C. & Sears, C. L. Microbiota dysbiosis in select human cancers: evidence of association and causality. Semin. Immunol. 32, 25–34 (2017).
Rajagopala, S. V. et al. The human microbiome and cancer. Cancer Prev. Res. 10, 226–234 (2017).
Fulbright, L. E., Ellermann, M. & Arthur, J. C. The microbiome and the hallmarks of cancer. PLoS Pathog. 13, e1006480 (2017).
Moschen, A. R. et al. Lipocalin 2 protects from inflammation and tumorigenesis associated with gut microbiota alterations. Cell Host Microbe 19, 455–469 (2016).
Rubinstein, M. R. et al. Fusobacterium nucleatum promotes colorectal cancer by inducing Wnt/beta-catenin modulator Annexin A1. EMBO Rep. 20, e47638 (2019).
Liu, N. et al. Helicobacter pylori promotes angiogenesis depending on Wnt/beta-catenin-mediated vascular endothelial growth factor via the cyclooxygenase-2 pathway in gastric cancer. BMC Cancer 16, 321 (2016).
Zadora, P. K. et al. Integrated phosphoproteome and transcriptome analysis reveals Chlamydia-induced epithelial-to-mesenchymal transition in host cells. Cell Rep. 26, 1286–1302 e1288 (2019).
Chase, D., Goulder, A., Zenhausern, F., Monk, B. & Herbst-Kralovetz, M. The vaginal and gastrointestinal microbiomes in gynecologic cancers: a review of applications in etiology, symptoms and treatment. Gynecol. Oncol. 138, 190–200 (2015).
Bray, F. et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 68, 394–424 (2018).
Siegel, R. L. et al. Cancer statistics for Hispanics/Latinos, 2015. CA Cancer J. Clin. 65, 457–480 (2015).
Viens, L. J. et al. Human papillomavirus-associated cancers — United States, 2008–2012. Morb. Mortal. Wkly Rep. 65, 661–666 (2016).
Marsh, M. Original site of cervical carcinoma; topographical relationship of carcinoma of the cervix to the external os and to the squamocolumnar junction. Obstet. Gynecol. 7, 444–452 (1956).
Herfs, M. et al. A discrete population of squamocolumnar junction cells implicated in the pathogenesis of cervical cancer. Proc. Natl Acad. Sci. USA 109, 10516–10521 (2012).
Walboomers, J. M. et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J. Pathol. 189, 12–19 (1999).
Shulzhenko, N., Lyng, H., Sanson, G. F. & Morgun, A. Menage a trois: an evolutionary interplay between human papillomavirus, a tumor, and a woman. Trends Microbiol. 22, 345–353 (2014).
Gravitt, P. E. & Winer, R. L. Natural history of HPV infection across the lifespan: role of viral latency. Viruses 9, E267 (2017).
Ryser, M. D., Rositch, A. & Gravitt, P. E. Modeling of US human papillomavirus (HPV) seroprevalence by age and sexual behavior indicates an increasing trend of hpv infection following the sexual revolution. J. Infect. Dis. 216, 604–611 (2017).
Eldridge, R. C. et al. Smoking and subsequent human papillomavirus infection: a mediation analysis. Ann. Epidemiol. 27, 724–730.e721 (2017).
Castle, P. E. et al. An association of cervical inflammation with high-grade cervical neoplasia in women infected with oncogenic human papillomavirus (HPV). Cancer Epidemiol. Biomarkers Prev. 10, 1021–1027 (2001).
Mhatre, M. et al. Cervical intraepithelial neoplasia is associated with genital tract mucosal inflammation. Sex. Transm. Dis. 39, 591–597 (2012).
Lehtinen, M. et al. Chlamydia trachomatis infection and risk of cervical intraepithelial neoplasia. Sex. Transm. Infect. 87, 372–376 (2011).
Mitra, A. et al. Cervical intraepithelial neoplasia disease progression is associated with increased vaginal microbiome diversity. Sci. Rep. 5, 16865 (2015).
Audirac-Chalifour, A. et al. Cervical microbiome and cytokine profile at various stages of cervical cancer: a pilot study. PLoS One 11, e0153274 (2016).
Ilhan, Z. E. et al. Deciphering the complex interplay between microbiota, HPV, inflammation and cancer through cervicovaginal metabolic profiling. EBioMedicine 44, 675–690 (2019).
Watts, D. H. et al. Effects of bacterial vaginosis and other genital infections on the natural history of human papillomavirus infection in HIV-1-infected and high-risk HIV-1-uninfected women. J. Infect. Dis. 191, 1129–1139 (2005).
Gillet, E. et al. Bacterial vaginosis is associated with uterine cervical human papillomavirus infection: a meta-analysis. BMC Infect. Dis. 11, 10 (2011).
Guo, Y. L., You, K., Qiao, J., Zhao, Y. M. & Geng, L. Bacterial vaginosis is conducive to the persistence of HPV infection. Int. J. STD AIDS 23, 581–584 (2012).
Gao, W., Weng, J., Gao, Y. & Chen, X. Comparison of the vaginal microbiota diversity of women with and without human papillomavirus infection: a cross-sectional study. BMC Infect. Dis. 13, 271 (2013).
Lee, J. E. et al. Association of the vaginal microbiota with human papillomavirus infection in a Korean twin cohort. PLoS One 8, e6351 (2013).
Brotman, R. M. et al. Interplay between the temporal dynamics of the vaginal microbiota and human papillomavirus detection. J. Infect. Dis. 210, 1723–1733 (2014).
Di Paola, M. et al. Characterization of cervico-vaginal microbiota in women developing persistent high-risk Human Papillomavirus infection. Sci. Rep. 7, 10200 (2017).
Tuominen, H., Rautava, S., Syrjanen, S., Collado, M. C. & Rautava, J. HPV infection and bacterial microbiota in the placenta, uterine cervix and oral mucosa. Sci. Rep. 8, 9787 (2018).
Oh, H. Y. et al. The association of uterine cervical microbiota with an increased risk for cervical intraepithelial neoplasia in Korea. Clin. Microbiol. Infect. 21, 674.e1–674.e9 (2015).
Brusselaers, N., Shrestha, S., Van De Wijgert, J. & Verstraelen, H. Vaginal dysbiosis, and the risk of human papillomavirus and cervical cancer: systematic review and meta-analysis. Am. J. Obstet. Gynecol. 21, 9–18.e8 (2018).
Norenhag, J. et al. The vaginal microbiota, HPV and cervical dysplasia: a systematic review and network meta-analysis. BJOG 127, 171–180 (2020).
Wang, H. et al. Associations of cervicovaginal lactobacilli with high-risk HPV infection, cervical intraepithelial neoplasia, and cancer: a systematic review and meta-analysis. J. Infect. Dis. 220, 1243–1254 (2019).
Mehta, F. F., Baik, S. & Chung, S. H. Recurrence of cervical cancer and its resistance to progestin therapy in a mouse model. Oncotarget 8, 2372–2380 (2017).
Larmour, L. I. et al. A patient derived xenograft model of cervical cancer and cervical dysplasia. PLoS One 13, e0206539 (2018).
Doorbar, J. Model systems of human papillomavirus-associated disease. J. Pathol. 238, 166–179 (2016).
Christensen, N. D., Budgeon, L. R., Cladel, N. M. & Hu, J. Recent advances in preclinical model systems for papillomaviruses. Virus Res. 231, 108–118 (2017).
Herbst-Kralovetz, M. M., Pyles, R. B., Ratner, A. J., Sycuro, L. K. & Mitchell, C. New systems for studying intercellular interactions in bacterial vaginosis. J. Infect. Dis. 214, S6–S13 (2016).
Gilbert, N. M., Lewis, W. G. & Lewis, A. L. Clinical features of bacterial vaginosis in a murine model of vaginal infection with Gardnerella vaginalis. PLoS One 8, e59539 (2013).
Barrila, J. et al. Organotypic 3D cell culture models: using the rotating wall vessel to study host-pathogen interactions. Nat. Rev. Microbiol. 8, 791–801 (2010).
Gardner, J. et al. IL-36gamma is elevated in cervicovaginal epithelial cells in women with bacterial vaginosis and in vitro after infection with microbes associated with bacterial vaginosis. J. Infect. Dis. https://doi.org/10.1093/infdis/jiz514 (2019).
Doerflinger, S. Y., Throop, A. L. & Herbst-Kralovetz, M. M. Bacteria in the vaginal microbiome alter the innate immune response and barrier properties of the human vaginal epithelia in a species-specific manner. J. Infect. Dis. 209, 1989–1999 (2014).
Radtke, A. L., Quayle, A. J. & Herbst-Kralovetz, M. M. Microbial products alter the expression of membrane-associated mucin and antimicrobial peptides in a three-dimensional human endocervical epithelial cell model. Biol. Reprod. 87, 132 (2012).
Hjelm, B. E., Berta, A. N., Nickerson, C. A., Arntzen, C. J. & Herbst-Kralovetz, M. M. Development and characterization of a three-dimensional organotypic human vaginal epithelial cell model. Biol. Reprod. 82, 617–627 (2010).
Łaniewski, P., Gomez, A., Hire, G., So, M. & Herbst-Kralovetz, M. M. Human three-dimensional endometrial epithelial cell model to study host interactions with vaginal bacteria and Neisseria gonorrhoeae. Infect. Immun. 85, e01049-16 (2017).
Radtke, A. L. & Herbst-Kralovetz, M. M. Culturing and applications of rotating wall vessel bioreactor derived 3D epithelial cell models. J. Vis. Exp. 62, 3868 (2012).
McGowin, C. L., Radtke, A. L., Abraham, K., Martin, D. H. & Herbst-Kralovetz, M. Mycoplasma genitalium infection activates cellular host defense and inflammation pathways in a 3-dimensional human endocervical epithelial cell model. J. Infect. Dis. 207, 1857–1868 (2013).
Łaniewski, P. et al. Features of the cervicovaginal microenvironment drive cancer biomarker signatures in patients across cervical carcinogenesis. Sci. Rep. 9, 7333 (2019).
Srinivasan, S. et al. Metabolic signatures of bacterial vaginosis. MBio 6, e00204-15 (2015).
Pavlova, N. N. & Thompson, C. B. The emerging hallmarks of cancer metabolism. Cell Metab. 23, 27–47 (2016).
Rauh-Hain, J. A. et al. Racial disparities in treatment of high-grade endometrial cancer in the Medicare population. Obstet. Gynecol. 125, 843–851 (2015).
Chatterjee, S., Gupta, D., Caputo, T. A. & Holcomb, K. Disparities in gynecological malignancies. Front. Oncol. 6, 36 (2016).
Doll, A. et al. Novel molecular profiles of endometrial cancer-new light through old windows. J. Steroid Biochem. Mol. Biol. 108, 221–229 (2008).
Doll, K. M. & Winn, A. N. Assessing endometrial cancer risk among US women: long-term trends using hysterectomy-adjusted analysis. Am. J. Obstet. Gynecol. 221, 318.e1–318.e9 (2019).
Doll, K. M., Snyder, C. R. & Ford, C. L. Endometrial cancer disparities: a race-conscious critique of the literature. Am. J. Obstet. Gynecol. 218, 474–482 e472 (2018).
Allen, N. E. et al. Endogenous sex hormones and endometrial cancer risk in women in the European Prospective Investigation into Cancer and Nutrition (EPIC). Endocr. Relat. Cancer 15, 485–497 (2008).
Dossus, L. et al. Obesity, inflammatory markers, and endometrial cancer risk: a prospective case-control study. Endocr. Relat. Cancer 17, 1007–1019 (2010).
Tilg, H., Moschen, A. R. & Kaser, A. Obesity and the microbiota. Gastroenterology 136, 1476–1483 (2009).
Candela, M. et al. Inflammation and colorectal cancer, when microbiota-host mutualism breaks. World J. Gastroenterol. 20, 908–922 (2014).
Si, J., You, H. J., Yu, J., Sung, J. & Ko, G. Prevotella as a hub for vaginal microbiota under the influence of host genetics and their association with obesity. Cell Host Microbe 21, 97–105 (2017).
Choi, S., Hwang, Y. J., Shin, M. J. & Yi, H. Difference in the gut microbiome between ovariectomy-induced obesity and diet-induced obesity. J. Microbiol. Biotechnol. 27, 2228–2236 (2017).
Cox-York, K. A. et al. Ovariectomy results in differential shifts in gut microbiota in low versus high aerobic capacity rats. Physiol. Rep. 3, e12488 (2015).
Shen, J. et al. Effects of low dose estrogen therapy on the vaginal microbiomes of women with atrophic vaginitis. Sci. Rep. 6, 24380 (2016).
Kwa, M., Plottel, C. S., Blaser, M. J. & Adams, S. The intestinal microbiome and estrogen receptor-positive female breast cancer. J. Natl Cancer Inst. 108, djw029 (2016).
Joshi, A. R. & Ellenson, L. H. in Molecular Genetics of Endometrial Carcinoma (ed L. H. Ellenson) 261–273 (Springer, 2017).
Torre, L. A. et al. Ovarian cancer statistics, 2018. CA Cancer J. Clin. 68, 284–296 (2018).
Zhou, B. et al. The biodiversity composition of microbiome in ovarian carcinoma patients. Sci. Rep. 9, 1691 (2019).
Banerjee, S. et al. The ovarian cancer oncobiome. Oncotarget 8, 36225–36245 (2017).
Shanmughapriya, S. et al. Viral and bacterial aetiologies of epithelial ovarian cancer. Eur. J. Clin. Microbiol. Infect. Dis. 31, 2311–2317 (2012).
Chan, P. J., Seraj, I. M., Kalugdan, T. H. & King, A. Prevalence of mycoplasma conserved DNA in malignant ovarian cancer detected using sensitive PCR-ELISA. Gynecol. Oncol. 63, 258–260 (1996).
Emara, M. M. et al. Synchronous occurrence of brucellosis and ovarian cancer — a case report. Austral. Asian J. Cancer 6, 257–259 (2016).
Pakish, J. B. & Jazaeri, A. A. Immunotherapy in gynecologic cancers: are we there yet? Curr. Treat. Options Oncol. 18, 59 (2017).
Routy, B. et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 359, 91–97 (2018).
Matson, V. et al. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science 359, 104–108 (2018).
Gopalakrishnan, V. et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science 359, 97–103 (2018).
Colbert, L. E. et al. The gut and cervical microbiome promote immune activation and response to chemoradiation in cervical cancer. Cancer Cell https://doi.org/10.2139/ssrn.3199993 (2018).
Sivan, A. et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science 350, 1084–1089 (2015).
Alexander, J. L. et al. Gut microbiota modulation of chemotherapy efficacy and toxicity. Nat. Rev. Gastroenterol. Hepatol. 14, 356–365 (2017).
Wilkinson, E. M., Ilhan, Z. E. & Herbst-Kralovetz, M. M. Microbiota-drug interactions: impact on metabolism and efficacy of therapeutics. Maturitas 112, 53–63 (2018).
Kurita, A. et al. Streptomycin alleviates irinotecan-induced delayed-onset diarrhea in rats by a mechanism other than inhibition of beta-glucuronidase activity in intestinal lumen. Cancer Chemother. Pharmacol. 67, 201–213 (2011).
Vetizou, M. et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science 350, 1079–1084 (2015).
Kamada, N., Seo, S. U., Chen, G. Y. & Nunez, G. Role of the gut microbiota in immunity and inflammatory disease. Nat. Rev. Immunol. 13, 321–335 (2013).
Wong, S. & Slavcev, R. A. Treating cancer with infection: a review on bacterial cancer therapy. Lett. Appl. Microbiol. 61, 107–112 (2015).
Taneva, E. et al. Vaginal microbiome modulates topical antiretroviral drug pharmacokinetics. JCI Insight 3, 99545 (2018).
Thurman, A. R. et al. Vaginal microbiota and mucosal pharmacokinetics of tenofovir in healthy women using tenofovir and tenofovir/levonorgestrel vaginal rings. PLoS One 14, e0217229 (2019).
Donahue Carlson, R. et al. The female genital tract microbiome is associated with vaginal antiretroviral drug concentrations in human immunodeficiency virus-infected women on antiretroviral therapy. J. Infect. Dis. 216, 990–999 (2017).
Vitali, B. et al. Vaginal microbiome and metabolome highlight specific signatures of bacterial vaginosis. Eur. J. Clin. Microbiol. Infect. Dis. 34, 2367–2376 (2015).
Maduro, J. H., Pras, E., Willemse, P. H. & de Vries, E. G. Acute and long-term toxicity following radiotherapy alone or in combination with chemotherapy for locally advanced cervical cancer. Cancer Treat. Rev. 29, 471–488 (2003).
Berkey, F. J. Managing the adverse effects of radiation therapy. Am. Fam. Physician 82, 381–388, 394 (2010).
Morris, L., Do, V., Chard, J. & Brand, A. H. Radiation-induced vaginal stenosis: current perspectives. Int. J. Womens Health 9, 273–279 (2017).
Lin, X. B. et al. The role of intestinal microbiota in development of irinotecan toxicity and in toxicity reduction through dietary fibres in rats. PLoS One 9, e83644 (2014).
Brandi, G. et al. Intestinal microflora and digestive toxicity of irinotecan in mice. Clin. Cancer Res. 12, 1299–1307 (2006).
Secombe, K. R., Coller, J. K., Gibson, R. J., Wardill, H. R. & Bowen, J. M. The bidirectional interaction of the gut microbiome and the innate immune system: Implications for chemotherapy-induced gastrointestinal toxicity. Int. J. Cancer 144, 2365–2376 (2019).
Stringer, A. M. et al. Biomarkers of chemotherapy-induced diarrhoea: a clinical study of intestinal microbiome alterations, inflammation and circulating matrix metalloproteinases. Support. Care Cancer 21, 1843–1852 (2013).
Audeh, M. W. et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer: a proof-of-concept trial. Lancet 376, 245–251 (2010).
Liu, Y., Meng, J. & Wang, G. Risk of selected gastrointestinal toxicities associated with poly (ADP-ribose) polymerase (PARP) inhibitors in the treatment of ovarian cancer: a meta-analysis of published trials. Drug. Des. Devel. Ther. 12, 3013–3019 (2018).
Vida, A., Kardos, G., Kovacs, T., Bodrogi, B. L. & Bai, P. Deletion of poly(ADPribose) polymerase-1 changes the composition of the microbiome in the gut. Mol. Med. Rep. 18, 4335–4341 (2018).
Wallace, B. D. et al. Alleviating cancer drug toxicity by inhibiting a bacterial enzyme. Science 330, 831–835 (2010).
Touchefeu, Y. et al. Systematic review: the role of the gut microbiota in chemotherapy- or radiation-induced gastrointestinal mucositis — current evidence and potential clinical applications. Aliment. Pharmacol. Ther. 40, 409–421 (2014).
Carvalho, R. et al. Gut microbiome modulation during treatment of mucositis with the dairy bacterium Lactococcus lactis and recombinant strain secreting human antimicrobial PAP. Sci. Rep. 8, 15072 (2018).
Acero Brand, F. Z. et al. Severe immune mucositis and esophagitis in metastatic squamous carcinoma of the larynx associated with pembrolizumab. J. Immunother. Cancer 6, 22 (2018).
Bruner, D. W. et al. Vaginal stenosis and sexual function following intracavitary radiation for the treatment of cervical and endometrial carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 27, 825–830 (1993).
Mac Bride, M. B., Rhodes, D. J. & Shuster, L. T. Vulvovaginal atrophy. Mayo Clin. Proc. 85, 87–94 (2010).
Stahl, J. M. et al. Extended duration of dilator use beyond 1 year may reduce vaginal stenosis after intravaginal high-dose-rate brachytherapy. Support. Care Cancer 27, 1425–1433 (2019).
Decruze, S. B., Guthrie, D. & Magnani, R. Prevention of vaginal stenosis in patients following vaginal brachytherapy. Clin. Oncol. 11, 46–48 (1999).
Bai, J., Jhaney, I., Daniel, G. & Watkins Bruner, D. Pilot study of vaginal microbiome using QIIME 2 in women with gynecologic cancer before and after radiation therapy. Oncol. Nurs. Forum 46, E48–E59 (2019).
Colman, R. J. & Rubin, D. T. Fecal microbiota transplantation as therapy for inflammatory bowel disease: a systematic review and meta-analysis. J. Crohns Colitis 8, 1569–1581 (2014).
Gough, E., Shaikh, H. & Manges, A. R. Systematic review of intestinal microbiota transplantation (fecal bacteriotherapy) for recurrent Clostridium difficile infection. Clin. Infect. Dis. 53, 994–1002 (2011).
Weiman, S. Harnessing the power of microbes as therapeutics: bugs as drugs. Report on an American Academy of Microbiology Colloquium held in San Diego, CA, in April 2014 (ed. Fox J.) (American Society for Microbiology, 2015).
Biancheri, P., Divekar, D. & Watson, A. J. M. Could fecal transplantation become part of PD-1-based immunotherapy, due to effects of the intestinal microbiome? Gastroenterology 154, 1845–1847 (2018).
Wang, Y., Ma, R., Liu, F., Lee, S. A. & Zhang, L. Modulation of gut microbiota: a novel paradigm of enhancing the efficacy of programmed death-1 and programmed death ligand-1 blockade therapy. Front. Immunol. 9, 374 (2018).
Alang, N. & Kelly, C. R. Weight gain after fecal microbiota transplantation. Open Forum Infect. Dis. 2, ofv004 (2015).
Weingarden, A. R. et al. Microbiota transplantation restores normal fecal bile acid composition in recurrent Clostridium difficile infection. Am. J. Physiol. Gastrointest. Liver Physiol. 306, G310–G319 (2014).
Cui, M. et al. Faecal microbiota transplantation protects against radiation-induced toxicity. EMBO Mol. Med. 9, 448–461 (2017).
Hefazi, M. et al. Safety and efficacy of fecal microbiota transplant for recurrent Clostridium difficile infection in patients with cancer treated with cytotoxic chemotherapy: a single-institution retrospective case series. Mayo Clin. Proc. 92, 1617–1624 (2017).
Wardill, H. R., Secombe, K. R., Bryant, R. V., Hazenberg, M. D. & Costello, S. P. Adjunctive fecal microbiota transplantation in supportive oncology: emerging indications and considerations in immunocompromised patients. EBioMedicine 44, 730–740 (2019).
Javurek, A. B. et al. Effects of exposure to bisphenol A and ethinyl estradiol on the gut microbiota of parents and their offspring in a rodent model. Gut Microbes 7, 471–485 (2016).
van Baarlen, P., Wells, J. M. & Kleerebezem, M. Regulation of intestinal homeostasis and immunity with probiotic lactobacilli. Trends Immunol. 34, 208–215 (2013).
Ngugi, B. M. et al. Effects of bacterial vaginosis-associated bacteria and sexual intercourse on vaginal colonization with the probiotic Lactobacillus crispatus CTV-05. Sex. Transm. Dis. 38, 1020–1027 (2011).
Hemmerling, A. et al. Phase 2a study assessing colonization efficiency, safety, and acceptability of Lactobacillus crispatus CTV-05 in women with bacterial vaginosis. Sex. Transm. Dis. 37, 745–750 (2010).
Stapleton, A. E. et al. Randomized, placebo-controlled phase 2 trial of a Lactobacillus crispatus probiotic given intravaginally for prevention of recurrent urinary tract infection. Clin. Infect. Dis. 52, 1212–1217 (2011).
Marrazzo, J. M. et al. Safety and efficacy of a novel vaginal anti-infective, TOL-463, in the treatment of bacterial vaginosis and vulvovaginal candidiasis: a randomized, single-blind, phase 2, controlled trial. Clin. Infect. Dis. 68, 803–809 (2019).
Chavoustie, S. E., Gersten, J. K., Samuel, M. J. & Schwebke, J. R. A phase 3, multicenter, prospective, open-label study to evaluate the safety of a single dose of secnidazole 2 g for the treatment of women and postmenarchal adolescent girls with bacterial vaginosis. J. Womens Health 27, 492–497 (2018).
Lev-Sagie, A. et al. Vaginal microbiome transplantation in women with intractable bacterial vaginosis. Nat. Med. 25, 1500–1504 (2019).
DeLong, K. et al. Conceptual design of a universal donor screening approach for vaginal microbiota transplant. Front. Cell Infect. Microbiol. 9, 306 (2019).
Scott, A. J. et al. International Cancer Microbiome Consortium consensus statement on the role of the human microbiome in carcinogenesis. Gut 68, 1624–1632 (2019).
Lokken, E. M. et al. Association between vaginal washing and detection of Lactobacillus by culture and quantitative PCR in HIV-seronegative Kenyan women: a cross-sectional analysis. Sex Transm. Infect. (2019).
Vandeputte, D. et al. Quantitative microbiome profiling links gut community variation to microbial load. Nature 551, 507–511 (2017).
Lengyel, E. et al. Epithelial ovarian cancer experimental models. Oncogene 33, 3619–3633 (2014).
Acknowledgements
We would like to acknowledge our clinical colleagues and past and present members of the Herbst-Kralovetz lab for thoughtful discussions on this topic. P.Ł., Z.E.I. and M.M.H.-K. have been supported by the Mary Kay Foundation Translational Research Grant (no. 017-48), the Valley Research Partnership Grant (no. VRP26), the Flinn Foundation Grant (no. 1974), the Alternatives Research and Development Foundation Grant, and the National Institutes of Health Grants from the National Institute of Allergy and Infectious Diseases (1R15AI113457-01A1) and the National Cancer Institute (NCI) and Office for Research on Women’s Health (P30CA023074 and 2U54CA143924-11).
Author information
Authors and Affiliations
Contributions
All authors contributed to researching data for the article, made substantial contributions to discussions of the content, and wrote and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
M.M.H.-K. has been a consultant for Lupin Pharmaceuticals and Beckton Dickinson. P.Ł. and Z.E.I. declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
ClinicalTrials.gov: https://www.clinicaltrials.gov
Glossary
- Microbiota
-
A community of microorganisms in a particular environment.
- Microbiome
-
The entire habitat, which includes microorganisms, their genomes, and the surrounding environment.
- Metagenome
-
The collection of genomes and genes from the members of a microbial community.
- Pathobionts
-
Resident microorganisms with pathogenic potential, harmless to the host under normal conditions.
- Osmolality
-
A concentration of osmotic solution expressed as the number of solute particles in 1kg of solvent.
- Microbial culturomics
-
An approach to identifying unknown bacteria that inhabit the human body utilizing bacterial culture techniques to provide unique insights into host–bacteria relationships.
- Metagenomics
-
An approach to characterizing microbial communities at genome and gene level without requiring culturing.
- Oestrobolome
-
The collection of microorganisms (and their genes) that are able to metabolize oestrogens.
- Metabolomic studies
-
Studies of small molecules (metabolites), which are substrates, intermediates and products of metabolism within microorganisms, cells, tissues or body fluids.
- Metabolomes
-
The collections of small molecules (metabolites) and interactions among these molecules within a biological system.
- Faecal microbiota transplantation
-
(FMT). A process of transplantation of faecal material from a healthy individual to a recipient for restoration of the gut microbiota.
- Probiotics
-
Live microorganisms that confer a health benefit on the host when taken as a dietary supplement in adequate amounts.
- Biofilm
-
An assemblage of microbial cells that form on and coat various surfaces.
- Vaginal microbiota transplantation
-
(VMT). A process of transplantation of vaginal secretions from a healthy individual to a recipient for restoration of the vaginal microbiota.
- Microbiomics
-
The study of microbial communities inhabiting a particular environment (for example, the human body).
Rights and permissions
About this article
Cite this article
Łaniewski, P., Ilhan, Z.E. & Herbst-Kralovetz, M.M. The microbiome and gynaecological cancer development, prevention and therapy. Nat Rev Urol 17, 232–250 (2020). https://doi.org/10.1038/s41585-020-0286-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41585-020-0286-z
This article is cited by
-
Effect of stress urinary incontinence on vaginal microbial communities
BMC Microbiology (2024)
-
Changes in microbial composition and interaction patterns of female urogenital tract and rectum in response to HPV infection
Journal of Translational Medicine (2024)
-
Metabolic profiles outperform the microbiota in assessing the response of vaginal microenvironments to the changed state of HPV infection
npj Biofilms and Microbiomes (2024)
-
Vaginal microbiomes of breast cancer survivors treated with aromatase inhibitors with and without vulvovaginal symptoms
Scientific Reports (2024)
-
The Vaginal Microbiota, Human Papillomavirus Infection, and Cervical Carcinogenesis: A Systematic Review in the Latina Population
Journal of Epidemiology and Global Health (2024)