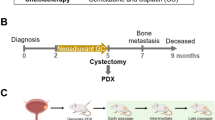
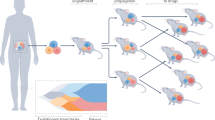
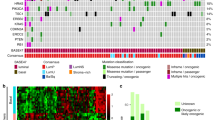
Abstract
Preclinical knowledge of dysregulated pathways and potential biomarkers for urological cancers has undergone limited translation into the clinic. Moreover, the low approval rate of new anticancer drugs and the heterogeneous drug responses in patients indicate that current preclinical models do not always reflect the complexity of malignant disease. Patient-derived tumour models used in preclinical uro-oncology research include 3D culture systems, organotypic tissue slices and patient-derived xenograft models. Technological innovations have enabled major improvements in the capacity of these tumour models to reproduce the clinical complexity of urological cancers. Each type of patient-derived model has inherent advantages and limitations that can be exploited, either alone or in combination, to gather specific knowledge on clinical challenges and address unmet clinical needs. Nevertheless, few opportunities exist for patients with urological cancers to benefit from personalized therapeutic approaches. Clinical validation of experimental data is needed to facilitate the translation and implementation of preclinical knowledge into treatment decision making.
Key points
-
Personalized therapeutic approaches currently have limited use in uro-oncology clinics.
-
Discrepancies between preclinical data and clinical outcomes, high drug attrition rates and heterogeneous drug responses indicate the need for additional clinically relevant patient-derived tumour models including 3D cultures, organotypic tissue slices and patient-derived xenograft models.
-
Each patient-derived model has advantages and limitations and can be used alone or in combination to gather knowledge on clinical challenges in uro-oncology.
-
Co-clinical trials and cross-validation of preclinical results with patient outcomes are expected to advance the implementation of patient-derived models in treatment decision-making.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Dy, G. W., Gore, J. L., Forouzanfar, M. H., Naghavi, M. & Fitzmaurice, C. Global burden of urologic cancers, 1990–2013. Eur. Urol. 71, 437–446 (2017).
Bray, F. et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 68, 394–424 (2018).
Spratlin, J. L., Serkova, N. J. & Eckhardt, S. G. Clinical applications of metabolomics in oncology: a review. Clin. Cancer Res. 15, 431–440 (2009).
Zhang, A., Yan, G., Han, Y. & Wang, X. Metabolomics approaches and applications in prostate cancer research. Appl. Biochem. Biotechnol. 174, 6–12 (2014).
Yadav, S. S., Li, J., Lavery, H. J., Yadav, K. K. & Tewari, A. K. Next-generation sequencing technology in prostate cancer diagnosis, prognosis, and personalized treatment. Urol. Oncol. 33, 267.e1–e13 (2015).
Mottet, N. et al. EAU-ESTRO-SIOG guidelines on prostate cancer. Part 1: screening, diagnosis, and local treatment with curative intent. Eur. Urol. 71, 618–629 (2017).
Lowrance, W. et al. Adavanced prostate cancer: AUA/ASTRO/SUO guideline. AUA https://www.auanet.org/guidelines/advanced-prostate-cancer (2020).
Yossepowitch, O. Digital rectal examination remains an important screening tool for prostate cancer. Eur. Urol. 54, 483–484 (2008).
Hessels, D. et al. DD3(PCA3)-based molecular urine analysis for the diagnosis of prostate cancer. Eur. Urol. 44, 8–15 (2003).
Deras, I. L. et al. PCA3: a molecular urine assay for predicting prostate biopsy outcome. J. Urol. 179, 1587–1592 (2008).
Nakanishi, H. et al. PCA3 molecular urine assay correlates with prostate cancer tumor volume: implication in selecting candidates for active surveillance. J. Urol. 179, 1804–1809; discussion 1809–1810 (2008).
Hessels, D. et al. Predictive value of PCA3 in urinary sediments in determining clinico-pathological characteristics of prostate cancer. Prostate 70, 10–16 (2010).
Van Neste, L. et al. Detection of high-grade prostate cancer using a urinary molecular biomarker-based risk score. Eur. Urol. 70, 740–748 (2016).
McKiernan, J. et al. A novel urine exosome gene expression assay to predict high-grade prostate cancer at initial biopsy. JAMA Oncol. 2, 882–889 (2016).
Oeyen, E. et al. Bladder cancer diagnosis and follow-up: the current status and possible role of extracellular vesicles. Int. J. Mol. Sci. 20, 821 (2019).
Ljungberg, B. et al. EAU guidelines on renal cell carcinoma: 2014 update. Eur. Urol. 67, 913–924 (2015).
Pastore, A. L. et al. Serum and urine biomarkers for human renal cell carcinoma. Dis. Markers 2015, 251403 (2015).
Scelo, G. et al. KIM-1 as a blood-based marker for early detection of kidney cancer: a prospective nested case-control study. Clin. Cancer Res. 24, 5594–5601 (2018).
Sim, S. H. et al. Prognostic utility of pre-operative circulating osteopontin, carbonic anhydrase IX and CRP in renal cell carcinoma. Br. J. Cancer 107, 1131–1137 (2012).
Mak, I. W., Evaniew, N. & Ghert, M. Lost in translation: animal models and clinical trials in cancer treatment. Am. J. Transl. Res. 6, 114–118 (2014).
Box, G.E.P. & Draper, N. R. Empirical Model-Building and Response Surfaces (Wiley, 1987).
Gheibi, P. et al. Microchamber cultures of bladder cancer: a platform for characterizing drug responsiveness and resistance in PDX and primary cancer cells. Sci. Rep. 7, 12277 (2017).
Fan, Q. et al. A novel 3-D bio-microfluidic system mimicking in vivo heterogeneous tumour microstructures reveals complex tumour-stroma interactions. Lab Chip 17, 2852–2860 (2017).
Baudoin, R., Griscom, L., Monge, M., Legallais, C. & Leclerc, E. Development of a renal microchip for in vitro distal tubule models. Biotechnol. Prog. 23, 1245–1253 (2007).
Kettunen, K. et al. Personalized drug sensitivity screening for bladder cancer using conditionally reprogrammed patient-derived cells. Eur. Urol. 76, 430–434 (2019).
Timofeeva, O. A. et al. Conditionally reprogrammed normal and primary tumor prostate epithelial cells: a novel patient-derived cell model for studies of human prostate cancer. Oncotarget 8, 22741–22758 (2017).
Saeed, K. et al. Comprehensive drug testing of patient-derived conditionally reprogrammed cells from castration-resistant prostate cancer. Eur. Urol. 71, 319–327 (2017).
Redekop, W. K. & Mladsi, D. The faces of personalized medicine: a framework for understanding its meaning and scope. Value Health 16, S4–S9 (2013).
Tomlins, S. A. et al. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science 310, 644–648 (2005).
Sibley, K., Cuthbert-Heavens, D. & Knowles, M. A. Loss of heterozygosity at 4p16.3 and mutation of FGFR3 in transitional cell carcinoma. Oncogene 20, 686–691 (2001).
Ledford, H. Translational research: 4 ways to fix the clinical trial. Nature 477, 526–528 (2011).
Attarwala, H. TGN1412: from discovery to disaster. J. Young Pharm. 2, 332–336 (2010).
Ogi, C. & Aruga, A. Immunological monitoring of anticancer vaccines in clinical trials. Oncoimmunology 2, e26012 (2013).
Hutchinson, L. & Kirk, R. High drug attrition rates — where are we going wrong? Nat. Rev. Clin. Oncol. 8, 189–190 (2011).
Arrowsmith, J. Trial watch: phase III and submission failures: 2007–2010. Nat. Rev. Drug Discov. 10, 87 (2011).
CenterWatch. FDA approved drugs. CenterWatch https://www.centerwatch.com/directories/1067 (2020).
Petrylak, D. P. Practical guide to the use of chemotherapy in castration resistant prostate cancer. Can. J. Urol. 21, 77–83 (2014).
Weiswald, L. B., Bellet, D. & Dangles-Marie, V. Spherical cancer models in tumor biology. Neoplasia 17, 1–15 (2015).
Karthaus, W. R. et al. Identification of multipotent luminal progenitor cells in human prostate organoid cultures. Cell 159, 163–175 (2014).
Chua, C. W. et al. Single luminal epithelial progenitors can generate prostate organoids in culture. Nat. Cell Biol. 16, 951–954 (2014).
Grassi, L. et al. Organoids as a new model for improving regenerative medicine and cancer personalized therapy in renal diseases. Cell Death Dis. 10, 201 (2019).
Lee, S. H. et al. Tumor evolution and drug response in patient-derived organoid models of bladder cancer. Cell 173, 515–528.e17 (2018).
Drost, J. et al. Organoid culture systems for prostate epithelial and cancer tissue. Nat. Protoc. 11, 347–358 (2016).
Mullenders, J. et al. Mouse and human urothelial cancer organoids: a tool for bladder cancer research. Proc. Natl Acad. Sci. USA 116, 4567–4574 (2019).
Puca, L. et al. Patient derived organoids to model rare prostate cancer phenotypes. Nat. Commun. 9, 2404 (2018).
Gao, D. et al. Organoid cultures derived from patients with advanced prostate cancer. Cell 159, 176–187 (2014).
Ma, L. et al. Organoid culture of human prostate cancer cell lines LNCaP and C4-2B. Am. J. Clin. Exp. Urol. 5, 25–33 (2017).
Beshiri, M. L. et al. A PDX/organoid biobank of advanced prostate cancers captures genomic and phenotypic heterogeneity for disease modeling and therapeutic screening. Clin. Cancer Res. 24, 4332–4345 (2018).
Gao, D. & Chen, Y. Organoid development in cancer genome discovery. Curr. Opin. Genet. Dev. 30, 42–48 (2015).
Shu, Y. & Chua, C. W. An organoid assay for long-term maintenance and propagation of mouse prostate luminal epithelial progenitors and cancer cells. Methods Mol. Biol. 1940, 231–254 (2019).
Wang, S., Gao, D. & Chen, Y. The potential of organoids in urological cancer research. Nat. Rev. Urol. 14, 401–414 (2017).
Gleave, A. M., Ci, X., Lin, D. & Wang, Y. A synopsis of prostate organoid methodologies, applications, and limitations. Prostate 80, 518–526 (2020).
Hribar, K. C. et al. A simple three-dimensional hydrogel platform enables ex vivo cell culture of patient and PDX tumors for assaying their response to clinically relevant therapies. Mol. Cancer Ther. 18, 718–725 (2019).
Fong, E. L. et al. Hydrogel-based 3D model of patient-derived prostate xenograft tumors suitable for drug screening. Mol. Pharm. 11, 2040–2050 (2014).
Fong, E. L. et al. A 3D in vitro model of patient-derived prostate cancer xenograft for controlled interrogation of in vivo tumor-stromal interactions. Biomaterials 77, 164–172 (2016).
Sachs, N. & Clevers, H. Organoid cultures for the analysis of cancer phenotypes. Curr. Opin. Genet. Dev. 24, 68–73 (2014).
Bleijs, M., van de Wetering, M., Clevers, H. & Drost, J. Xenograft and organoid model systems in cancer research. EMBO J. 38, e101654 (2019).
van de Wetering, M. et al. Prospective derivation of a living organoid biobank of colorectal cancer patients. Cell 161, 933–945 (2015).
Conteduca, V. et al. Clinical features of neuroendocrine prostate cancer. Eur. J. Cancer 121, 7–18 (2019).
Pauli, C. et al. Personalized in vitro and in vivo cancer models to guide precision medicine. Cancer Discov. 7, 462–477 (2017).
Ben-David, U., Beroukhim, R. & Golub, T. R. Genomic evolution of cancer models: perils and opportunities. Nat. Rev. Cancer 19, 97–109 (2019).
Galletti, G., Leach, B. I., Lam, L. & Tagawa, S. T. Mechanisms of resistance to systemic therapy in metastatic castration-resistant prostate cancer. Cancer Treat. Rev. 57, 16–27 (2017).
Li, Y. et al. Diverse AR gene rearrangements mediate resistance to androgen receptor inhibitors in metastatic prostate cancer. Clin. Cancer Res. 26, 1965–1976 (2020).
Eder, T. et al. Cancer-associated fibroblasts modify the response of prostate cancer cells to androgen and anti-androgens in three-dimensional spheroid culture. Int. J. Mol. Sci. 17, 1458 (2016).
Chambers, K. F., Mosaad, E. M., Russell, P. J., Clements, J. A. & Doran, M. R. 3D Cultures of prostate cancer cells cultured in a novel high-throughput culture platform are more resistant to chemotherapeutics compared to cells cultured in monolayer. PLoS One 9, e111029 (2014).
Mosaad, E., Chambers, K., Futrega, K., Clements, J. & Doran, M. R. Using high throughput microtissue culture to study the difference in prostate cancer cell behavior and drug response in 2D and 3D co-cultures. BMC Cancer 18, 592 (2018).
Frankel, A., Man, S., Elliott, P., Adams, J. & Kerbel, R. S. Lack of multicellular drug resistance observed in human ovarian and prostate carcinoma treated with the proteasome inhibitor PS-341. Clin. Cancer Res. 6, 3719–3728 (2000).
Harma, V. et al. A comprehensive panel of three-dimensional models for studies of prostate cancer growth, invasion and drug responses. PLoS One 5, e10431 (2010).
Richards, Z. et al. Prostate stroma increases the viability and maintains the branching phenotype of human prostate organoids. iScience 12, 304–317 (2019).
Ramamoorthy, P. et al. Metastatic tumor-in-a-dish, a novel multicellular organoid to study lung colonization and predict therapeutic response. Cancer Res. 79, 1681–1695 (2019).
Clohessy, J. G. & Pandolfi, P. P. Mouse hospital and co-clinical trial project — from bench to bedside. Nat. Rev. Clin. Oncol. 12, 491–498 (2015).
Nardella, C., Lunardi, A., Patnaik, A., Cantley, L. C. & Pandolfi, P. P. The APL paradigm and the “co-clinical trial” project. Cancer Discov. 1, 108–116 (2011).
Centenera, M. M., Raj, G. V., Knudsen, K. E., Tilley, W. D. & Butler, L. M. Ex vivo culture of human prostate tissue and drug development. Nat. Rev. Urol. 10, 483–487 (2013).
van de Merbel, A. F. et al. An ex vivo tissue culture model for the assessment of individualized drug responses in prostate and bladder cancer. Front. Oncol. 8, 400 (2018).
Annels, N. E. et al. Oncolytic immunotherapy for bladder cancer using coxsackie A21 virus. Mol. Ther. Oncolytics 9, 1–12 (2018).
Zhang, W. et al. Ex vivo treatment of prostate tumor tissue recapitulates in vivo therapy response. Prostate 79, 390–402 (2018).
Davies, E. J. et al. Capturing complex tumour biology in vitro: histological and molecular characterisation of precision cut slices. Sci. Rep. 5, 17187 (2015).
Maund, S. L., Nolley, R. & Peehl, D. M. Optimization and comprehensive characterization of a faithful tissue culture model of the benign and malignant human prostate. Lab. Invest. 94, 208–221 (2014).
Shafi, A. A. et al. Patient-derived models reveal impact of the tumor microenvironment on therapeutic response. Eur. Urol. Oncol. 1, 325–337 (2018).
Varani, J., Dame, M. K., Wojno, K., Schuger, L. & Johnson, K. J. Characteristics of nonmalignant and malignant human prostate in organ culture. Lab. Invest. 79, 723–731 (1999).
Parrish, A. R. et al. Culturing precision-cut human prostate slices as an in vitro model of prostate pathobiology. Cell Biol. Toxicol. 18, 205–219 (2002).
Fleck, C. et al. Ex vivo stimulation of renal tubular p-aminohippurate transport by dexamethasone and triiodothyronine in human renal cell carcinoma. Urol. Res. 28, 383–390 (2000).
Vickers, A. E. et al. Kidney slices of human and rat to characterize cisplatin-induced injury on cellular pathways and morphology. Toxicol. Pathol. 32, 577–590 (2004).
van der Horst, G. et al. Cationic amphiphilic drugs as potential anti-cancer therapy for bladder cancer. Mol. Oncol. https://doi.org/10.1002/1878-0261.12793 (2020).
Centenera, M. M. et al. A patient-derived explant (PDE) model of hormone-dependent cancer. Mol. Oncol. 12, 1608–1622 (2018).
Jiang, X., Seo, Y. D., Sullivan, K. M. & Pillarisetty, V. G. Establishment of slice cultures as a tool to study the cancer immune microenvironment. Methods Mol. Biol. 1884, 283–295 (2019).
Lim, C. Y. et al. Organotypic slice cultures of pancreatic ductal adenocarcinoma preserve the tumor microenvironment and provide a platform for drug response. Pancreatology 18, 913–927 (2018).
Ozdemir, B. C. et al. The molecular signature of the stroma response in prostate cancer-induced osteoblastic bone metastasis highlights expansion of hematopoietic and prostate epithelial stem cell niches. PLoS One 9, e114530 (2014).
van der Horst, G., Bos, L. & van der Pluijm, G. Epithelial plasticity, cancer stem cells, and the tumor-supportive stroma in bladder carcinoma. Mol. Cancer Res. 10, 995–1009 (2012).
Voorwerk, L. et al. Immune induction strategies in metastatic triple-negative breast cancer to enhance the sensitivity to PD-1 blockade: the TONIC trial. Nat. Med. 25, 920–928 (2019).
Majumder, B. et al. Predicting clinical response to anticancer drugs using an ex vivo platform that captures tumour heterogeneity. Nat. Commun. 6, 6169 (2015).
Malaney, P., Nicosia, S. V. & Davé, V. One mouse, one patient paradigm: new avatars of personalized cancer therapy. Cancer Lett. 344, 1–12 (2014).
Davies, A. H., Wang, Y. & Zoubeidi, A. Patient-derived xenografts: a platform for accelerating translational research in prostate cancer. Mol. Cell. Endocrinol. 462, 17–24 (2018).
Gao, H. et al. High-throughput screening using patient-derived tumor xenografts to predict clinical trial drug response. Nat. Med. 21, 1318–1325 (2015).
Okada, S., Vaeteewoottacharn, K. & Kariya, R. Application of highly immunocompromised mice for the establishment of patient-derived xenograft (PDX) models. Cells 8, 889 (2019).
Lawrence, M. G. et al. Establishment of primary patient-derived xenografts of palliative TURP specimens to study castrate-resistant prostate cancer. Prostate 75, 1475–1483 (2015).
Russell, P. J. et al. Establishing prostate cancer patient derived xenografts: lessons learned from older studies. Prostate 75, 628–636 (2015).
Wang, Y. et al. Development and characterization of efficient xenograft models for benign and malignant human prostate tissue. Prostate 64, 149–159 (2005).
Brennen, W. N. & Isaacs, J. T. The what, when, and why of human prostate cancer xenografts. Prostate 78, 646–654 (2018).
van Weerden, W. M. et al. Development of seven new human prostate tumor xenograft models and their histopathological characterization. Am. J. Pathol. 149, 1055–1062 (1996).
van Weerden, W. M. & Romijn, J. C. Use of nude mouse xenograft models in prostate cancer research. Prostate 43, 263–271 (2000).
Namekawa, T., Ikeda, K., Horie-Inoue, K. & Inoue, S. Application of prostate cancer models for preclinical study: advantages and limitations of cell lines, patient-derived xenografts, and three-dimensional culture of patient-derived cells. Cells 8, 74 (2019).
Navone, N. M. et al. Movember GAP1 PDX project: an international collection of serially transplantable prostate cancer patient-derived xenograft (PDX) models. Prostate 78, 1262–1282 (2018).
Patel, A. et al. Patient-derived xenograft models to optimize kidney cancer therapies. Transl. Androl. Urol. 8, S156–S165 (2019).
Lang, H. et al. Establishment of a large panel of patient-derived preclinical models of human renal cell carcinoma. Oncotarget 7, 59336–59359 (2016).
Bernardo, C., Costa, C., Sousa, N., Amado, F. & Santos, L. Patient-derived bladder cancer xenografts: a systematic review. Transl. Res. 166, 324–331 (2015).
Castillo-Avila, W. et al. Sunitinib inhibits tumor growth and synergizes with cisplatin in orthotopic models of cisplatin-sensitive and cisplatin-resistant human testicular germ cell tumors. Clin. Cancer Res. 15, 3384–3395 (2009).
Lam, H. M. et al. Durable response of enzalutamide-resistant prostate cancer to supraphysiological testosterone is associated with a multifaceted growth suppression and impaired DNA damage response transcriptomic program in patient-derived xenografts. Eur. Urol. 77, 144–155 (2019).
Zeng, S. X. et al. The phosphatidylinositol 3-kinase pathway as a potential therapeutic target in bladder cancer. Clin. Cancer Res. 23, 6580–6591 (2017).
Pan, C. X. et al. Development and characterization of bladder cancer patient-derived xenografts for molecularly guided targeted therapy. PLoS One 10, e0134346 (2015).
Lange, T. et al. Development and characterization of a spontaneously metastatic patient-derived xenograft model of human prostate cancer. Sci. Rep. 8, 17535 (2018).
Thong, A. E. et al. Tissue slice grafts of human renal cell carcinoma: an authentic preclinical model with high engraftment rate and metastatic potential. Urol. Oncol. 32, 43.e23–30 (2014).
Valta, M. P. et al. Development of a realistic in vivo bone metastasis model of human renal cell carcinoma. Clin. Exp. Metastasis 31, 573–584 (2014).
Schneeberger, V. E., Allaj, V., Gardner, E. E., Poirier, J. T. & Rudin, C. M. Quantitation of murine stroma and selective purification of the human tumor component of patient-derived xenografts for genomic analysis. PLoS One 11, e0160587 (2016).
Mestas, J. & Hughes, C. C. Of mice and not men: differences between mouse and human immunology. J. Immunol. 172, 2731–2738 (2004).
Rickinson, A. & Kieff, E. in Fields Virology 5th edn (ed. Knipe, D. M. & Howley, P.M.) 2655–2700 (Lippincott Williams & Wilkins, 2001).
Wetterauer, C. et al. Early development of human lymphomas in a prostate cancer xenograft program using triple knock-out immunocompromised mice. Prostate 75, 585–592 (2015).
Taurozzi, A. J. et al. Spontaneous development of Epstein-Barr Virus associated human lymphomas in a prostate cancer xenograft program. PLoS One 12, e0188228 (2017).
Williams, A. P. et al. Corruption of neuroblastoma patient derived xenografts with human T cell lymphoma. J. Pediatr. Surg. 54, 2117–2119 (2018).
Bondarenko, G. et al. Patient-derived tumor xenografts are susceptible to formation of human lymphocytic tumors. Neoplasia 17, 735–741 (2015).
Choi, Y. Y. et al. Establishment and characterisation of patient-derived xenografts as paraclinical models for gastric cancer. Sci. Rep. 6, 22172 (2016).
Fujii, E. et al. Characterization of EBV-related lymphoproliferative lesions arising in donor lymphocytes of transplanted human tumor tissues in the NOG mouse. Exp. Anim. 63, 289–296 (2014).
Kalavska, K. et al. Lymphoma transformation of tumor infiltrating lymphocytes observed in testicular patient-derived xenograft models. Oncol. Rep. 40, 3593–3602 (2018).
Yao, L. C. et al. Creation of PDX-bearing humanized mice to study immuno-oncology. Methods Mol. Biol. 1953, 241–252 (2019).
Capasso, A. et al. Characterization of immune responses to anti-PD-1 mono and combination immunotherapy in hematopoietic humanized mice implanted with tumor xenografts. J. Immunother. Cancer 7, 37 (2019).
Lin, S. et al. Establishment of peripheral blood mononuclear cell-derived humanized lung cancer mouse models for studying efficacy of PD-L1/PD-1 targeted immunotherapy. mAbs 10, 1301–1311 (2018).
Wang, M. et al. Humanized mice in studying efficacy and mechanisms of PD-1-targeted cancer immunotherapy. FASEB J. 32, 1537–1549 (2018).
Williams, J. A. Using PDX for preclinical cancer drug discovery: the evolving field. J. Clin. Med. 7, 41 (2018).
Hidalgo, M. et al. A pilot clinical study of treatment guided by personalized tumorgrafts in patients with advanced cancer. Mol. Cancer Ther. 10, 1311–1316 (2011).
Koga, Y. & Ochiai, A. Systematic review of patient-derived xenograft models for preclinical studies of anti-cancer drugs in solid tumors. Cells 8, 418 (2019).
Sia, D., Moeini, A., Labgaa, I. & Villanueva, A. The future of patient-derived tumor xenografts in cancer treatment. Pharmacogenomics 16, 1671–1683 (2015).
Clohessy, J. G. & Pandolfi, P. P. The mouse hospital and its integration in ultra-precision approaches to cancer care. Front. Oncol. 8, 340 (2018).
Vlachogiannis, G. et al. Patient-derived organoids model treatment response of metastatic gastrointestinal cancers. Science 359, 920–926 (2018).
Beltran, H. et al. A phase II trial of the aurora kinase A inhibitor alisertib for patients with castration-resistant and neuroendocrine prostate cancer: efficacy and biomarkers. Clin. Cancer Res. 25, 43–51 (2019).
Dong, Y. et al. Tumor xenografts of human clear cell renal cell carcinoma but not corresponding cell lines recapitulate clinical response to sunitinib: feasibility of using biopsy samples. Eur. Urol. Focus 3, 590–598 (2017).
Acknowledgements
The authors’ research work is supported by a personalized medicine grant from the Dutch Cancer Society (KWF) and Alpe D’Huzes (UL2014-7058) (to A.F.v.d.M., G.v.d.H. and G.v.d.P.).
Author information
Authors and Affiliations
Contributions
A.F.v.d.M researched data for the manuscript, A.F.v.d.M, G.v.d.H and G.v.d.P. wrote the manuscript, and all authors made substantial contributions to discussions of content and reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Urology thanks C.-X. Pan and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Jackson Laboratory: https://www.jax.org
National Cancer Institute Patient-Derived Models Repository: https://pdmr.cancer.gov
Supplementary information
Rights and permissions
About this article
Cite this article
van de Merbel, A.F., van der Horst, G. & van der Pluijm, G. Patient-derived tumour models for personalized therapeutics in urological cancers. Nat Rev Urol 18, 33–45 (2021). https://doi.org/10.1038/s41585-020-00389-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41585-020-00389-2
This article is cited by
-
New advances of the androgen receptor in prostate cancer: report from the 1st International Androgen Receptor Symposium
Journal of Translational Medicine (2024)
-
Preclinical models of prostate cancer — modelling androgen dependency and castration resistance in vitro, ex vivo and in vivo
Nature Reviews Urology (2023)
-
AF9 targets acetyl-modified STAT6 to diminish purine metabolism and accelerate cell apoptosis during metastasis
Cell Death & Differentiation (2023)
-
The future of patient-derived xenografts in prostate cancer research
Nature Reviews Urology (2023)
-
Patient-derived tumor models: a suitable tool for preclinical studies on esophageal cancer
Cancer Gene Therapy (2023)