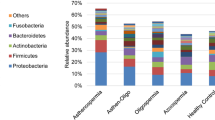
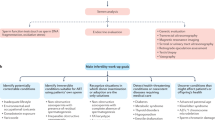
Abstract
Owing to the fact that there are more microbial than human cells in our body and that humans contain more microbial than human genes, the microbiome has huge potential to influence human physiology, both in health and in disease. The use of next-generation sequencing technologies has helped to elucidate functional, quantitative and mechanistic aspects of the complex microorganism–host interactions that underlie human physiology and pathophysiology. The microbiome of semen is a field of increasing scientific interest, although this microbial niche is currently understudied compared with other areas of microbiome research. However, emerging evidence is beginning to indicate that the seminal microbiome has important implications for the reproductive health of men, the health of the couple and even the health of offspring, owing to transfer of microorganisms to the partner and offspring. As this field expands, further carefully designed and well-powered studies are required to unravel the true nature and role of the seminal microbiome.
Key points
-
Semen has a unique microbiome; however, its origin and function need to be further investigated in order to understand its role in health and disease.
-
Alterations in the bacterial composition of semen have been linked to a variety of disorders, including subinfertility and poor semen quality, prostatitis and HIV infection.
-
The seminal microbiome might influence a couple’s health and even that of their offspring, as well as affecting pregnancy outcomes.
-
When studying the male seminal microbiome, the partner’s reproductive tract microbiome and the sexual behaviours of both partners should also be considered.
-
Study of the seminal microbiome is still in its infancy, and further well-designed, large-cohort, functional studies are required.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Power, M. L., Quaglieri, C. & Schulkin, J. Reproductive microbiomes. Reprod. Sci. 24, 1482–1492 (2017).
Sender, R., Fuchs, S. & Milo, R. Are we really vastly outnumbered? Revisiting the ratio of bacterial to host cells in humans. Cell 164, 337–340 (2016).
Sender, R., Fuchs, S. & Milo, R. Revised estimates for the number of human and bacteria cells in the body. PLOS Biol. 14, e1002533 (2016).
Grice, E. A. & Segre, J. A. The human microbiome: our second genome. Annu. Rev. Genomics Hum. Genet. 13, 151–170 (2012).
Wang, B., Yao, M., Lv, L., Ling, Z. & Li, L. The human microbiota in health and disease. Engineering 3, 71–82 (2017).
Kroon, S. J., Ravel, J. & Huston, W. M. Cervicovaginal microbiota, women’s health, and reproductive outcomes. Fertil. Steril. 110, 327–336 (2018).
Benner, M., Ferwerda, G., Joosten, I. & van der Molen, R. G. How uterine microbiota might be responsible for a receptive, fertile endometrium. Hum. Reprod. Update 24, 393–415 (2018).
Baker, J. M., Chase, D. M. & Herbst-Kralovetz, M. M. Uterine microbiota: residents, tourists, or invaders? Front. Immunol. 9, 208 (2018).
Altmäe, S. Commentary: uterine microbiota: residents, tourists, or invaders? Front. Immunol. 9, 1874 (2018).
Franasiak, J. M. & Scott, R. T. Endometrial microbiome. Curr. Opin. Obstet. Gynecol. 29, 146–152 (2017).
Moreno, I. & Franasiak, J. M. Endometrial microbiota-new player in town. Fertil. Steril. 108, 32–39 (2017).
Moreno, I. et al. Evidence that the endometrial microbiota has an effect on implantation success or failure. Am. J. Obstet. Gynecol. 215, 684–703 (2016).
Altmäe, S. Uterine microbiota: a role beyond infection. EMJ Reprod. Heal. 6, 70–75 (2018).
Franasiak, J. M. & Scott, R. T. Reproductive tract microbiome in assisted reproductive technologies. Fertil. Steril. 104, 1364–1371 (2015).
Nguyen, P. V., Kafka, J. K., Ferreira, V. H., Roth, K. & Kaushic, C. Innate and adaptive immune responses in male and female reproductive tracts in homeostasis and following HIV infection. Cell. Mol. Immunol. 11, 410–427 (2014).
Weng, S. L. et al. Bacterial communities in semen from men of infertile couples: metagenomic sequencing reveals relationships of seminal microbiota to semen quality. PLOS ONE 9, e110152 (2014).
Chen, H., Luo, T., Chen, T. & Wang, G. Seminal bacterial composition in patients with obstructive and non‑obstructive azoospermia. Exp. Ther. Med. 15, 2884–2890 (2018).
Monteiro, C. et al. Characterization of microbiota in male infertility cases uncovers differences in seminal hyperviscosity and oligoasthenoteratozoospermia possibly correlated with increased prevalence of infectious bacteria. Am. J. Reprod. Immunol. 79, e12838 (2018).
Mändar, R. et al. Seminal microbiome in men with and without prostatitis. Int. J. Urol. 24, 1–6 (2017).
Koedooder, R. et al. Identification and evaluation of the microbiome in the female and male reproductive tract. Hum. Reprod. Update 25, 298–325 (2019).
Liu, C. M. et al. The semen microbiome and its relationship with local immunology and viral load in HIV infection. PLOS Pathog. 10, e1004262 (2014).
Hou, D. et al. Microbiota of the seminal fluid from healthy and infertile men. Fertil. Steril. 100, 1261–1269.e3 (2013).
Baud, D. et al. Sperm microbiota and its impact on semen parameters. Front. Microbiol. 10, 234 (2019).
Mändar, R. et al. Complementary seminovaginal microbiome in couples. Res. Microbiol. 166, 440–447 (2015).
Mändar, R., Türk, S., Korrovits, P., Ausmees, K. & Punab, M. Impact of sexual debut on culturable human seminal microbiota. Andrology 6, 510–512 (2018).
Reece, A. S. Dying for love: perimenopausal degeneration of vaginal microbiome drives the chronic inflammation-malignant transformation of benign prostatic hyperplasia to prostatic adenocarcinoma. Med. Hypotheses 101, 44–47 (2017).
Kjaergaard, N. et al. Pyospermia and preterm, prelabor, rupture of membranes. Acta Obstet. Gynecol. Scand. 76, 528–531 (1997).
Wittemer, C. et al. [Abnormal bacterial colonisation of the vagina and implantation during assisted reproduction]. Gynecol. Obstet. Fertil. 32, 135–139 (2004).
Kenny, L. C. & Kell, D. B. Immunological tolerance, pregnancy, and preeclampsia: the roles of semen microbes and the father. Front. Med. 4, 239 (2018).
Sisti, G., Kanninen, T. T. & Witkin, S. S. Maternal immunity and pregnancy outcome: focus on preconception and autophagy. Genes Immun. 17, 1–7 (2016).
Rando, O. J. & Simmons, R. A. I’m eating for two: parental dietary effects on offspring metabolism. Cell 161, 93–105 (2015).
Ricci, S. et al. Impact of asymptomatic genital tract infections on in vitro fertilization (IVF) outcome. PLOS ONE 13, e0207684 (2018).
Damke, E., Kurscheidt, F. A., Irie, M. M. T., Gimenes, F. & Consolaro, M. E. L. Male partners of infertile couples with seminal positivity for markers of bacterial vaginosis have impaired fertility. Am. J. Mens Health 12, 2104–2115 (2018).
La Vignera, S., Vicari, E., Condorelli, R. A., D’Agata, R. & Calogero, A. E. Male accessory gland infection and sperm parameters (review). Int. J. Androl. 34, e330–e347 (2011).
Merino, G. et al. Bacterial infection and semen characteristics in infertile men. Arch. Androl. 35, 43–47 (1995).
Lacroix, J.-M., Jarvi, K., Batra, S. D., Heritz, D. M. & Mittelmana, M. PCR-based technique for the detection of bacteria in semen and urine. J. Microbiol. Methods 26, 61–71 (1996).
Elsner, P. & Hartmann, A. A. Gardnerella vaginalis in the male upper genital tract: a possible source of reinfection of the female partner. Sex. Transm. Dis. 14, 122–123 (1987).
Ferlin, A. OR15-5: Effects of low sperm count go beyond fertility. Presented at The Endocrine Society Annual Meeting (2018).
Hanson, B. M., Eisenberg, M. L. & Hotaling, J. M. Male infertility: a biomarker of individual and familial cancer risk. Fertil. Steril. 109, 6–19 (2018).
Ferlin, A. et al. Sperm count and hypogonadism as markers of general male health. Eur. Urol. Focus https://doi.org/10.1016/j.euf.2019.08.001 (2019).
Javurek, A. B. et al. Discovery of a novel seminal fluid microbiome and influence of estrogen receptor alpha genetic status. Sci. Rep. 6, 1–14 (2016).
Hanson, H. A. et al. Risk of childhood mortality in family members of men with poor semen quality. Hum. Reprod. 32, 239–247 (2016).
Sermondade, N. et al. BMI in relation to sperm count: an updated systematic review and collaborative meta-analysis. Hum. Reprod. Update 19, 221–231 (2013).
Bieniek, J. M. et al. Influence of increasing body mass index on semen and reproductive hormonal parameters in a multi-institutional cohort of subfertile men. Fertil. Steril. 106, 1070–1075 (2016).
Hart, R. J. et al. Features of the metabolic syndrome in late adolescence are associated with impaired testicular function at 20 years of age. Hum. Reprod. 34, 389–402 (2019).
Younes, J. A. et al. Women and their microbes: the unexpected friendship. Trends Microbiol. 26, 16–32 (2018).
Valles-Colomer, M. et al. The neuroactive potential of the human gut microbiota in quality of life and depression. Nat. Microbiol. 4, 623–632 (2019).
Cryan, J. F. & Dinan, T. G. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat. Rev. Neurosci. 13, 701–712 (2012).
Mohajeri, M. H., La Fata, G., Steinert, R. E. & Weber, P. Relationship between the gut microbiome and brain function. Nutr. Rev. 76, 481–496 (2018).
Winter, G., Hart, R. A., Charlesworth, R. P. G. & Sharpley, C. F. Gut microbiome and depression: what we know and what we need to know. Rev. Neurosci. 29, 629–643 (2018).
Smith, L. K. & Wissel, E. F. Microbes and the mind: how bacteria shape affect, neurological processes, cognition, social relationships, development, and pathology. Perspect. Psychol. Sci. 14, 397–418 (2019).
Takagi, T. et al. Differences in gut microbiota associated with age, sex, and stool consistency in healthy Japanese subjects. J. Gastroenterol. 54, 53–63 (2018).
Hopkins, M. J., Sharp, R. & Macfarlane, G. T. Age and disease related changes in intestinal bacterial populations assessed by cell culture, 16S rRNA abundance, and community cellular fatty acid profiles. Gut 48, 198–205 (2001).
He, Y. et al. Regional variation limits applications of healthy gut microbiome reference ranges and disease models. Nat. Med. 24, 1532–1535 (2018).
Deschasaux, M. et al. Depicting the composition of gut microbiota in a population with varied ethnic origins but shared geography. Nat. Med. 24, 1526–1531 (2018).
Goodrich, J. K. et al. Human genetics shape the gut microbiome. Cell 159, 789–799 (2014).
Dominianni, C. et al. Sex, body mass index, and dietary fiber intake influence the human gut microbiome. PLOS ONE 10, e0124599 (2015).
Postler, T. S. & Ghosh, S. Understanding the holobiont: how microbial metabolites affect human health and shape the immune system. Cell Metab. 26, 110–130 (2017).
Goodrich, J. K. et al. Conducting a microbiome study. Cell 158, 250–262 (2014).
Suez, J. & Elinav, E. The path towards microbiome-based metabolite treatment. Nat. Microbiol. 2, 17075 (2017).
Zeevi, D. et al. Personalized nutrition by prediction of glycemic responses. Cell 163, 1079–1094 (2015).
Lynch, S. V. & Pedersen, O. The human intestinal microbiome in health and disease. N. Engl. J. Med. 375, 2369–2379 (2016).
Price, L. B. et al. The effects of circumcision on the penis microbiome. PLOS ONE 5, e8422 (2010).
Vodstrcil, L. A. et al. The influence of sexual activity on the vaginal microbiota and Gardnerella vaginalis clade diversity in young women. PLOS ONE 12, e0171856 (2017).
Javurek, A. B. et al. Consumption of a high-fat diet alters the seminal fluid and gut microbiomes in male mice. Reprod. Fertil. Dev. 29, 1602–1612 (2017).
Costea, P. I. et al. Towards standards for human fecal sample processing in metagenomic studies. Nat. Biotechnol. 35, 1069–1076 (2017).
Santiago, A. et al. Processing faecal samples: a step forward for standards in microbial community analysis. BMC Microbiol. 14, 112 (2014).
O’Donnell, M. M. et al. Preparation of a standardised faecal slurry for ex-vivo microbiota studies which reduces inter-individual donor bias. J. Microbiol. Methods 129, 109–116 (2016).
Karstens, L. et al. Community profiling of the urinary microbiota: considerations for low-biomass samples. Nat. Rev. Urol. 15, 735–749 (2018).
Castillo, J., Jodar, M. & Oliva, R. The contribution of human sperm proteins to the development and epigenome of the preimplantation embryo. Hum. Reprod. Update 24, 535–555 (2018).
Ronquist, G. K. et al. Prostasomal DNA characterization and transfer into human sperm. Mol. Reprod. Dev. 78, 467–476 (2011).
Aalberts, M., Stout, T. A. E. & Stoorvogel, W. Prostasomes: extracellular vesicles from the prostate. Reproduction 147, R1–R14 (2014).
Drabovich, A. P., Saraon, P., Jarvi, K. & Diamandis, E. P. Seminal plasma as a diagnostic fluid for male reproductive system disorders. Nat. Rev. Urol. 11, 278–288 (2014).
Vojtech, L. et al. Exosomes in human semen carry a distinctive repertoire of small non-coding RNAs with potential regulatory functions. Nucleic Acids Res. 42, 7290–7304 (2014).
Chiasserini, D. et al. Identification and partial characterization of two populations of prostasomes by a combination of dynamic light scattering and proteomic analysis. J. Membr. Biol. 248, 991–1004 (2015).
Jodar, M., Sendler, E. & Krawetz, S. A. The protein and transcript profiles of human semen. Cell Tissue Res. 363, 85–96 (2016).
Mändar, R. Microbiota of male genital tract: impact on the health of man and his partner. Pharmacol. Res. 69, 32–41 (2013).
Kiessling, A. A., Desmarais, B. M., Yin, H.-Z. Z., Loverde, J. & Eyre, R. C. Detection and identification of bacterial DNA in semen. Fertil. Steril. 90, 1744–1756 (2008).
Jarvi, K. et al. Polymerase chain reaction-based detection of bacteria in semen. Fertil. Steril. 66, 463–467 (1996).
Kermes, K., Punab, M., Lõivukene, K. & Mändar, R. Anaerobic seminal fluid micro-flora in chronic prostatitis/chronic pelvic pain syndrome patients. Anaerobe 9, 117–123 (2003).
Cavarretta, I. et al. The microbiome of the prostate tumor microenvironment. Eur. Urol. 72, 625–631 (2017).
Alfano, M. et al. Testicular microbiome in azoospermic men-first evidence of the impact of an altered microenvironment. Hum. Reprod. 33, 1212–1217 (2018).
Jeon, S. J. et al. Blood as a route of transmission of uterine pathogens from the gut to the uterus in cows. Microbiome 5, 109 (2017).
Levy, M., Kolodziejczyk, A. A., Thaiss, C. A. & Elinav, E. Dysbiosis and the immune system. Nat. Rev. Immunol. 17, 219–232 (2017).
Luca, F., Kupfer, S. S., Knights, D., Khoruts, A. & Blekhman, R. Functional genomics of host-microbiome interactions in humans. Trends Genet. 34, 30–40 (2018).
Opazo, M. C. et al. Intestinal microbiota influences non-intestinal related autoimmune diseases. Front. Microbiol. 9, 1–20 (2018).
Nishimura, M. & Naito, S. Tissue-specific mRNA expression profiles of human toll-like receptors and related genes. Biol. Pharm. Bull. 28, 886–892 (2005).
Pudney, J. & Anderson, D. J. Expression of toll-like receptors in genital tract tissues from normal and HIV-infected men. Am. J. Reprod. Immunol. 65, 28–43 (2011).
Girling, J. E. & Hedger, M. P. Toll-like receptors in the gonads and reproductive tract: emerging roles in reproductive physiology and pathology. Immunol. Cell Biol. 85, 481–489 (2007).
Wira, C. R., Grant-Tschudy, K. S. & Crane-Godreau, M. A. Epithelial cells in the female reproductive tract: a central role as sentinels of immune protection. Am. J. Reprod. Immunol. 53, 65–76 (2005).
Round, J. L. et al. The toll-like receptor 2 pathway establishes colonization by a commensal of the human microbiota. Science 332, 974–977 (2011).
Petnicki-Ocwieja, T. et al. Nod2 is required for the regulation of commensal microbiota in the intestine. Proc. Natl. Acad. Sci. USA 106, 15813–15818 (2009).
Türk, S., Mazzoli, S., Stšepetova, J., Kuznetsova, J. & Mändar, R. Coryneform bacteria in human semen: inter-assay variability in species composition detection and biofilm production ability. Microb. Ecol. Health Dis. 25, 1–6 (2014).
Magri, V. et al. Multidisciplinary approach to prostatitis. Arch. Ital. Urol. Androl. 90, 227–248 (2019).
Cai, T. et al. Prostate calcifications: a case series supporting the microbial biofilm theory. Investig. Clin. Urol. 59, 187–193 (2018).
Bartoletti, R. et al. The impact of biofilm-producing bacteria on chronic bacterial prostatitis treatment: results from a longitudinal cohort study. World J. Urol. 32, 737–742 (2014).
D’Amore, R. et al. A comprehensive benchmarking study of protocols and sequencing platforms for 16S rRNA community profiling. BMC Genomics 17, 55 (2016).
Glassing, A., Dowd, S. E., Galandiuk, S., Davis, B. & Chiodini, R. J. Inherent bacterial DNA contamination of extraction and sequencing reagents may affect interpretation of microbiota in low bacterial biomass samples. Gut Pathog. 8, 24 (2016).
de Goffau, M. C. et al. Human placenta has no microbiome but can contain potential pathogens. Nature 572, 329–334 (2019).
Eisenhofer, R. et al. Contamination in low microbial biomass microbiome studies: issues and recommendations. Trends Microbiol. 27, 105–117 (2019).
Salter, S. J. et al. Reagent and laboratory contamination can critically impact sequence-based microbiome analyses. BMC Biol. 12, 87 (2014).
Hallmaier-Wacker, L. K., Lueert, S., Roos, C. & Knauf, S. The impact of storage buffer, DNA extraction method, and polymerase on microbial analysis. Sci. Rep. 8, 6292 (2018).
Chen, Z. et al. Impact of preservation method and 16S rRNA hypervariable region on gut microbiota profiling. mSystems 4, e00271–18 (2019).
Lim, M. Y., Song, E.-J., Kim, S. H., Lee, J. & Nam, Y.-D. Comparison of DNA extraction methods for human gut microbial community profiling. Syst. Appl. Microbiol. 41, 151–157 (2018).
Thomas, V., Clark, J. & Doré, J. Fecal microbiota analysis: an overview of sample collection methods and sequencing strategies. Future Microbiol. 10, 1485–1504 (2015).
Lambert, J. A. et al. Novel PCR-based methods enhance characterization of vaginal microbiota in a bacterial vaginosis patient before and after treatment. Appl. Environ. Microbiol. 79, 4181–4185 (2013).
Gohl, D. M. et al. Systematic improvement of amplicon marker gene methods for increased accuracy in microbiome studies. Nat. Biotechnol. 34, 942–949 (2016).
Wu, J.-Y. Y. et al. Effects of polymerase, template dilution and cycle number on PCR based 16 S rRNA diversity analysis using the deep sequencing method. BMC Microbiol. 10, 255 (2010).
Clooney, A. G. et al. Comparing apples and oranges?: Next generation sequencing and its impact on microbiome analysis. PLOS ONE 11, e0148028 (2016).
Multinu, F. et al. Systematic bias introduced by genomic dna template dilution in 16S rRNA gene-targeted microbiota profiling in human stool homogenates. mSphere 3, e00560–17 (2018).
Aron-Wisnewsky, J. & Clément, K. The gut microbiome, diet, and links to cardiometabolic and chronic disorders. Nat. Rev. Nephrol. 12, 169–181 (2016).
Plummer, E., Twin, J., Bulach, D. M., Garland, S. M. & Tabrizi, S. N. A comparison of three bioinformatics pipelines for the analysis of preterm gut microbiota using 16S rRNA gene sequencing data. J. Proteom. Bioinform Cit. 8, 283–291 (2015).
Callahan, B. J., McMurdie, P. J. & Holmes, S. P. Exact sequence variants should replace operational taxonomic units in marker-gene data analysis. ISME J. 11, 2639–2643 (2017).
Ma, Z. & Li, L. Semen microbiome biogeography: an analysis based on a chinese population study. Front. Microbiol. 9, 3333 (2019).
Rehewy, M. S., Hafez, E. S., Thomas, A. & Brown, W. J. Aerobic and anaerobic bacterial flora in semen from fertile and infertile groups of men. Arch. Androl. 2, 263–268 (1979).
Willén, M., Holst, E., Myhre, E. B. & Olsson, A. M. The bacterial flora of the genitourinary tract in healthy fertile men. Scand. J. Urol. Nephrol. 30, 387–393 (1996).
Ivanov, I. B., Kuzmin, M. D. & Gritsenko, V. A. Microflora of the seminal fluid of healthy men and men suffering from chronic prostatitis syndrome. Int. J. Androl. 32, 462–467 (2009).
Punab, M., Lõivukene, K., Kermes, K. & Mändar, R. The limit of leucocytospermia from the microbiological viewpoint. Andrologia 35, 271–278 (2003).
Korrovits, P., Punab, M., Türk, S. & Mändar, R. Seminal microflora in asymptomatic inflammatory (NIH IV category) prostatitis. Eur. Urol. 50, 1338–1344; discussion 1344–1346 (2006).
Türk, S., Korrovits, P., Punab, M. & Mändar, R. Coryneform bacteria in semen of chronic prostatitis patients. Int. J. Androl. 30, 123–128 (2007).
Petrova, M. I., Lievens, E., Malik, S., Imholz, N. & Lebeer, S. Lactobacillus species as biomarkers and agents that can promote various aspects of vaginal health. Front. Physiol. 6, 81 (2015).
Nelson, D. E. et al. Characteristic male urine microbiomes associate with asymptomatic sexually transmitted infection. PLOS ONE 5, e14116 (2010).
World Health Organization. Laboratory Manual for the Examination and Processing of Human Semen 5th edn (Geneva, Switzerland, 2011).
Barbonetti, A. et al. Effect of vaginal probiotic lactobacilli on in vitro-induced sperm lipid peroxidation and its impact on sperm motility and viability. Fertil. Steril. 95, 2485–2488 (2011).
Agarwal, A., Mulgund, A., Hamada, A. & Chyatte, M. R. A unique view on male infertility around the globe. Reprod. Biol. Endocrinol. 13, 37 (2015).
Jungwirth, A. et al. European Association of Urology guidelines on male infertility: the 2012 update. Eur. Urol. 62, 324–332 (2012).
Winters, B. R. & Walsh, T. J. The epidemiology of male infertility. Urol. Clin. North Am. 41, 195–204 (2014).
Calogero, A. E., Duca, Y., Condorelli, R. A. & La Vignera, S. Male accessory gland inflammation, infertility, and sexual dysfunctions: a practical approach to diagnosis and therapy. Andrology 5, 1064–1072 (2017).
Du Plessis, S. S., Gokul, S. & Agarwal, A. Semen hyperviscosity: causes, consequences, and cures. Front. Biosci. (Elite Ed). 5, 224–231 (2013).
Punab, M., Kullisaar, T. & Mändar, R. Male infertility workup needs additional testing of expressed prostatic secretion and/or post-massage urine. PLOS ONE 8, e82776 (2013).
Condorelli, R. A., Russo, G. I., Calogero, A. E., Morgia, G. & La Vignera, S. Chronic prostatitis and its detrimental impact on sperm parameters: a systematic review and meta-analysis. J. Endocrinol. Invest. 40, 1209–1218 (2017).
Mogra, N., Dhruva, A. & Kothari, L. K. Non-specific seminal tract infection and male infertility: a bacteriological study. J. Postgrad. Med. 27, 99–104 (1981).
Mashaly, M., Masallat, D. T., Elkholy, A. A., Abdel-Hamid, I. A. & Mostafa, T. Seminal Corynebacterium strains in infertile men with and without leucocytospermia. Andrologia 48, 355–359 (2016).
Esfandiari, N., Saleh, R. A., Abdoos, M., Rouzrokh, A. & Nazemian, Z. Positive bacterial culture of semen from infertile men with asymptomatic leukocytospermia. Int. J. Fertil. Womens. Med. 47, 265–270 (2002).
De Francesco, M. A., Negrini, R., Ravizzola, G., Galli, P. & Manca, N. Bacterial species present in the lower male genital tract: a five-year retrospective study. Eur. J. Contracept. Reprod. Health Care 16, 47–53 (2011).
Domes, T. et al. The incidence and effect of bacteriospermia and elevated seminal leukocytes on semen parameters. Fertil. Steril. 97, 1050–1055 (2012).
Filipiak, E. et al. Presence of aerobic micro-organisms and their influence on basic semen parameters in infertile men. Andrologia 47, 826–831 (2015).
Palini, S. et al. A new micro swim-up procedure for sperm preparation in ICSI treatments: preliminary microbiological testing. JBRA Assist. Reprod. 20, 94–98 (2016).
Cumming, J. A., Dawes, J. & Hargreave, T. B. Granulocyte elastase levels do not correlate with anaerobic and aerobic bacterial growth in seminal plasma from infertile men. Int. J. Androl. 13, 273–277 (1990).
Gregoriou, O. et al. Culture of seminal fluid in infertile men and relationship to semen evaluation. Int. J. Gynaecol. Obstet. 28, 149–153 (1989).
Colpi, G. M., Zanollo, A., Roveda, M. L., Tommasini-Degna, A. & Beretta, G. Anaerobic and aerobic bacteria in secretions of prostate and seminal vesicles of infertile men. Arch. Androl. 9, 175–181 (1982).
Balmelli, T. et al. Bacteroides ureolyticus in men consulting for infertility. Andrologia 26, 35–38 (1994).
Virecoulon, F. et al. Bacterial flora of the low male genital tract in patients consulting for infertility. Andrologia 37, 160–165 (2005).
Edström, A. M. L. et al. The major bactericidal activity of human seminal plasma is zinc-dependent and derived from fragmentation of the semenogelins. J. Immunol. 181, 3413–3421 (2008).
Flint, M., du Plessis, S. S. & Menkveld, R. Revisiting the assessment of semen viscosity and its relationship to leucocytospermia. Andrologia 46, 837–841 (2014).
Gimenes, F. et al. Male infertility: a public health issue caused by sexually transmitted pathogens. Nat. Rev. Urol. 11, 672–687 (2014).
Combaz-Söhnchen, N. & Kuhn, A. A systematic review of mycoplasma and ureaplasma in urogynaecology. Geburtshilfe Frauenheilkd. 77, 1299–1303 (2017).
Boeri, L. et al. High-risk human papillomavirus in semen is associated with poor sperm progressive motility and a high sperm DNA fragmentation index in infertile men. Hum. Reprod. 34, 209–217 (2019).
Yow, M. A. et al. Characterisation of microbial communities within aggressive prostate cancer tissues. Infect. Agent. Cancer 12, 4 (2017).
Brede, C. M. & Shoskes, D. A. The etiology and management of acute prostatitis. Nat. Rev. Urol. 8, 207–212 (2011).
Nickel, J. The prostatitis manual (Bladon Medical Publishing, 2002).
Domingue, G. J. & Hellstrom, W. J. Prostatitis. Clin. Microbiol. Rev. 11, 604–613 (1998).
Vicari, L. O. et al. Effect of levofloxacin treatment on semen hyperviscosity in chronic bacterial prostatitis patients. Andrologia 48, 380–388 (2016).
Heras-Cañas, V. et al. [Chronic bacterial prostatitis. Clinical and microbiological study of 332 cases]. Med. Clin. 147, 144–147 (2016).
Iovene, M. R. et al. Enrichment of semen culture in the diagnosis of bacterial prostatitis. J. Microbiol. Methods 154, 124–126 (2018).
Mobley, D. F. Semen cultures in the diagnosis of bacterial prostatitis. J. Urol. 114, 83–85 (1975).
Eijsten, A., Hauri, D. & Knönagel, H. [Bacteriology of the ejaculate — a useful study?]. Urologe. A 27, 340–342 (1988).
Weidner, W., Jantos, C., Schiefer, H. G., Haidl, G. & Friedrich, H. J. Semen parameters in men with and without proven chronic prostatitis. Arch. Androl. 26, 173–183 (1991).
Magri, V. et al. Microscopic and microbiological findings for evaluation of chronic prostatitis. Arch. Ital. di Urol. Androl. 77, 135–138 (2005).
Rizzo, M., Marchetti, F., Travaglini, F., Trinchieri, A. & Nickel, J. C. Clinical characterization of the prostatitis patient in Italy: a prospective urology outpatient study. World J. Urol. 23, 61–66 (2005).
Budía, A. et al. Value of semen culture in the diagnosis of chronic bacterial prostatitis: a simplified method. Scand. J. Urol. Nephrol. 40, 326–331 (2006).
Zegarra Montes, L. Z. M. R., Sanchez Mejia, A. A., Loza Munarriz, C. A. & Gutierrez, E. C. Semen and urine culture in the diagnosis of chronic bacterial prostatitis. Int. Braz. J. Urol. 34, 30–37; discussion 38–40 (2008).
Magri, V. et al. Semen analysis in chronic bacterial prostatitis: diagnostic and therapeutic implications. Asian J. Androl. 11, 461–477 (2009).
Nickel, J. C. et al. Search for microorganisms in men with urologic chronic pelvic pain syndrome: a culture-independent analysis in the mapp research network. J. Urol. 194, 127–135 (2015).
Hou, D.-S. et al. Characterisation of the bacterial community in expressed prostatic secretions from patients with chronic prostatitis/chronic pelvic pain syndrome and infertile men: a preliminary investigation. Asian J. Androl. 14, 566–573 (2012).
Mårdh, P. A. & Colleen, S. Search for uro-genital tract infections in patients with symptoms of prostatitis. Studies on aerobic and strictly anaerobic bacteria, mycoplasmas, fungi, trichomonads and viruses. Scand. J. Urol. Nephrol. 9, 8–16 (1975).
Schaeffer, A. J. et al. Leukocyte and bacterial counts do not correlate with severity of symptoms in men with chronic prostatitis: the National Institutes of Health Chronic Prostatitis Cohort Study. J. Urol. 168, 1048–1053 (2002).
Nickel, J. C. et al. Leukocytes and bacteria in men with chronic prostatitis/chronic pelvic pain syndrome compared to asymptomatic controls. J. Urol. 170, 818–822 (2003).
Liu, L., Yang, J. & Lu, F. Urethral dysbacteriosis as an underlying, primary cause of chronic prostatitis: potential implications for probiotic therapy. Med. Hypotheses 73, 741–743 (2009).
Siqueira, J. F. & Rôças, I. N. Microbiology and treatment of acute apical abscesses. Clin. Microbiol. Rev. 26, 255–273 (2013).
Brook, I. Bacterial synergy in pelvic inflammatory disease. Arch. Gynecol. Obstet. 241, 133–143 (1987).
Vicari, E., Calogero, A. E., Condorelli, R. A., Vicari, L. O. & La Vignera, S. Male accessory gland infection frequency in infertile patients with chronic microbial prostatitis and irritable bowel syndrome. Int. J. Androl. 35, 183–189 (2012).
Santoianni, J. E., De Paulis, A. N., Cardoso, E. M., Gonzalez, B. N. & Predari, S. C. Assessment in the diagnosis of male chronic genital tract infection. Medicina 60, 331–334 (2000).
Manzoor, M. A. P. & Rekha, P.-D. Prostate cancer: microbiome — the ‘unforeseen organ’. Nat. Rev. Urol. 14, 521–522 (2017).
Sfanos, K. S., Yegnasubramanian, S., Nelson, W. G. & De Marzo, A. M. The inflammatory microenvironment and microbiome in prostate cancer development. Nat. Rev. Urol. 15, 11–24 (2018).
Porter, C. M., Shrestha, E., Peiffer, L. B. & Sfanos, K. S. The microbiome in prostate inflammation and prostate cancer. Prostate Cancer Prostatic Dis. 21, 345–354 (2018).
Amirian, E. et al. Potential role of gastrointestinal microbiota composition in prostate cancer risk. Infect. Agent. Cancer 8, 42 (2013).
Golombos, D. M. et al. The role of gut microbiome in the pathogenesis of prostate cancer: a prospective, pilot study. Urology 111, 122–128 (2018).
Wolk, A. Diet, lifestyle and risk of prostate cancer. Acta Oncol. 44, 277–281 (2005).
Puhr, M. et al. Inflammation, microbiota, and prostate cancer. Eur. Urol. Focus. 2, 374–382 (2016).
Sheflin, A. M., Whitney, A. K. & Weir, T. L. Cancer-promoting effects of microbial dysbiosis. Curr. Oncol. Rep. 16, 406 (2014).
Alfano, M. et al. The interplay of extracellular matrix and microbiome in urothelial bladder cancer. Nat. Rev. Urol. 13, 77–90 (2016).
Schwabe, R. F. & Jobin, C. The microbiome and cancer. Nat. Rev. Cancer 13, 800–812 (2013).
Cavalieri, E. et al. Catechol estrogen quinones as initiators of breast and other human cancers: implications for biomarkers of susceptibility and cancer prevention. Biochim. Biophys. Acta Rev. Cancer 1766, 63–78 (2006).
Feng, Y. et al. Metagenomic analysis reveals a rich bacterial content in high-risk prostate tumors from African men. Prostate 79, 1731–1738 (2019).
Xie, H. et al. [Detection of 16S ribosomal RNA gene of bacteria in prostate tissues of adults]. Zhonghua Yi Xue Za Zhi 86, 976–978 (2006).
Hochreiter, W. W., Duncan, J. L. & Schaeffer, A. J. Evaluation of the bacterial flora of the prostate using a 16S rRNA gene based polymerase chain reaction. J. Urol. 163, 127–130 (2000).
Krieger, J. N. & Riley, D. E. Prostatitis: what is the role of infection. Int. J. Antimicrob. Agents 19, 475–479 (2002).
Leskinen, M. J. et al. Negative bacterial polymerase chain reaction (PCR) findings in prostate tissue from patients with symptoms of chronic pelvic pain syndrome (CPPS) and localized prostate cancer. Prostate 55, 105–110 (2003).
Shrestha, E. et al. Profiling the urinary microbiome in men with positive versus negative biopsies for prostate cancer. J. Urol. 199, 161–171 (2018).
Lupo, F. & Ingersoll, M. A. Is bacterial prostatitis a urinary tract infection? Nat. Rev. Urol. 16, 203–204 (2019).
Kim, C. J. et al. Can probiotics reduce inflammation and enhance gut immune health in people living with HIV: study designs for the Probiotic Visbiome for Inflammation and Translocation (PROOV IT) pilot trials. HIV Clin. Trials 17, 147–157 (2016).
Hladik, F. & McElrath, M. J. Setting the stage: host invasion by HIV. Nat. Rev. Immunol. 8, 447–457 (2008).
Baeten, J. M. et al. Genital HIV-1 RNA predicts risk of heterosexual HIV-1 transmission. Sci. Transl. Med. 3, 77ra29 (2011).
Kalichman, S. C., Di Berto, G. & Eaton, L. Human immunodeficiency virus viral load in blood plasma and semen: review and implications of empirical findings. Sex. Transm. Dis. 35, 55–60 (2008).
Schwebke, J. R., Richey, C. M. & Weiss, H. L. Correlation of behaviors with microbiological changes in vaginal flora. J. Infect. Dis. 180, 1632–1636 (1999).
Vallor, A. C., Antonio, M. A., Hawes, S. E. & Hillier, S. L. Factors associated with acquisition of, or persistent colonization by, vaginal lactobacilli: role of hydrogen peroxide production. J. Infect. Dis. 184, 1431–1436 (2001).
Beigi, R. H., Wiesenfeld, H. C., Hillier, S. L., Straw, T. & Krohn, M. A. Factors associated with absence of H2O2-producing Lactobacillus among women with bacterial vaginosis. J. Infect. Dis. 191, 924–929 (2005).
Cherpes, T. L., Hillier, S. L., Meyn, L. A., Busch, J. L. & Krohn, M. A. A delicate balance: risk factors for acquisition of bacterial vaginosis include sexual activity, absence of hydrogen peroxide-producing lactobacilli, black race, and positive herpes simplex virus type 2 serology. Sex. Transm. Dis. 35, 78–83 (2008).
Brotman, R. M., Ravel, J., Cone, R. A. & Zenilman, J. M. Rapid fluctuation of the vaginal microbiota measured by gram stain analysis. Sex. Transm. Infect. 86, 297–302 (2010).
Plummer, E. L. et al. Combined oral and topical antimicrobial therapy for male partners of women with bacterial vaginosis: acceptability, tolerability and impact on the genital microbiota of couples — a pilot study. PLOS ONE 13, e0190199 (2018).
Morison, L. et al. Bacterial vaginosis in relation to menstrual cycle, menstrual protection method, and sexual intercourse in rural Gambian women. Sex. Transm. Infect. 81, 242–247 (2005).
Hay, P. E., Ugwumadu, A. & Chowns, J. Sex, thrush and bacterial vaginosis. Int. J. STD AIDS 8, 603–608 (1997).
Verstraelen, H., Verhelst, R., Vaneechoutte, M. & Temmerman, M. The epidemiology of bacterial vaginosis in relation to sexual behaviour. BMC Infect. Dis. 10, 81 (2010).
Tandogdu, Z. & Wagenlehner, F. M. E. Global epidemiology of urinary tract infections. Curr. Opin. Infect. Dis. 29, 73–79 (2016).
Lisboa, C. et al. Genital candidosis in heterosexual couples. J. Eur. Acad. Dermatology Venereol. 25, 145–151 (2011).
Nelson, D. E. et al. Bacterial communities of the coronal sulcus and distal urethra of adolescent males. PLOS ONE 7, 1–9 (2012).
Kelley, C. F. et al. The rectal mucosa and condomless receptive anal intercourse in HIV-negative MSM: implications for HIV transmission and prevention. Mucosal. Immunol. 10, 996–1007 (2017).
Armstrong, A. J. S. et al. An exploration of prevotella-rich microbiomes in HIV and men who have sex with men. Microbiome 6, 198 (2018).
Noguera-Julian, M. et al. Gut microbiota linked to sexual preference and HIV infection. EBioMedicine 5, 135–146 (2016).
Pan, W.-H. et al. Exposure to the gut microbiota drives distinct methylome and transcriptome changes in intestinal epithelial cells during postnatal development. Genome Med. 10, 27 (2018).
Thion, M. S. et al. Microbiome influences prenatal and adult microglia in a sex-specific manner. Cell 172, 500–516.e16 (2018).
Lannon, S. M. R. et al. Parallel detection of lactobacillus and bacterial vaginosis-associated bacterial DNA in the chorioamnion and vagina of pregnant women at term. J. Matern. Fetal. Neonatal Med. 32, 2702–2710 (2019).
Watkins, A. J. & Sinclair, K. D. Paternal low protein diet affects adult offspring cardiovascular and metabolic function in mice. Am. J. Physiol. Circ. Physiol. 306, H1444–H1452 (2014).
Carone, B. R. et al. Paternally induced transgenerational environmental reprogramming of metabolic gene expression in mammals. Cell 143, 1084–1096 (2010).
Dardmeh, F. et al. Lactobacillus rhamnosus PB01 (DSM 14870) supplementation affects markers of sperm kinematic parameters in a diet-induced obesity mice model. PLOS ONE 12, e0185964 (2017).
Inatomi, T. & Otomaru, K. Effect of dietary probiotics on the semen traits and antioxidative activity of male broiler breeders. Sci. Rep. 8, 5874 (2018).
Valcarce, D. G. et al. Probiotic administration improves sperm quality in asthenozoospermic human donors. Benef. Microbes 8, 193–206 (2017).
Maretti, C. & Cavallini, G. The association of a probiotic with a prebiotic (Flortec, Bracco) to improve the quality/quantity of spermatozoa in infertile patients with idiopathic oligoasthenoteratospermia: a pilot study. Andrology 5, 439–444 (2017).
Senok, A. C., Verstraelen, H., Temmerman, M. & Botta, G. A. Probiotics for the treatment of bacterial vaginosis. Cochrane Database Syst. Rev. 4, CD006289 (2009).
Collins, S. L. et al. Promising prebiotic candidate established by evaluation of lactitol, lactulose, raffinose, and oligofructose for maintenance of a lactobacillus-dominated vaginal microbiota. Appl. Environ. Microbiol. 84, e02200–e02217 (2018).
Khalesi, S. et al. A review of probiotic supplementation in healthy adults: helpful or hype? Eur. J. Clin. Nutr. 73, 24–37 (2018).
Wong, A. C. & Levy, M. New approaches to microbiome-based therapies. mSystems 4, e00122–19 (2019).
Scarpellini, E. et al. The human gut microbiota and virome: potential therapeutic implications. Dig. Liver Dis. 47, 1007–1012 (2015).
Focà, A. et al. Gut inflammation and immunity: what is the role of the human gut virome? Mediators Inflamm. 2015, 326032 (2015).
Lugli, G. A. et al. Prophages of the genus Bifidobacterium as modulating agents of the infant gut microbiota. Environ. Microbiol. 18, 2196–2213 (2016).
Miller-Ensminger, T. et al. Bacteriophages of the urinary microbiome. J. Bacteriol. 200, e00738–17 (2018).
Moustafa, A. et al. Microbial metagenome of urinary tract infection. Sci. Rep. 8, 4333 (2018).
Ma, J. et al. Association between BMI and semen quality: an observational study of 3966 sperm donors. Hum. Reprod. 34, 155–162 (2019).
Zangara, M. T. & McDonald, C. How diet and the microbiome shape health or contribute to disease: a mini-review of current models and clinical studies. Exp. Biol. Med. 244, 484–493 (2019).
Acknowledgements
S.A. is funded by the Spanish Ministry of Economy, Industry and Competitiveness (MINECO) and the European Regional Development Fund (FEDER): grants RYC-2016-21199 and ENDORE SAF2017-87526; Programa Operativo FEDER Andalucía (B-CTS-500-UGR18) and by the University of Granada Plan Propio de Investigación 2016 —Excellence actions: Unit of Excellence on Exercise and Health (UCEES) — and Plan Propio de Investigación 2018 — Programa Contratos-Puente, and the Junta de Andalucía, Consejería de Conocimiento, Investigación y Universidades, European Regional Development Funds (ref. SOMM17/6107/UGR). R.M. is funded by the Estonian Research Council (grant No. IUT34-19), the Estonian Ministry of Education and Research (grant No. KOGU-HUMB) and Enterprise Estonia (grant No. EU48695).
Author information
Authors and Affiliations
Contributions
All the authors researched the data for the article, participated in the discussion of its content, wrote the article and critically reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Microbiota
-
A community of microorganisms present in a defined environment.
- Next-generation sequencing
-
(NGS). Technique to detect microbial communities.
- Tourists
-
Transient microbial population that is readily eliminated.
- Invaders
-
Microorganisms that are present in particular diseased states, a transient population that contribute to disease.
- Residents
-
‘Healthy’ bacterial residents that maintain homeostasis, a stable population.
- Microbiome
-
The entire habitat that includes the microorganisms (bacteria, viruses, archaea, and lower and higher eukaryotes), their genomes and the surrounding environmental conditions, including the products of the microbiota and the host environment.
- Seminal microbiome
-
The microbiome of the male ejaculate and reproductive tract.
- Postbiotics
-
Metabolic by-products of live (probiotic) bacteria.
- qPCR
-
Quantitative polymerase chain reaction (to detect specific microorganisms).
- Decomplementary activity
-
Microbial inhibitor of complement.
- Oestrobolome
-
The collection of microorganisms capable of metabolizing oestrogens.
- Shannon Index
-
A measure of the richness and evenness in a given sample.
- Methylome
-
Whole set of nucleic acid methylation modifications in genome.
- Transcriptome
-
Whole set of messenger RNA molecules expressed from the genome.
- Prebiotics
-
Compounds in food that induce growth or activity of beneficial microorganisms.
- Probiotics
-
Live bacteria and yeasts promoted as having various health benefits.
- Synbiotics
-
Food ingredients or dietary supplements combining probiotics and prebiotics in a form of synergism.
Rights and permissions
About this article
Cite this article
Altmäe, S., Franasiak, J.M. & Mändar, R. The seminal microbiome in health and disease. Nat Rev Urol 16, 703–721 (2019). https://doi.org/10.1038/s41585-019-0250-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41585-019-0250-y
This article is cited by
-
Reproductive tract microbiome and therapeutics of infertility
Middle East Fertility Society Journal (2023)
-
Alterations in microbiota of patients with COVID-19: potential mechanisms and therapeutic interventions
Signal Transduction and Targeted Therapy (2022)
-
Genitourinary microbial screening for all infertile men?
Nature Reviews Urology (2022)
-
HPV infection and bacterial microbiota in the semen from healthy men
BMC Infectious Diseases (2021)
-
Effect of bacterial infection on sperm quality and DNA fragmentation in subfertile men with Leukocytospermia
BMC Molecular and Cell Biology (2021)