Abstract
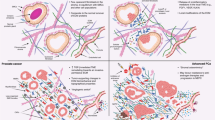
The prostate is the only organ in a man that continues to grow with age. John McNeal proposed, 40 years ago, that this BPH is characterized by an age-related reinitiation of benign neoplastic growth selectively in developmentally abortive distal ducts within the prostate transition–periurethral zone (TPZ), owing to a reawakening of inductive stroma selectively within these zones. An innovative variant of this hypothesis is that, owing to its location, the TPZ is continuously exposed to urinary components and/or autoantigens, which produces an inflammatory TPZ microenvironment that promotes recruitment of bone marrow-derived mesenchymal stem cells (MSCs) and generates a paracrine-inductive stroma that reinitiates benign neoplastic nodular growth. In support of this hypothesis, MSCs infiltrate human BPH tissue and have the ability to stimulate epithelial stem cell growth. These results provide a framework for defining both the aetiology of BPH in ageing men and insights into new therapeutic approaches.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Isaacs, J. T. Prostate stem cells and benign prostatic hyperplasia. Prostate 68, 1025–1034 (2008).
McNeal, J. E. Regional morphology and pathology of the prostate. Am. J. Clin. Pathol. 49, 347–357 (1968).
McNeal, J. E. The prostate and prostatic urethra: a morphologic synthesis. J. Urol. 107, 1008–1016 (1972).
McNeal, J. E. Origin and evolution of benign prostatic enlargement. Invest. Urol. 15, 340–345 (1978).
McNeal, J. E. The zonal anatomy of the prostate. Prostate 2, 35–49 (1981).
McNeal, J. E. Anatomy of the prostate and morphogenesis of BPH. Prog. Clin. Biol. Res. 145, 27–53 (1984).
Isaacs, J. T. & Coffey, D. S. Etiology and disease process of benign prostatic hyperplasia. Prostate Suppl. 2, 33–50 (1989).
Hollingsworth, J. M. & Wilt, T. J. Lower urinary tract symptoms in men. BMJ. 349, g4474 (2014).
Sausville, J. & Naslund, M. Benign prostatic hyperplasia and prostate cancer: an overview for primary care physicians. Int. J. Clin. Pract. 64, 1740–1745 (2010).
Cindolo, L. et al. Drug adherence and clinical outcomes for patients under pharmacological therapy for lower urinary tract symptoms related to benign prostatic hyperplasia: population-based cohort study. Eur. Urol. 68, 418–425 (2015).
Bosch, J. L., Tilling, K., Bohnen, A. M., Bangma, C. H. & Donovan, J. L. Establishing normal reference ranges for prostate volume change with age in the population-based Krimpen-study: prediction of future prostate volume in individual men. Prostate 67, 1816–1824 (2007).
Park, J. et al. Establishment of reference ranges for prostate volume and annual prostate volume change rate in Korean adult men: analyses of a nationwide screening population. J. Kor. Med. Sci. 30, 1136–1142 (2015).
Hayward, S. W., Cunha, G. R. & Dahiya, R. Normal development and carcinogenesis of the prostate. A unifying hypothesis. Ann. NY Acad. Sci. 784, 50–62 (1996).
Hayward, S. W. et al. Interactions between adult human prostatic epithelium and rat urogenital sinus mesenchyme in a tissue recombination model. Differentiation 63, 131–140 (1998).
Thomson, A. A., Timms, B. G., Barton, L., Cunha, G. R. & Grace, O. C. The role of smooth muscle in regulating prostatic induction. Development 129, 1905–1912 (2002).
Marker, P. C., Donjacour, A. A., Dahiya, R. & Cunha, G. R. Hormonal, cellular, and molecular control of prostatic development. Dev. Biol. 253, 165–174 (2003).
Yan, G., Fukabori, Y., Nikolaropoulos, S., Wang, F. & McKeehan, W. L. Heparin-binding keratinocyte growth factor is a candidate stromal-to-epithelial-cell andromedin. Mol. Endocrinol. 6, 2123–2128 (1992).
Richard, C. et al. Androgens modulate the balance between VEGF and angiopoietin expression in prostate epithelial and smooth muscle cells. Prostate 50, 83–91 (2002).
Litvinov, I. V., De Marzo, A. M. & Isaacs, J. T. Is the Achilles’ heel for prostate cancer therapy a gain of function in androgen receptor signaling? J. Clin. Endocrinol. Metab. 88, 2972–2982 (2003).
Ohlson, N., Bergh, A., Stattin, P. & Wikstrom, P. Castration-induced epithelial cell death in human prostate tissue is related to locally reduced IGF-1 levels. Prostate 67, 32–40 (2007).
Brennen, W. N. et al. Mesenchymal stem cell infiltration during neoplastic transformation of the human prostate. Oncotarget 8, 46710–46727 (2017).
Lamm, M. L. et al. Sonic hedgehog activates mesenchymal Gli1 expression during prostate ductal bud formation. Dev. Biol. 249, 349–366 (2002).
Karhadkar, S. S. et al. Hedgehog signalling in prostate regeneration, neoplasia and metastasis. Nature 431, 707–712 (2004).
Peng, Y. C., Levine, C. M., Zahid, S., Wilson, E. L. & Joyner, A. L. Sonic hedgehog signals to multiple prostate stromal stem cells that replenish distinct stromal subtypes during regeneration. Proc. Natl Acad. Sci. USA 110, 20611–20616 (2013).
Peehl, D. M. & Sellers, R. G. Basic FGF, EGF, and PDGF modify TGFbeta-induction of smooth muscle cell phenotype in human prostatic stromal cells. Prostate 35, 125–134 (1998).
Zhu, M. L. & Kyprianou, N. Androgen receptor and growth factor signaling cross-talk in prostate cancer cells. Endocr. Relat. Cancer 15, 841–849 (2008).
Yoon, G., Kim, J. Y., Choi, Y. K., Won, Y. S. & Lim, I. K. Direct activation of TGF-beta1 transcription by androgen and androgen receptor complex in Huh7 human hepatoma cells and its tumor in nude mice. J. Cell. Biochem. 97, 393–411 (2006).
Kanda, T., Jiang, X. & Yokosuka, O. Androgen receptor signaling in hepatocellular carcinoma and pancreatic cancers. World J. Gastroenterol. 20, 9229–9236 (2014).
Singh, R., Artaza, J. N., Taylor, W. E., Gonzalez-Cadavid, N. F. & Bhasin, S. Androgens stimulate myogenic differentiation and inhibit adipogenesis in C3H 10T1/2 pluripotent cells through an androgen receptor-mediated pathway. Endocrinology 144, 5081–5088 (2003).
Singh, R. et al. Regulation of myogenic differentiation by androgens: cross talk between androgen receptor/ beta-catenin and follistatin/transforming growth factor-beta signaling pathways. Endocrinology 150, 1259–1268 (2009).
Timms, B. G. Prostate development: a historical perspective. Differentiation 76, 565–577 (2008).
Lowsley, O. Embryology, anatomy and surgery of the prostate gland with report of operative results. Am. J. Surg. 8, 526–541 (1930).
Moore, R. A. Benign hypertrophy and carcinoma of the prostate, occurence and experimental production in animals. Surgery 16, 152–167 (1944).
Claus, S., Wrenger, M., Senge, T. & Schulze, H. Immunohistochemical determination of age related proliferation rates in normal and benign hyperplastic human prostates. Urol. Res. 21, 305–308 (1993).
Claus, S., Berges, R., Senge, T. & Schulze, H. Cell kinetic in epithelium and stroma of benign prostatic hyperplasia. J. Urol. 158, 217–221 (1997).
Shapiro, E., Becich, M. J., Hartanto, V. & Lepor, H. The relative proportion of stromal and epithelial hyperplasia is related to the development of symptomatic benign prostate hyperplasia. J. Urol. 147, 1293–1297 (1992).
Cunha, G. R. & Lung, B. The possible influence of temporal factors in androgenic responsiveness of urogenital tissue recombinants from wild-type and androgen-insensitive (Tfm) mice. J. Exp. Zool. 205, 181–193 (1978).
Cunha, G. R., Hayward, S. W., Dahiya, R. & Foster, B. A. Smooth muscle-epithelial interactions in normal and neoplastic prostatic development. Acta Anat. (Basel) 155, (63–72 (1996).
Wang, Y., Hayward, S., Cao, M., Thayer, K. & Cunha, G. Cell differentiation lineage in the prostate. Differentiation 68, 270–279 (2001).
Schalken, J. A. & van Leenders, G. Cellular and molecular biology of the prostate: stem cell biology. Urology 62, 11–20 (2003).
Goto, K. et al. Proximal prostatic stem cells are programmed to regenerate a proximal-distal ductal axis. Stem Cells 24, 1859–1868 (2006).
Vander Griend, D. J. et al. The role of CD133 in normal human prostate stem cells and malignant cancer-initiating cells. Cancer Res. 68, 9703–9711 (2008).
Moad, M. et al. Multipotent basal stem cells, maintained in localized proximal niches, support directed long-ranging epithelial flows in human prostates. Cell Rep. 20, 1609–1622 (2017).
Guo, C. et al. Epcam, CD44, and CD49f distinguish sphere-forming human prostate basal cells from a subpopulation with predominant tubule initiation capability. PLOS ONE 7, e34219 (2012).
Antony, L., van der Schoor, F., Dalrymple, S. L. & Isaacs, J. T. Androgen receptor (AR) suppresses normal human prostate epithelial cell proliferation via AR/beta-catenin/TCF-4 complex inhibition of c-MYC transcription. Prostate 74, 1118–1131 (2014).
Vander Griend, D. J., Litvinov, I. V. & Isaacs, J. T. Conversion of androgen receptor signaling from a growth suppressor in normal prostate epithelial cells to an oncogene in prostate cancer cells involves a gain of function in c-Myc regulation. Int. J. Biol. Sci. 10, 627–642 (2014).
Peters, C. A. & Walsh, P. C. The effect of nafarelin acetate, a luteinizing-hormone-releasing hormone agonist, on benign prostatic hyperplasia. N. Engl. J. Med. 317, 599–604 (1987).
Joseph, I. B., Nelson, J. B., Denmeade, S. R. & Isaacs, J. T. Androgens regulate vascular endothelial growth factor content in normal and malignant prostatic tissue. Clin. Cancer Res. 3, 2507–2511 (1997).
Macoska, J. A. Chemokines and BPH/LUTS. Differentiation 82, 253–260 (2011).
McLaren, I. D., Jerde, T. J. & Bushman, W. Role of interleukins, IGF and stem cells in BPH. Differentiation 82, 237–243 (2011).
Quillard, T. & Charreau, B. Impact of notch signaling on inflammatory responses in cardiovascular disorders. Int. J. Mol. Sci. 14, 6863–6888 (2013).
Hahn, A. M., Myers, J. D., McFarland, E. K., Lee, S. & Jerde, T. J. Interleukin-driven insulin-like growth factor promotes prostatic inflammatory hyperplasia. J. Pharmacol. Exp. Ther. 351, 605–615 (2014).
Edeling, M., Ragi, G., Huang, S., Pavenstadt, H. & Susztak, K. Developmental signalling pathways in renal fibrosis: the roles of Notch, Wnt and Hedgehog. Nat. Rev. Nephrol. 12, 426–439 (2016).
Fazio, C. & Ricciardiello, L. Inflammation and Notch signaling: a crosstalk with opposite effects on tumorigenesis. Cell Death Dis. 7, e2515 (2016).
Shang, Y., Smith, S. & Hu, X. Role of Notch signaling in regulating innate immunity and inflammation in health and disease. Protein Cell 7, 159–174 (2016).
Balistreri, C. R., Madonna, R., Melino, G. & Caruso, C. The emerging role of Notch pathway in ageing: focus on the related mechanisms in age-related diseases. Ageing Res. Rev. 29, 50–65 (2016).
Ma, B. & Hottiger, M. O. Crosstalk between Wnt/beta-catenin and NF-kappaB signaling pathway during Inflammation. Front. Immunol. 7, 378 (2016).
Kohnen, P. W. & Drach, G. W. Patterns of inflammation in prostatic hyperplasia: a histologic and bacteriologic study. J. Urol. 121, 755–760 (1979).
Kramer, G., Mitteregger, D. & Marberger, M. Is benign prostatic hyperplasia (BPH) an immune inflammatory disease? Eur. Urol. 51, 1202–1216 (2007).
Nickel, J. C. Inflammation and benign prostatic hyperplasia. Urol. Clin. North Amer. 35, 109–115 (2008).
Robert, G. et al. Inflammation in benign prostatic hyperplasia: a 282 patients’ immunohistochemical analysis. Prostate 69, 1774–1780 (2009).
Steiner, G. E. et al. Cytokine expression pattern in benign prostatic hyperplasia infiltrating T cells and impact of lymphocytic infiltration on cytokine mRNA profile in prostatic tissue. Lab Invest. 83, 1131–1146 (2003).
De Nunzio, C., Presicce, F. & Tubaro, A. Inflammatory mediators in the development and progression of benign prostatic hyperplasia. Nat. Rev. Urol. 13, 613–626 (2016).
Nickel, J. C. et al. The relationship between prostate inflammation and lower urinary tract symptoms: examination of baseline data from the REDUCE trial. Eur. Urol. 54, 1379–1384 (2008).
Yu, H. et al. Urinary microbiota in patients with prostate cancer and benign prostatic hyperplasia. Arch. Med. Sci. 11, 385–394 (2015).
Vignozzi, L., Gacci, M. & Maggi, M. Lower urinary tract symptoms, benign prostatic hyperplasia and metabolic syndrome. Nat. Rev. Urol. 13, 108–119 (2016).
Tilg, H. & Moschen, A. R. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat. Rev. Immunol. 6, 772–783 (2006).
Persson, B. E. & Ronquist, G. Evidence for a mechanistic association between nonbacterial prostatitis and levels of urate and creatinine in expressed prostatic secretion. J. Urol. 155, 958–960 (1996).
Motrich, R. D. et al. Uric acid crystals in the semen of a patient with symptoms of chronic prostatitis. Fertil Steril. 85, 751 (2006).
Kirby, R. S., Lowe, D., Bultitude, M. I. & Shuttleworth, K. E. Intra-prostatic urinary reflux: an aetiological factor in abacterial prostatitis. Br. J. Urol. 54, 729–731 (1982).
Luo, J. et al. Gene expression signature of benign prostatic hyperplasia revealed by cDNA microarray analysis. Prostate 51, 189–200 (2002).
O’Malley, K. J. et al. Proteomic analysis of patient tissue reveals PSA protein in the stroma of benign prostatic hyperplasia. Prostate 74, 892–900 (2014).
Mikolajczyk, S. D. et al. “BPSA,” a specific molecular form of free prostate-specific antigen, is found predominantly in the transition zone of patients with nodular benign prostatic hyperplasia. Urology 55, 41–45 (2000).
Horwitz, E. M. et al. Clarification of the nomenclature for MSC: the international society for cellular therapy position statement. Cytotherapy 7, 393–395 (2005).
Zuk, P. A. et al. Human adipose tissue is a source of multipotent stem cells. Mol. Biol. Cell 13, 4279–4295 (2002).
da Silva Meirelles, L., Chagastelles, P. C. & Nardi, N. B. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J. Cell Sci. 119, 2204–2213 (2006).
Crisan, M. et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 3, 301–313 (2008).
Lin, V. K. et al. Prostatic stromal cells derived from benign prostatic hyperplasia specimens possess stem cell like property. Prostate 67, 1265–1276 (2007).
Brennen, W. N., Chen, S., Denmeade, S. R. & Isaacs, J. T. Quantification of mesenchymal stem cells (MSCs) at sites of human prostate cancer. Oncotarget 4, 106–117 (2013).
Kim, W. et al. RUNX1 is essential for mesenchymal stem cell proliferation and myofibroblast differentiation. Proc Natl Acad. Sci. USA 111, 16389–16394 (2014).
Brennen, W. N., Kisteman, L. N. & Isaacs, J. T. Rapid selection of mesenchymal stem and progenitor cells in primary prostate stromal cultures. Prostate 76, 552–564 (2016).
Brennen, W. N. et al. Assessing angiogenic responses induced by primary human prostate stromal cells in a three-dimensional fibrin matrix assay. Oncotarget 7, 71298–71308 (2016).
Colter, D. C., Sekiya, I. & Prockop, D. J. Identification of a subpopulation of rapidly self-renewing and multipotential adult stem cells in colonies of human marrow stromal cells. Proc Natl Acad. Sci. USA 98, 7841–7845 (2001).
Sakaguchi, Y., Sekiya, I., Yagishita, K. & Muneta, T. Comparison of human stem cells derived from various mesenchymal tissues: superiority of synovium as a cell source. Arthritis Rheum. 52, 2521–2529 (2005).
Musina, R. A., Bekchanova, E. S., Belyavskii, A. V. & Sukhikh, G. T. Differentiation potential of mesenchymal stem cells of different origin. Bull. Exp. Biol. Med. 141, 147–151 (2006).
Park, H. W., Shin, J. S. & Kim, C. W. Proteome of mesenchymal stem cells. Proteomics 7, 2881–2894 (2007).
Noel, D. et al. Cell specific differences between human adipose-derived and mesenchymal-stromal cells despite similar differentiation potentials. Exp. Cell Res. 314, 1575–1584 (2008).
Petrini, M. et al. Identification and purification of mesodermal progenitor cells from human adult bone marrow. Stem Cells Dev. 18, 857–866 (2009).
Jansen, B. J. et al. Functional differences between mesenchymal stem cell populations are reflected by their transcriptome. Stem Cells Dev. 19, 481–490 (2010).
Russell, K. C. et al. Clonal analysis of the proliferation potential of human bone marrow mesenchymal stem cells as a function of potency. Biotechnol. Bioeng. 108, 2716–2726 (2011).
Strioga, M., Viswanathan, S., Darinskas, A., Slaby, O. & Michalek, J. Same or not the same? Comparison of adipose tissue-derived versus bone marrow-derived mesenchymal stem and stromal cells. Stem Cells Dev. 21, 2724–2752 (2012).
Pacini, S. et al. Mesangiogenic progenitor cells derived from one novel CD64(bright)CD31(bright)CD14(neg) population in human adult bone marrow. Stem Cells Dev. 25, 661–673 (2016).
Spaeth, E., Klopp, A., Dembinski, J., Andreeff, M. & Marini, F. Inflammation and tumor microenvironments: defining the migratory itinerary of mesenchymal stem cells. Gene Ther. 15, 730–738 (2008).
Brennen, W. N., Denmeade, S. R. & Isaacs, J. T. Mesenchymal stem cells as a vector for the inflammatory prostate microenvironment. Endocr. Relat. Cancer 20, R269–R290 (2013).
Lourenco, S. et al. Macrophage migration inhibitory factor-CXCR4 is the dominant chemotactic axis in human mesenchymal stem cell recruitment to tumors. J. Immunol. 194, 3463–3474 (2015).
Wang, L. et al. Aberrant transforming growth factor-beta activation recruits mesenchymal stem cells during prostatic hyperplasia. Stem Cells Transl Med. 6, 394–404 (2017).
Tanaka, S. T. et al. Recruitment of bone marrow derived cells to the bladder after bladder outlet obstruction. J. Urol. 182, 1769–1774 (2009).
Placencio, V. R., Li, X., Sherrill, T. P., Fritz, G. & Bhowmick, N. A. Bone marrow derived mesenchymal stem cells incorporate into the prostate during regrowth. PLOS One 5, e12920 (2010).
Garcia, N. P. et al. Kinetics of mesenchymal and hematopoietic stem cells mobilization by G-CSF and its impact on the cytokine microenvironment in primary cultures. Cell. Immunol. 293, 1–9 (2015).
Begley, L., Monteleon, C., Shah, R. B., Macdonald, J. W. & Macoska, J. A. CXCL12 overexpression and secretion by aging fibroblasts enhance human prostate epithelial proliferation in vitro. Aging Cell 4, 291–298 (2005).
Fujita, K. et al. Monocyte chemotactic protein-1 (MCP-1/CCL2) is associated with prostatic growth dysregulation and benign prostatic hyperplasia. Prostate 70, 473–481 (2010).
Nakata, W. et al. Bone marrow-derived cells contribute to regeneration of injured prostate epithelium and stroma. Prostate 75, 806–814 (2015).
Sokolova, I. B., Zin’kova, N. N., Shvedova, E. V., Kruglyakov, P. V. & Polyntsev, D. G. Distribution of mesenchymal stem cells in the area of tissue inflammation after transplantation of the cell material via different routes. Bull. Exp. Biol. Med. 143, 143–146 (2007).
Saffarini, C. M. et al. Maturation of the developing human fetal prostate in a rodent xenograft model. Prostate 73, 1761–1775 (2013).
Caplan, A. I. Why are MSCs therapeutic? New data: new insight. J. Pathol. 217, 318–324 (2009).
Newman, R. E., Yoo, D., LeRoux, M. A. & Danilkovitch-Miagkova, A. Treatment of inflammatory diseases with mesenchymal stem cells. Inflamm Allergy Drug Targets 8, 110–123 (2009).
English, K. & Mahon, B. P. Allogeneic mesenchymal stem cells: agents of immune modulation. J. Cell. Biochem. 112, 1963–1968 (2011).
Guess, H. A., Arrighi, H. M., Metter, E. J. & Fozard, J. L. Cumulative prevalence of prostatism matches the autopsy prevalence of benign prostatic hyperplasia. Prostate 17, 241–246 (1990).
Berry, S. J., Coffey, D. S., Walsh, P. C. & Ewing, L. L. The development of human benign prostatic hyperplasia with age. J. Urol. 132, 474–479 (1984).
Franks, L. M. Benign nodular hyperplasia of the prostate: a review. Ann. R. Coll. Surg. Engl. 14, 92–106 (1954).
Ross, J. J. et al. Cytokine-induced differentiation of multipotent adult progenitor cells into functional smooth muscle cells. J. Clin. Invest. 116, 3139–3149 (2006).
Narita, Y., Yamawaki, A., Kagami, H., Ueda, M. & Ueda, Y. Effects of transforming growth factor-beta 1 and ascorbic acid on differentiation of human bone-marrow-derived mesenchymal stem cells into smooth muscle cell lineage. Cell Tissue Res. 333, 449–459 (2008).
Mishra, P. J. et al. Carcinoma-associated fibroblast-like differentiation of human mesenchymal stem cells. Cancer Res. 68, 4331–4339 (2008).
Paunescu, V. et al. Tumour-associated fibroblasts and mesenchymal stem cells: more similarities than differences. J. Cell. Mol. Med. 15, 635–646 (2011).
Zhao, L. & Hantash, B. M. TGF-beta1 regulates differentiation of bone marrow mesenchymal stem cells. Vitam. Horm. 87, 127–141 (2011).
Liu, J., Wang, Y., Wu, Y., Ni, B. & Liang, Z. Sodium butyrate promotes the differentiation of rat bone marrow mesenchymal stem cells to smooth muscle cells through histone acetylation. PLOS ONE 9, e116183 (2014).
Shi, N., Guo, X. & Chen, S. Y. Olfactomedin 2, a novel regulator for transforming growth factor-beta-induced smooth muscle differentiation of human embryonic stem cell-derived mesenchymal cells. Mol. Biol. Cell 25, 4106–4114 (2014).
Visweswaran, M. et al. Multi-lineage differentiation of mesenchymal stem cells - to Wnt, or not Wnt. Int. J. Biochem. Cell Biol. 68, 139–147 (2015).
Almalki, S. G. & Agrawal, D. K. Key transcription factors in the differentiation of mesenchymal stem cells. Differentiation 92, 41–51 (2016).
Russell, K. C. et al. In vitro high-capacity assay to quantify the clonal heterogeneity in trilineage potential of mesenchymal stem cells reveals a complex hierarchy of lineage commitment. Stem Cells 28, 788–798 (2010).
Lin, V. K. et al. Myosin heavy chain gene expression in normal and hyperplastic human prostate tissue. Prostate 44, 193–203 (2000).
Schauer, I. G., Ressler, S. J., Tuxhorn, J. A., Dang, T. D. & Rowley, D. R. Elevated epithelial expression of interleukin-8 correlates with myofibroblast reactive stroma in benign prostatic hyperplasia. Urology 72, 205–213 (2008).
Schauer, I. G. & Rowley, D. R. The functional role of reactive stroma in benign prostatic hyperplasia. Differentiation. 82, 200–210 (2011).
Tuxhorn, J. A. et al. Reactive stroma in human prostate cancer: induction of myofibroblast phenotype and extracellular matrix remodeling. Clin. Cancer Res. 8, 2912–2923 (2002).
Kidd, S. et al. Origins of the tumor microenvironment: quantitative assessment of adipose-derived and bone marrow-derived stroma. PLOS ONE 7, e30563 (2012).
Shangguan, L. et al. Inhibition of TGF-beta/Smad signaling by BAMBI blocks differentiation of human mesenchymal stem cells to carcinoma-associated fibroblasts and abolishes their protumor effects. Stem Cells 30, 2810–2819 (2012).
Sufrin, G., Heston, W. D., Hazra, T. & Coffey, D. S. The effect of radiation on prostatic growth. Invest. Urol. 13, 418–423 (1976).
Mallepell, S., Krust, A., Chambon, P. & Brisken, C. Paracrine signaling through the epithelial estrogen receptor alpha is required for proliferation and morphogenesis in the mammary gland. Proc. Natl Acad. Sci. USA 103, 2196–2201 (2006).
Fass, D., Steinfeld, A., Brown, J. & Tessler, A. Radiotherapeutic prophylaxis of estrogen-induced gynecomastia: a study of late sequela. Int. J. Radiat. Oncol. Biol. Phys. 12, 407–408 (1986).
Alfthan, O. & Kettunen, K. The effect of roentgen ray treatment of gynecomastia in patients with prostatic carcinoma treated with estrogenic hormones: a preliminary communication. J. Urol. 94, 604–606 (1965).
Koukourakis, M. et al. Transurethral radiotherapy for benign prostatic hypertrophy-related urethral obstruction. Dosimetry, ethics, and preliminary results. Med. Dosim 19, 67–72 (1994).
Zhao, P., Lu, S., Yang, Y., Shao, Q. & Liang, J. Three-dimensional conformal radiation therapy of spontaneous benign prostatic hyperplasia in canines. Oncol. Res. 19, 225–235 (2011).
Williams, S. A. et al. A prostate-specific antigen-activated channel-forming toxin as therapy for prostatic disease. J. Natl Cancer Inst. 99, 376–385 (2007).
Magistro, G. et al. Emerging minimally invasive treatment options for male lower urinary tract symptoms. Eur. Urol. 72, 986–997 (2017).
Denmeade, S. R. et al. Phase 1 and 2 studies demonstrate the safety and efficacy of intraprostatic injection of PRX302 for the targeted treatment of lower urinary tract symptoms secondary to benign prostatic hyperplasia. Eur. Urol. 59, 747–754 (2011).
Elhilali, M. M. et al. Prospective, randomized, double-blind, vehicle controlled, multicenter phase IIb clinical trial of the pore forming protein PRX302 for targeted treatment of symptomatic benign prostatic hyperplasia. J. Urol. 189, 1421–1426 (2013).
Scanlan, M. J. et al. Molecular cloning of fibroblast activation protein alpha, a member of the serine protease family selectively expressed in stromal fibroblasts of epithelial cancers. Proc. Natl Acad. Sci. USA 91, 5657–5661 (1994).
Brennen, W. N., Isaacs, J. T. & Denmeade, S. R. Rationale behind targeting fibroblast activation protein-expressing carcinoma-associated fibroblasts as a novel chemotherapeutic strategy. Mol. Cancer Ther. 11, 257–266 (2012).
Bae, S. et al. Fibroblast activation protein alpha identifies mesenchymal stromal cells from human bone marrow. Br. J. Haematol. 142, 827–830 (2008).
Aggarwal, S. et al. Fibroblast activation protein peptide substrates identified from human collagen I derived gelatin cleavage sites. Biochemistry 47, 1076–1086 (2008).
Brennen, W. N., Rosen, D. M., Wang, H., Isaacs, J. T. & Denmeade, S. R. Targeting carcinoma-associated fibroblasts within the tumor stroma with a fibroblast activation protein-activated prodrug. J. Natl Cancer Inst. 104, 1320–1334 (2012).
LeBeau, A. M., Brennen, W. N., Aggarwal, S. & Denmeade, S. R. Targeting the cancer stroma with a fibroblast activation protein-activated promelittin protoxin. Mol. Cancer Ther. 8, 1378–1386 (2009).
Acknowledgements
The authors thank B. Zhang and I. P. Garraway (University of California–Los Angeles) for generously providing urogenital sinus tissue, A. Meeker for his assistance in developing the immunofluorescence assays, and the Sidney Kimmel Comprehensive Cancer Center (SKCCC) Immunohistochemistry Core supported by the SKCCC Cancer Center Support Grant (CCSG; P30 CA006973). The authors also acknowledge the following sources of financial support: the Prostate Cancer Foundation Young Investigator Award (W.N.B.), SKCCC CCSG developmental funds (P30 CA006973 (W.N.B.)), the U.S. Department of Defense (W81XWH-17-1-0528 (W.N.B.)), and the US NIH Prostate SPORE Grant (P50 CA058236 (J.T.I.)).
Reviewer information
Nature Reviews Urology thanks W. -J. Lin and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
W.N.B. and J.T.I. researched data for the article, substantially contributed to discussion of content, and wrote, reviewed, and edited the article.
Corresponding authors
Ethics declarations
Competing interests
J.T.I. declares financial interest with Sophiris Bio related to topsalysin (also known as PRX302). W.N.B. declares no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Brennen, W.N., Isaacs, J.T. Mesenchymal stem cells and the embryonic reawakening theory of BPH. Nat Rev Urol 15, 703–715 (2018). https://doi.org/10.1038/s41585-018-0087-9
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41585-018-0087-9
This article is cited by
-
Identification and functional activity of Nik related kinase (NRK) in benign hyperplastic prostate
Journal of Translational Medicine (2024)
-
Aberrant activated Notch1 promotes prostate enlargement driven by androgen signaling via disrupting mitochondrial function in mouse
Cellular and Molecular Life Sciences (2024)
-
Prognostic and therapeutic potential of senescent stromal fibroblasts in prostate cancer
Nature Reviews Urology (2023)
-
Upregulation of mir-1199-5p is associated with reduced type 2 5-α reductase expression in benign prostatic hyperplasia
BMC Urology (2022)
-
Prostate luminal progenitor cells: from mouse to human, from health to disease
Nature Reviews Urology (2022)