Abstract
Invasive squamous cell carcinoma (SCC) of the penis is almost always treated with surgical therapy at the primary site. However, almost all other SCC primary sites, such as anal, vulvar, uterine cervix, head and neck, and oesophagus, and their involved nodal basins, can be successfully treated with radiotherapy or combined chemotherapy and radiation, reserving surgery as a salvage option. Review of the penile cancer literature and examination of data from more common SCC primary sites make a case for complete organ preservation of the penis using definitive combined chemotherapy and radiation, reserving surgical therapies as salvage options.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



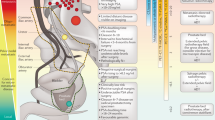
Figure reproduced from ref.91, Crook, J. Curr. Opin. Urol. 27(1), 62–67, Lippincott Williams and Wilkins Ltd https://journals.lww.com/co-urology/pages/default.aspx.
Similar content being viewed by others
References
Friedenwald, H. The prayer of Maimonides. Bull. Johns Hopkins Hospital 18, 260–261 (1917).
Kieffer, J. M. et al. Quality of life for patients treated for penile cancer. J. Urol. 192, 1105–1110 (2014).
Opjordsmoen, S. & Fossa, S. D. Quality of life in patients treated for penile cancer. A follow-up study. Br. J. Urol. 74, 652–657 (1994).
Schover, L. R., von Eschenbach, A. C., Smith, D. B. & Gonzalez, J. Sexual rehabilitation of urologic cancer patients: a practical approach. CA Cancer J. Clin. 34, 66–74 (1984).
Witty, K. et al. The impact of surgical treatment for penile cancer — patients’ perspectives. Eur. J. Oncol. Nurs. 17, 661–667 (2013).
Cady, B. Basic principles in surgical oncology. Arch. Surg. 132, 338–346 (1997).
Burt, L. M., Shrieve, D. C. & Tward, J. D. Stage presentation, care patterns, and treatment outcomes for squamous cell carcinoma of the penis. Int. J. Radiat. Oncol. Biol. Phys. 88, 94–100 (2014).
Gotsadze, D., Matveev, B., Zak, B. & Mamaladze, V. Is conservative organ-sparing treatment of penile carcinoma justified? Eur. Urol. 38, 306–312 (2000).
Lont, A. P., Gallee, M. P., Meinhardt, W., van Tinteren, H. & Horenblas, S. Penis conserving treatment for T1 and T2 penile carcinoma: clinical implications of a local recurrence. J. Urol. 176, 575–580; discussion 580 (2006).
Mistry, T., Jones, R. W., Dannatt, E., Prasad, K. K. & Stockdale, A. D. A. 10-year retrospective audit of penile cancer management in the UK. BJU Int. 100, 1277–1281 (2007).
Ozsahin, M. et al. Treatment of penile carcinoma: to cut or not to cut? Int. J. Radiat. Oncol. Biol. Phys. 66, 674–679 (2006).
Tang, D. H. et al. Adjuvant pelvic radiation is associated with improved survival and decreased disease recurrence in pelvic node-positive penile cancer after lymph node dissection: a multi-institutional study. Urol. Oncol. 35, 605.e17–605.e23 (2017).
Zouhair, A. et al. Radiation therapy alone or combined surgery and radiation therapy in squamous-cell carcinoma of the penis? Eur. J. Cancer 37, 198–203 (2001).
Cordoba, A. et al. Low-dose brachytherapy for early stage penile cancer: a 20-year single-institution study (73 patients). Radiat. Oncol. 11, 96 (2016).
Crook, J., Ma, C. & Grimard, L. Radiation therapy in the management of the primary penile tumor: an update. World J. Urol. 27, 189–196 (2009).
de Crevoisier, R. et al. Long-term results of brachytherapy for carcinoma of the penis confined to the glans (N− or NX). Int. J. Radiat. Oncol. Biol. Phys. 74, 1150–1156 (2009).
Delannes, M., Malavaud, B., Douchez, J., Bonnet, J. & Daly, N. J. Iridium-192 interstitial therapy for squamous cell carcinoma of the penis. Int. J. Radiat. Oncol. Biol. Phys. 24, 479–483 (1992).
Escande, A. et al. Brachytherapy for conservative treatment of invasive penile carcinoma: prognostic factors and long-term analysis of outcome. Int. J. Radiat. Oncol. Biol. Phys. 99, 563–570 (2017).
Kiltie, A. E., Elwell, C., Close, H. J. & Ash, D. V. Iridium-192 implantation for node-negative carcinoma of the penis: the Cookridge Hospital experience. Clin. Oncol. (R. Coll. Radiol) 12, 25–31 (2000).
Mazeron, J. J. et al. Interstitial radiation therapy for carcinoma of the penis using iridium 192 wires: the Henri Mondor experience 1970–1979. Int. J. Radiat. Oncol. Biol. Phys. 10, 1891–1895 (1984).
McLean, M. et al. The results of primary radiation therapy in the management of squamous cell carcinoma of the penis. Int. J. Radiat. Oncol. Biol. Phys. 25, 623–628 (1993).
Rozan, R. et al. Interstitial brachytherapy for penile carcinoma: a multicentric survey (259 patients). Radiother. Oncol. 36, 83–93 (1995).
Crook, J. M., Jezioranski, J., Grimard, L., Esche, B. & Pond, G. Penile brachytherapy: results for 49 patients. Int. J. Radiat. Oncol. Biol. Phys. 62, 460–467 (2005).
Philippou, P. et al. Conservative surgery for squamous cell carcinoma of the penis: resection margins and long-term oncological control. J. Urol. 188, 803–808 (2012).
Pietrzak, P., Corbishley, C. & Watkin, N. Organ-sparing surgery for invasive penile cancer: early follow-up data. BJU Int. 94, 1253–1257 (2004).
Ubrig, B., Waldner, M., Fallahi, M. & Roth, S. Preputial flap for primary closure after excision of tumors on the glans penis. Urology 58, 274–276 (2001).
van Bezooijen, B. P., Horenblas, S., Meinhardt, W. & Newling, D. W. Laser therapy for carcinoma in situ of the penis. J. Urol. 166, 1670–1671 (2001).
Amin, M. B. et al. (eds) AJCC Cancer Staging Manual 8th edn (Springer, 2016).
Ficarra, V. et al. Nomogram predictive of pathological inguinal lymph node involvement in patients with squamous cell carcinoma of the penis. J. Urol. 175, 1700–1705 (2006).
Haas, G. P. et al. Cisplatin, methotrexate and bleomycin for the treatment of carcinoma of the penis: a Southwest Oncology Group study. J. Urol. 161, 1823–1825 (1999).
Necchi, A. et al. First-line therapy with dacomitinib, an orally available pan-HER tyrosine kinase inhibitor, for locally advanced or metastatic penile squamous cell carcinoma: results of an open-label, single-arm, single-centre, phase 2 study. BJU Int. 121, 348–356 (2018).
Pagliaro, L. C. et al. Neoadjuvant paclitaxel, ifosfamide, and cisplatin chemotherapy for metastatic penile cancer: a phase II study. J. Clin. Oncol. 28, 3851–3857 (2010).
Sharma, P. et al. Adjuvant chemotherapy is associated with improved overall survival in pelvic node-positive penile cancer after lymph node dissection: a multi-institutional study. Urol. Oncol. 33, 496.e17–496.e23 (2015).
Franks, K. N. et al. Radiotherapy for node positive penile cancer: experience of the Leeds teaching hospitals. J. Urol. 186, 524–529 (2011).
Necchi, A. et al. Clinical outcomes of perioperative chemotherapy in patients with locally advanced penile squamous-cell carcinoma: results of a multicenter analysis. Clin. Genitourinary Cancer 15, 548–555.e3 (2017).
Edsmyr, F., Andersson, L. & Esposti, P. L. Combined bleomycin and radiation therapy in carcinoma of the penis. Cancer 56, 1257–1263 (1985).
Maiche, A. G. Combined bleomycin and radiation treatment of penile carcinoma. Acta Oncol. 28, 548–549 (1989).
Modig, H., Duchek, M. & Sjodin, J. G. Carcinoma of the penis. Treatment by surgery or combined bleomycin and radiation therapy. Acta Oncol. 32, 653–655 (1993).
Pond, G. R. et al. Concurrent chemoradiotherapy for men with locally advanced penile squamous cell carcinoma. Clin. Genitourin Cancer 12, 440–446 (2014).
Neave, F., Neal, A. J., Hoskin, P. J. & Hope-Stone, H. F. Carcinoma of the penis: a retrospective review of treatment with iridium mould and external beam irradiation. Clin. Oncol. (R. Coll. Radiol) 5, 207–210 (1993).
Sarin, R., Norman, A. R., Steel, G. G. & Horwich, A. Treatment results and prognostic factors in 101 men treated for squamous carcinoma of the penis. Int. J. Radiat. Oncol. Biol. Phys. 38, 713–722 (1997).
Brown, M. D., Zachary, C. B., Grekin, R. C. & Swanson, N. A. Penile tumors: their management by Mohs micrographic surgery. J. Dermatol. Surg. Oncol. 13, 1163–1167 (1987).
Shindel, A. W. et al. Mohs micrographic surgery for penile cancer: management and long-term followup. J. Urol. 178, 1980–1985 (2007).
Bandieramonte, G. et al. Peniscopically controlled CO2 laser excision for conservative treatment of in situ and T1 penile carcinoma: report on 224 patients. Eur. Urol. 54, 875–882 (2008).
Frimberger, D. et al. Penile carcinoma. Is Nd:YAG laser therapy radical enough? J. Urol. 168, 2418–2421; discussion 2421 (2002).
Meijer, R. P., Boon, T. A., van Venrooij, G. E. & Wijburg, C. J. Long-term follow-up after laser therapy for penile carcinoma. Urology 69, 759–762 (2007).
Schlenker, B. et al. Organ-preserving neodymium-yttrium-aluminium-garnet laser therapy for penile carcinoma: a long-term follow-up. BJU Int. 106, 786–790 (2010).
Tietjen, D. N. & Malek, R. S. Laser therapy of squamous cell dysplasia and carcinoma of the penis. Urology 52, 559–565 (1998).
Windahl, T. & Andersson, S. O. Combined laser treatment for penile carcinoma: results after long-term followup. J. Urol. 169, 2118–2121 (2003).
Bissada, N. K. et al. Multi-institutional long-term experience with conservative surgery for invasive penile carcinoma. J. Urol. 169, 500–502 (2003).
Leijte, J. A., Kirrander, P., Antonini, N., Windahl, T. & Horenblas, S. Recurrence patterns of squamous cell carcinoma of the penis: recommendations for follow-up based on a two-centre analysis of 700 patients. Eur. Urol. 54, 161–168 (2008).
McDaniel, A. S. et al. Genomic profiling of penile squamous cell carcinoma reveals new opportunities for targeted therapy. Cancer Res. 75, 5219–5227 (2015).
Cubilla., L. A. et al. The World Health Organisation 2016 classification of penile carcinomas: a review and update from the International Society of Urological Pathology expert-driven recommendations. Histopathology 72, 893–904 (2018).
Ali, S. M. et al. Comprehensive genomic profiling of advanced penile carcinoma suggests a high frequency of clinically relevant genomic alterations. Oncologist 21, 33–39 (2016).
Chung, J. H. et al. Comprehensive genomic profiling of anal squamous cell carcinoma reveals distinct genomically defined classes. Ann. Oncol. 27, 1336–1341 (2016).
Elvin, J. A. et al. Comprehensive genomic profiling (CGP) of cervical squamous cell carcinoma (cSCC) to identifiy targeted therapy options. J. Clin. Oncol. 33, 5602–5602 (2015).
Ojesina, A. I. et al. Landscape of genomic alterations in cervical carcinomas. Nature 506, 371–375 (2013).
Chaux, A., Velazquez, E. F., Barreto, J. E., Ayala, E. & Cubilla, A. L. New pathologic entities in penile carcinomas: an update of the 2004 World Health Organization Classification. Semin. Diagn. Pathol. 29, 59–66 (2012).
Wolf, G. T. et al. Induction chemotherapy plus radiation compared with surgery plus radiation in patients with advanced laryngeal cancer. N. Engl. J. Med. 324, 1685–1690 (1991).
Whitney, C. W. et al. Randomized comparison of fluorouracil plus cisplatin versus hydroxyurea as an adjunct to radiation therapy in stage IIB-IVA carcinoma of the cervix with negative para-aortic lymph nodes: a Gynecologic Oncology Group and Southwest Oncology Group study. J. Clin. Oncol. 17, 1339–1348 (1999).
Eifel, P. J. et al. Pelvic irradiation with concurrent chemotherapy versus pelvic and para-aortic irradiation for high-risk cervical cancer: an update of radiation therapy oncology group trial (RTOG) 90–01. J. Clin. Oncol. 22, 872–880 (2004).
Rose, P. G. et al. Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. N. Engl. J. Med. 340, 1144–1153 (1999).
Peters, W. A. 3rd et al. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J. Clin. Oncol. 18, 1606–1613 (2000).
Keys, H. M. et al. Cisplatin, radiation, and adjuvant hysterectomy compared with radiation and adjuvant hysterectomy for bulky stage IB cervical carcinoma. N. Engl. J. Med. 340, 1154–1161 (1999).
Josefson, D. Adding chemotherapy improves survival in cervical cancer. BMJ 318, 623–623 (1999).
Chemoradiotherapy for Cervical Cancer Meta-analysis Collaboration. Reducing uncertainties about the effects of chemoradiotherapy for cervical cancer: individual patient data meta-analysis. Cochrane Database Syst. Rev. 1, CD008285 (2010).
Adelstein, D. J. et al. An intergroup phase III comparison of standard radiation therapy and two schedules of concurrent chemoradiotherapy in patients with unresectable squamous cell head and neck cancer. J. Clin. Oncol. 21, 92–98 (2003).
Calais, G. et al. Randomized trial of radiation therapy versus concomitant chemotherapy and radiation therapy for advanced-stage oropharynx carcinoma. J. Natl Cancer Inst. 91, 2081–2086 (1999).
Tobias, J. S. et al. Chemoradiotherapy for locally advanced head and neck cancer: 10-year follow-up of the UK Head and Neck (UKHAN1) trial. Lancet. Oncol. 11, 66–74 (2010).
Zakotnik, B. et al. Patterns of failure in patients with locally advanced head and neck cancer treated postoperatively with irradiation or concomitant irradiation with mitomycin C and bleomycin. Int. J. Radiat. Oncol. Biol. Phys. 67, 685–690 (2007).
Kunos, C., Simpkins, F., Gibbons, H., Tian, C. & Homesley, H. Radiation therapy compared with pelvic node resection for node-positive vulvar cancer: a randomized controlled trial. Obstetr. Gynecol. 114, 537–546 (2009).
Nigro, N. D., Vaitkevicius, V. K. & Considine, B. Jr. Combined therapy for cancer of the anal canal: a preliminary report. Dis. Colon Rectum 17, 354–356 (1974).
Nigro, N. D. An evaluation of combined therapy for squamous cell cancer of the anal canal. Dis. Colon Rectum 27, 763–766 (1984).
Bartelink, H. et al. Concomitant radiotherapy and chemotherapy is superior to radiotherapy alone in the treatment of locally advanced anal cancer: results of a phase III randomized trial of the European Organization for Research and Treatment of Cancer Radiotherapy and Gastrointestinal Cooperative Groups. J. Clin. Oncol. 15, 2040–2049 (1997).
Northover, J. et al. Chemoradiation for the treatment of epidermoid anal cancer: 13-year follow-up of the first randomised UKCCCR Anal Cancer Trial (ACT I). Br. J. Cancer 102, 1123–1128 (2010).
Ajani, J. A. et al. Fluorouracil, mitomycin, and radiotherapy vs fluorouracil, cisplatin, and radiotherapy for carcinoma of the anal canal: a randomized controlled trial. JAMA 299, 1914–1921 (2008).
James, R. D. et al. Mitomycin or cisplatin chemoradiation with or without maintenance chemotherapy for treatment of squamous-cell carcinoma of the anus (ACT II): a randomised, phase 3, open-label, 2×2 factorial trial. Lancet. Oncol. 14, 516–524 (2013).
National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Anal Carcinoma. NCCN https://www.tri-kobe.org/nccn/guideline/colorectal/english/anal.pdf (2017).
Henderson, M. A. et al. Adjuvant lymph-node field radiotherapy versus observation only in patients with melanoma at high risk of further lymph-node field relapse after lymphadenectomy (ANZMTG 01.02/TROG 02.01): 6-year follow-up of a phase 3, randomised controlled trial. Lancet Oncol. 16, 1049–1060 (2015).
Lopes, A. et al. Prognostic factors in carcinoma of the penis: multivariate analysis of 145 patients treated with amputation and lymphadenectomy. J. Urol. 156, 1637–1642 (1996).
National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Penile Cancer. NCCN https://www.tri-kobe.org/nccn/guideline/urological/english/penile.pdf (2016).
National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Vulvar Cancer. NCCN https://www.tri-kobe.org/nccn/guideline/gynecological/english/vulvar.pdf (2016).
National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Head and Neck Cancers. NCCN https://oralcancerfoundation.org/wp-content/uploads/2016/09/head-and-neck.pdf (2016).
National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Esophageal and Esophagogastric Cancer. NCCN http://tsqb.hnszlyy.com/ueditor/upload/NCCN%20Guidelines%20esophageal%20cancer%20(2017%20V1)_2017112723507.pdf (2017).
National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Non-Small Cell Lung Cancer. NCCN https://www.tri-kobe.org/nccn/guideline/lung/english/non_small.pdf (2015).
National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Cervical cancer. NCCN https://www.tri-kobe.org/nccn/guideline/gynecological/english/cervical.pdf (2016).
Hakenberg, O. W. et al. EAU guidelines on penile cancer: 2014 update. Eur. Urol. 67, 142–150 (2015).
O’Neil, B., Brant, W. O., Slack, S. D., Tward, J. D. & Myers, J. B. Penile cancer: contemporary considerations in management of local disease. Curr. Urol. 5, 62–71 (2011).
Chhabra, A. et al. Neoadjuvant concurrent chemoradiation for curative treatment of penile squamous cell carcinoma. Case Rep. Oncol. Med. 2014, 4 (2014).
Lapierre, A., Riou, O., Flechon, A., Mottet, N. & Azria, D. Advanced penile cancer with iliac lymph node involvement treated with radiation and concurrent gemcitabine. Cancer Radiother. 21, 134–137 (2017).
Crook, J. Radiotherapy approaches for locally advanced penile cancer: neoadjuvant and adjuvant. Curr. Opin. Urol. 27, 62–67 (2017).
Stacey, D., Samant, R. & Bennett, C. Decision making in oncology: a review of patient decision aids to support patient participation. CA Cancer J. Clin. 58, 293–304 (2008).
Street, R. L., Elwyn, G. & Epstein, R. M. Patient preferences and healthcare outcomes: an ecological perspective. Expert Rev. Pharmacoeconom. Outcomes Res. 12, 167–180 (2012).
Zucca, A., Sanson-Fisher, R., Waller, A. & Carey, M. Patient-centred care: making cancer treatment centres accountable. Supportive Care Cancer 22, 1989–1997 (2014).
Institute of Medicine (US) Committee on Quality of Health care in America. Crossing the Quality Chasm: A New Health System for the 21st Century (National Academies Press, Washington, 2001).
Gerteis, M., Edgman-Levitan, S., Daley, J. & Delbanco, T. L. (eds). Through the Patient’s Eyes: Understanding and Promoting Patient-Centered Care. (Jossey-Bass, San Francisco, 1993).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Tward, J. The case for nonsurgical therapy of nonmetastatic penile cancer. Nat Rev Urol 15, 574–584 (2018). https://doi.org/10.1038/s41585-018-0040-y
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41585-018-0040-y
This article is cited by
-
The role of radiotherapy in the management of squamous cell cancer of the penis
World Journal of Urology (2023)
-
Immune-based therapies in penile cancer
Nature Reviews Urology (2022)
-
HPV positivity and radiotherapy outcomes in PSCC
Nature Reviews Urology (2021)
-
Understanding genomics and the immune environment of penile cancer to improve therapy
Nature Reviews Urology (2020)