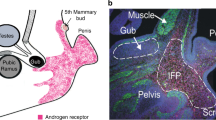
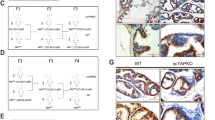
Abstract
The biology of masculinization is fundamentally important for understanding the embryonic developmental processes that are involved in the development of the male reproductive tract, external genitalia, and also the tumorigenesis of prostate cancer. The molecular mechanisms of masculinization are of interest to many researchers and clinicians involved in varied fields, including molecular developmental biology, cancer research, endocrinology, and urology. Androgen signalling is mediated by the nuclear androgen receptor, which has fundamental roles in masculinization during development. Various modes of androgen signalling, including 5α-dihydrotestosterone-induced regulation of mesenchymal cell proliferation, have been observed in masculinization. Such regulation is essential for regulating urogenital tissue development, including external genitalia development. Androgen-induced genes, such as MAFB, which belongs to the activator protein 1 (AP-1) superfamily of genes, have essential roles in male urethral formation, and disruption of its signalling can interfere with urethral formation, which often results in hypospadias. Another AP-1 superfamily gene, ATF3, could be responsible for some instances of hypospadias in humans. These androgen-dependent signals and downstream events are crucial for not only developmental processes but also processes of diseases such as hypospadias and prostate cancer.
Key points
-
The molecular mechanisms of masculinization are fundamental topics of many fields of science, including molecular developmental biology, cancer research, endocrinology, and urology.
-
One of the activator protein 1 (AP-1) superfamily genes, MAFB, has been identified as an androgen target gene and has essential roles in male-type urethral formation.
-
Mesenchymal cell proliferation can be regulated by testosterone and 5α-dihydrotestosterone via the androgen receptor.
-
Putatively similar mesenchymal cell characteristics in embryos and prostate-cancer-associated fibroblasts have been described, including the identification of AP-1 superfamily genes.
-
Genes such as ATF3 that are involved in various signalling pathways are affected by oestrogen receptor-mediated cellular processes.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Nordenskjöld, A. et al. Screening for mutations in candidate genes for hypospadias. Urol. Res. 27, 49–55 (1999).
Beleza-Meireles, A. et al. FGFR2, FGF8, FGF10 and BMP7 as candidate genes for hypospadias. Eur. J. Hum. Genet. 15, 405–410 (2007).
Murashima, A., Kishigami, S., Thomson, A. & Yamada, G. Androgens and mammalian male reproductive tract development. Biochim. Biophys. Acta 1849, 163–170 (2015).
Ahmed, S. F. & Hughes, I. A. The genetics of male undermasculinization. Clin. Endocrinol. 56, 1–18 (2002).
Doehnert, U., Bertelloni, S., Werner, R., Dati, E. & Hiort, O. Characteristic features of reproductive hormone profiles in late adolescent and adult females with complete androgen insensitivity syndrome. Sex. Dev. 9, 69–74 (2015).
Yamada, G., Satoh, Y., Baskin, L. S. & Cunha, G. R. Cellular and molecular mechanisms of development of the external genitalia. Differentiation 71, 445–460 (2003).
Omori, A. et al. Essential roles of epithelial bone morphogenetic protein signaling during prostatic development. Endocrinology 155, 2534–2544 (2014).
Ashley, G. R., Grace, O. C., Vanpoucke, G. & Thomson, A. A. Identification of ephrinb1 expression in prostatic mesenchyme and a role for ephb-ephrinb signalling in prostate development. Differentiation 80, 89–98 (2010).
Thomson, A. A. Mesenchymal mechanisms in prostate organogenesis. Differentiation 76, 587–598 (2008).
Kasper, S. et al. Development, progression, and androgen-dependence of prostate tumors in probasin-large T antigen transgenic mice: a model for prostate cancer. Lab Invest. 78, 319–333 (1998).
Yamada, G. et al. Molecular genetic cascades for external genitalia formation: an emerging organogenesis program. Dev. Dyn. 235, 1738–1752 (2006).
Murakami, R. & Mizuno, T. Proximal-distal sequence of development of the skeletal tissues in the penis of rat and the inductive effect of epithelium. J. Embryol. Exp. Morphol. 92, 133–143 (1986).
Suzuki, H., Suzuki, K. & Yamada, G. Systematic analyses of murine masculinization processes based on genital sex differentiation parameters. Dev. Growth Differ. 57, 639–647 (2015).
Lyon, M. F. & Hawkes, S. G. X-Linked gene for testicular feminization in the mouse. Nature 227, 1217–1219 (1970).
Ipulan, L. A. et al. Investigation of sexual dimorphisms through mouse models and hormone/hormone-disruptor treatments. Differentiation 91, 78–89 (2016).
Tsai, M. Y. et al. Differential effects of spermatogenesis and fertility in mice lacking androgen receptor in individual testis cells. Proc. Natl Acad. Sci. USA 103, 18975–18980 (2006).
Wang, R. S., Yeh, S., Tzeng, C. R. & Chang, C. Androgen receptor roles in spermatogenesis and fertility: lessons from testicular cell-specific androgen receptor knockout mice. Endocr. Rev. 30, 119–132 (2009).
Chang, C. et al. Infertility with defective spermatogenesis and hypotestosteronemia in male mice lacking the androgen receptor in Sertoli cells. Proc. Natl Acad. Sci. USA 101, 6876–6881 (2004).
De Gendt, K. et al. A Sertoli cell-selective knockout of the androgen receptor causes spermatogenic arrest in meiosis. Proc. Natl Acad. Sci. USA 101, 1327–1332 (2004).
Holdcraft, R. W. & Braun, R. E. Androgen receptor function is required in Sertoli cells for the terminal differentiation of haploid spermatids. Development 131, 459–467 (2004).
Miyagawa, S. et al. Genetic interactions of the androgen and Wnt/beta-catenin pathways for the masculinization of external genitalia. Mol. Endocrinol. 23, 871–880 (2009).
Sajjad, Y. Development of the genital ducts and external genitalia in the early human embryo. J. Obstet. Gynaecol. Res. 36, 929–937 (2010).
Baskin, L. S., Himes, K. & Colborn, T. Hypospadias and endocrine disruption: is there a connection? Environ. Health Perspect. 109, 1175–1183 (2001).
Baskin, L. S., Erol, A., Li, Y. W. & Cunha, G. R. Anatomical studies of hypospadias. J. Urol. 160, 1108–1115; discussion 1137 (1998).
Baskin, L. S. et al. Urethral seam formation and hypospadias. Cell Tissue Res. 305, 379–387 (2001).
Cox, K. et al. Shorter anogenital and anoscrotal distances correlate with the severity of hypospadias: a prospective study. J. Pediatr. Urol. 13, 57.e1–57.e5 (2016).
Beleza-Meireles, A. et al. Activating transcription factor 3: a hormone responsive gene in the etiology of hypospadias. Eur. J. Endocrinol. 158, 729–739 (2008).
Ahmed, S. F. et al. UK guidance on the initial evaluation of an infant or an adolescent with a suspected disorder of sex development. Clin. Endocrinol. 75, 12–26 (2011).
Utsch, B., Albers, N. & Ludwig, M. Genetic and molecular aspects of hypospadias. Eur. J. Pediatr. Surg. 14, 297–302 (2004).
Phillips, T. R., Wright, D. K., Gradie, P. E., Johnston, L. A. & Pask, A. J. A. Comprehensive atlas of the adult mouse penis. Sex. Dev. 9, 162–172 (2015).
Kojima, Y., Kohri, K. & Hayashi, Y. Genetic pathway of external genitalia formation and molecular etiology of hypospadias. J. Pediatr. Urol. 6, 346–354 (2010).
Kaftanovskaya, E. M. et al. Cryptorchidism in mice with an androgen receptor ablation in gubernaculum testis. Mol. Endocrinol. 26, 598–607 (2012).
Zheng, Z., Armfield, B. A. & Cohn, M. J. Timing of androgen receptor disruption and estrogen exposure underlies a spectrum of congenital penile anomalies. Proc. Natl Acad. Sci. USA 112, E7194–E7203 (2015).
Welsh, M. et al. Identification in rats of a programming window for reproductive tract masculinization, disruption of which leads to hypospadias and cryptorchidism. J. Clin. Invest. 118, 1479–1490 (2008).
Welsh, M., Suzuki, H. & Yamada, G. The masculinization programming window. Endocr. Dev. 27, 17–27 (2014).
Suzuki, H., Matsushita, S., Suzuki, K. & Yamada, G. 5α-Dihydrotestosterone negatively regulates cell proliferation of the periurethral ventral mesenchyme during urethral tube formation in the murine male genital tubercle. Andrology 5, 146–152 (2017).
Ipulan, L. A. et al. Nonmyocytic androgen receptor regulates the sexually dimorphic development of the embryonic bulbocavernosus muscle. Endocrinology 155, 2467–2479 (2014).
Welsh, M., Saunders, P. T. & Sharpe, R. M. The critical time window for androgen-dependent development of the Wolffian duct in the rat. Endocrinology 148, 3185–3195 (2007).
Karimian, A., Ahmadi, Y. & Yousefi, B. Multiple functions of p21 in cell cycle, apoptosis and transcriptional regulation after DNA damage. DNA Repair 42, 63–71 (2016).
Lu, S., Liu, M., Epner, D. E., Tsai, S. Y. & Tsai, M. J. Androgen regulation of the cyclin-dependent kinase inhibitor p21 gene through an androgen response element in the proximal promoter. Mol. Endocrinol. 13, 376–384 (1999).
Wilson, J. D. The critical role of androgens in prostate development. Endocrinol. Metab. Clin. North Am. 40, 577–590 (2011).
Veyssière, G. et al. [Sexual organogenesis and circulating androgens in the rabbit fetus. Study after active immunization of mothers against testosterone (author’s transl)]. Arch. Anat. Microsc. Morphol. Exp. 69, 17–28 (1980).
Mahendroo, M. S. & Russell, D. W. Male and female isoenzymes of steroid 5alpha-reductase. Rev. Reprod. 4, 179–183 (1999).
Suzuki, K. et al. Sexually dimorphic expression of Mafb regulates masculinization of the embryonic urethral formation. Proc. Natl Acad. Sci. USA 111, 16407–16412 (2014).
Pfaff, D. W. Hormones, Brain, and Behavior (Academic Press, 2009).
Rahman, M., Miyamoto, H. & Chang, C. Androgen receptor coregulators in prostate cancer: mechanisms and clinical implications. Clin. Cancer Res. 10, 2208–2219 (2004).
Montgomery, R. B. et al. Maintenance of intratumoral androgens in metastatic prostate cancer: a mechanism for castration-resistant tumor growth. Cancer Res. 68, 4447–4454 (2008).
Soto, A. M. et al. Variants of the human prostate LNCaP cell line as tools to study discrete components of the androgen-mediated proliferative response. Oncol. Res. 7, 545–558 (1995).
Heisler, L. E. et al. Androgen-dependent cell cycle arrest and apoptotic death in PC-3 prostatic cell cultures expressing a full-length human androgen receptor. Mol. Cell Endocrinol. 126, 59–73 (1997).
Niu, Y. et al. Differential androgen receptor signals in different cells explain why androgen-deprivation therapy of prostate cancer fails. Oncogene 29, 3593–3604 (2010).
Ogino, Y. et al. Essential functions of androgen signaling emerged through the developmental analysis of vertebrate sex characteristics. Evol. Dev. 13, 315–325 (2011).
Haraguchi, R. et al. Molecular analysis of external genitalia formation: the role of fibroblast growth factor (Fgf) genes during genital tubercle formation. Development 127, 2471–2479 (2000).
Haraguchi, R. et al. Unique functions of sonic hedgehog signaling during external genitalia development. Development 128, 4241–4250 (2001).
Suzuki, K. et al. Regulation of outgrowth and apoptosis for the terminal appendage: external genitalia development by concerted actions of BMP signaling [corrected]. Development 130, 6209–6220 (2003).
Perriton, C. L., Powles, N., Chiang, C., Maconochie, M. K. & Cohn, M. J. Sonic hedgehog signaling from the urethral epithelium controls external genital development. Dev. Biol. 247, 26–46 (2002).
Haraguchi, R. et al. Molecular analysis of coordinated bladder and urogenital organ formation by hedgehog signaling. Development 134, 525–533 (2007).
Huang, Y. C., Chen, F. & Li, X. Clarification of mammalian cloacal morphogenesis using high-resolution episcopic microscopy. Dev. Biol. 409, 106–113 (2016).
Satoh, Y. et al. Regulation of external genitalia development by concerted actions of FGF ligands and FGF receptors. Anat. Embryol. 208, 479–486 (2004).
Petiot, A., Perriton, C. L., Dickson, C. & Cohn, M. J. Development of the mammalian urethra is controlled by Fgfr2-IIIb. Development 132, 2441–2450 (2005).
Harada, M. et al. Tissue-specific roles of FGF signaling in external genitalia development. Dev. Dyn. 244, 759–773 (2015).
Gredler, M. L., Seifert, A. W. & Cohn, M. J. Tissue-specific roles of Fgfr2 in development of the external genitalia. Development 142, 2203–2212 (2015).
Ogino, Y., Katoh, H. & Yamada, G. Androgen dependent development of a modified anal fin, gonopodium, as a model to understand the mechanism of secondary sexual character expression in vertebrates. FEBS Lett. 575, 119–126 (2004).
Chung, J. W., Pask, A. J. & Renfree, M. B. Seminiferous cord formation is regulated by hedgehog signaling in the marsupial. Biol. Reprod. 86, 80 (2012).
Miyagawa, S. et al. The role of sonic hedgehog-Gli2 pathway in the masculinization of external genitalia. Endocrinology 152, 2894–2903 (2011).
He, F. et al. Adult Gli2+/−;Gli3Δ699/ + male and female mice display a spectrum of genital malformation. PLoS ONE 11, e0165958 (2016).
Yao, H. H., Whoriskey, W. & Capel, B. Desert hedgehog/patched 1 signaling specifies fetal Leydig cell fate in testis organogenesis. Genes Dev. 16, 1433–1440 (2002).
Jameson, S. A., Lin, Y. T. & Capel, B. Testis development requires the repression of Wnt4 by Fgf signaling. Dev. Biol. 370, 24–32 (2012).
Zhu, H. et al. Analysis of Wnt gene expression in prostate cancer: mutual inhibition by WNT11 and the androgen receptor. Cancer Res. 64, 7918–7926 (2004).
Bengoa-Vergniory, N. et al. Identification of noncanonical wnt receptors required for Wnt-3a-induced early differentiation of human neural stem cells. Mol. Neurobiol. 54, 6213–6224 (2016).
Reutter, H. et al. Genome-wide association study and mouse expression data identify a highly conserved 32 kb intergenic region between WNT3 and WNT9b as possible susceptibility locus for isolated classic exstrophy of the bladder. Hum. Mol. Genet. 23, 5536–5544 (2014).
Miyagawa, S. et al. Dosage-dependent hedgehog signals integrated with Wnt/beta-catenin signaling regulate external genitalia formation as an appendicular program. Development 136, 3969–3978 (2009).
Miyagawa, S. et al. Disruption of the temporally regulated cloaca endodermal β-catenin signaling causes anorectal malformations. Cell Death Differ. 21, 990–997 (2014).
Lin, C., Yin, Y., Long, F. & Ma, L. Tissue-specific requirements of beta-catenin in external genitalia development. Development 135, 2815–2825 (2008).
Lin, C. et al. Delineating a conserved genetic cassette promoting outgrowth of body appendages. PLoS Genet. 9, e1003231 (2013).
Pawlowski, J. E. et al. Liganded androgen receptor interaction with beta-catenin: nuclear co-localization and modulation of transcriptional activity in neuronal cells. J. Biol. Chem. 277, 20702–20710 (2002).
von Ahrens, D., Bhagat, T. D., Nagrath, D., Maitra, A. & Verma, A. The role of stromal cancer-associated fibroblasts in pancreatic cancer. J. Hematol. Oncol. 10, 76 (2017).
Leach, D. A. et al. Cell-lineage specificity and role of AP-1 in the prostate fibroblast androgen receptor cistrome. Mol. Cell Endocrinol. 439, 261–272 (2017).
Wikström, P., Marusic, J., Stattin, P. & Bergh, A. Low stroma androgen receptor level in normal and tumor prostate tissue is related to poor outcome in prostate cancer patients. Prostate 69, 799–809 (2009).
Tanner, M. J. et al. Effects of androgen receptor and androgen on gene expression in prostate stromal fibroblasts and paracrine signaling to prostate cancer cells. PLoS ONE 6, e16027 (2011).
Yang, Y. A. & Yu, J. Current perspectives on FOXA1 regulation of androgen receptor signaling and prostate cancer. Genes Dis. 2, 144–151 (2015).
Mazahery, A. R. et al. Functional analysis of ectodermal β-catenin during external genitalia formation. Congenit. Anom. 53, 34–41 (2013).
Matsushita, S. et al. Androgen regulates Mafb expression through its 3’UTR during mouse urethral masculinization. Endocrinology 157, 844–857 (2016).
Kataoka, K., Fujiwara, K. T., Noda, M. & Nishizawa, M. MafB, a new Maf family transcription activator that can associate with Maf and Fos but not with Jun. Mol. Cell. Biol. 14, 7581–7591 (1994).
Moriguchi, T. et al. MafB is essential for renal development and F4/80 expression in macrophages. Mol. Cell. Biol. 26, 5715–5727 (2006).
Wagner, E. F. Functions of AP1 (Fos/Jun) in bone development. Ann Rheum Dis 61(Suppl 2), ii40–ii42 (2002).
Shaulian, E. & Karin, M. AP-1 as a regulator of cell life and death. Nat. Cell Biol. 4, E131–E136 (2002).
Shaulian, E. & Karin, M. AP-1 in cell proliferation and survival. Oncogene 20, 2390–2400 (2001).
Jochum, W., Passegué, E. & Wagner, E. F. AP-1 in mouse development and tumorigenesis. Oncogene 20, 2401–2412 (2001).
Villaseñor, T. et al. Activation of the Wnt Pathway by Mycobacterium tuberculosis: a Wnt-Wnt Situation. Front. Immunol. 8, 50 (2017).
Suda, N. et al. Dimeric combinations of MafB, cFos and cJun control the apoptosis-survival balance in limb morphogenesis. Development 141, 2885–2894 (2014).
Kelly, L. M., Englmeier, U., Lafon, I., Sieweke, M. H. & Graf, T. MafB is an inducer of monocytic differentiation. EMBO J. 19, 1987–1997 (2000).
Kim, K. et al. MafB negatively regulates RANKL-mediated osteoclast differentiation. Blood 109, 3253–3259 (2007).
Abdellatif, A. M. et al. Role of large MAF transcription factors in the mouse endocrine pancreas. Exp. Anim. 64, 305–312 (2015).
Tillmanns, S. et al. SUMO modification regulates MafB-driven macrophage differentiation by enabling Myb-dependent transcriptional repression. Mol. Cell. Biol. 27, 5554–5564 (2007).
van Stralen, E. et al. Identification of primary MAFB target genes in multiple myeloma. Exp. Hematol. 37, 78–86 (2009).
Zankl, A. et al. Multicentric carpotarsal osteolysis is caused by mutations clustering in the amino-terminal transcriptional activation domain of MAFB. Am. J. Hum. Genet. 90, 494–501 (2012).
Beaty, T. H. et al. A genome-wide association study of cleft lip with and without cleft palate identifies risk variants near MAFB and ABCA4. Nat. Genet. 42, 525–529 (2010).
Joss, S. K., Paterson, W., Donaldson, M. D. & Tolmie, J. L. Cleft palate, hypotelorism, and hypospadias: Schilbach-Rott syndrome. Am. J. Med. Genet. 113, 105–107 (2002).
Leirós, G. J., Ceruti, J. M., Castellanos, M. L., Kusinsky, A. G. & Balañá, M. E. Androgens modify Wnt agonists/antagonists expression balance in dermal papilla cells preventing hair follicle stem cell differentiation in androgenetic alopecia. Mol. Cell Endocrinol. 439, 26–34 (2017).
Zitzmann, M. & Nieschlag, E. The CAG repeat polymorphism within the androgen receptor gene and maleness. Int. J. Androl 26, 76–83 (2003).
Heemers, H. V. & Tindall, D. J. Androgen receptor (AR) coregulators: a diversity of functions converging on and regulating the AR transcriptional complex. Endocr. Rev. 28, 778–808 (2007).
Schneider, J. A. & Logan, S. K. Revisiting the role of Wnt/ß-catenin signaling in prostate cancer. Mol. Cell Endocrinol. 15, 3–8 (2017).
Sturgeon, K. et al. Cdx1 refines positional identity of the vertebrate hindbrain by directly repressing Mafb expression. Development 138, 65–74 (2011).
Menéndez-Gutiérrez, M. P. et al. Retinoid X receptors orchestrate osteoclast differentiation and postnatal bone remodeling. J. Clin. Invest. 125, 809–823 (2015).
Yao, H. H., Tilmann, C., Zhao, G. Q. & Capel, B. The battle of the sexes: opposing pathways in sex determination. Novartis Found. Symp. 244, 187–198; discussion 198–206, 253–257 (2002).
DeFalco, T. et al. Macrophages contribute to the spermatogonial niche in the adult testis. Cell Rep. 12, 1107–1119 (2015).
Yoshida, S. et al. Neurogenin3 delineates the earliest stages of spermatogenesis in the mouse testis. Dev. Biol. 269, 447–458 (2004).
Febbo, P. G. et al. Androgen mediated regulation and functional implications of fkbp51 expression in prostate cancer. J. Urol. 173, 1772–1777 (2005).
Yong, W. et al. Essential role for co-chaperone Fkbp52 but not Fkbp51 in androgen receptor-mediated signaling and physiology. J. Biol. Chem. 282, 5026–5036 (2007).
Chen, H. et al. Fkbp52 regulates androgen receptor transactivation activity and male urethra morphogenesis. J. Biol. Chem. 285, 27776–27784 (2010).
Storer Samaniego, C. et al. The FKBP52 cochaperone acts in synergy with β-catenin to potentiate androgen receptor signaling. PLoS ONE 10, e0134015 (2015).
Nishida, H. et al. Gene expression analyses on embryonic external genitalia: identification of regulatory genes possibly involved in masculinization processes. Congenit. Anom. 48, 63–67 (2008).
Ni, L. et al. FKBP51 promotes assembly of the Hsp90 chaperone complex and regulates androgen receptor signaling in prostate cancer cells. Mol. Cell. Biol. 30, 1243–1253 (2010).
Makkonen, H., Kauhanen, M., Paakinaho, V., Jääskeläinen, T. & Palvimo, J. J. Long-range activation of FKBP51 transcription by the androgen receptor via distal intronic enhancers. Nucleic Acids Res. 37, 4135–4148 (2009).
Yucel, S., Dravis, C., Garcia, N., Henkemeyer, M. & Baker, L. A. Hypospadias and anorectal malformations mediated by Eph/ephrin signaling. J. Pediatr. Urol. 3, 354–363 (2007).
Ipulan, L. A. et al. Development of the external genitalia and their sexual dimorphic regulation in mice. Sex. Dev. 8, 297–310 (2014).
Bennett, N. C., Gardiner, R. A., Hooper, J. D., Johnson, D. W. & Gobe, G. C. Molecular cell biology of androgen receptor signalling. Int. J. Biochem. Cell Biol. 42, 813–827 (2010).
Chauvin, T. R. & Griswold, M. D. Androgen-regulated genes in the murine epididymis. Biol. Reprod. 71, 560–569 (2004).
Bowman, C. J. et al. Altered gene expression during rat Wolffian duct development following di(n-butyl) phthalate exposure. Toxicol. Sci. 86, 161–174 (2005).
Turner, K. J. et al. Altered gene expression during rat Wolffian duct development in response to in utero exposure to the antiandrogen linuron. Toxicol. Sci. 74, 114–128 (2003).
Hannema, S. E. & Hughes, I. A. Regulation of Wolffian duct development. Horm. Res. 67, 142–151 (2007).
Hu, S. et al. Research resource: genome-wide mapping of in vivo androgen receptor binding sites in mouse epididymis. Mol. Endocrinol. 24, 2392–2405 (2010).
Murashima, A. et al. Essential roles of androgen signaling in Wolffian duct stabilization and epididymal cell differentiation. Endocrinology 152, 1640–1651 (2011).
Suzuki, K. et al. Abnormal urethra formation in mouse models of split-hand/split-foot malformation type 1 and type 4. Eur. J. Hum. Genet. 16, 36–44 (2008).
Nagel, S. C. & Bromfield, J. J. Bisphenol a: a model endocrine disrupting chemical with a new potential mechanism of action. Endocrinology 154, 1962–1964 (2013).
Oliveira, C. A. et al. Differential hormonal regulation of estrogen receptors ERalpha and ERbeta and androgen receptor expression in rat efferent ductules. Reproduction 128, 73–86 (2004).
Sinclair, A. W., Cao, M., Pask, A., Baskin, L. & Cunha, G. R. Flutamide-induced hypospadias in rats: a critical assessment. Differentiation 94, 37–57 (2017).
Cunha, G. R., Sinclair, A., Risbridger, G., Hutson, J. & Baskin, L. S. Current understanding of hypospadias: relevance of animal models. Nat. Rev. Urol. 12, 271–280 (2015).
Hiort, O. The differential role of androgens in early human sex development. BMC Med. 11, 152 (2013).
Toppari, J., Virtanen, H. E., Main, K. M. & Skakkebaek, N. E. Cryptorchidism and hypospadias as a sign of testicular dysgenesis syndrome (TDS): environmental connection. Birth Defects Res. A Clin. Mol. Teratol. 88, 910–919 (2010).
Yiee, J. H. & Baskin, L. S. Environmental factors in genitourinary development. J. Urol. 184, 34–41 (2010).
Diamanti-Kandarakis, E. et al. Endocrine-disrupting chemicals: an endocrine society scientific statement. Endocr. Rev. 30, 293–342 (2009).
Lubahn, D. B. et al. Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proc. Natl Acad. Sci. USA 90, 11162–11166 (1993).
Miyagawa, S. et al. Characterization of diethylstilbestrol-induced hypospadias in female mice. Anat. Rec. 266, 43–50 (2002).
Schramm, C. et al. De novo microduplication at 22q11.21 in a patient with VACTERL association. Eur. J. Med. Genet. 54, 9–13 (2011).
Moggs, J. G. et al. Anti-proliferative effect of estrogen in breast cancer cells that re-express ERalpha is mediated by aberrant regulation of cell cycle genes. J. Mol. Endocrinol. 34, 535–551 (2005).
Sabbah, M., Courilleau, D., Mester, J. & Redeuilh, G. Estrogen induction of the cyclin D1 promoter: involvement of a cAMP response-like element. Proc. Natl Acad. Sci. USA 96, 11217–11222 (1999).
Ma, L. M. et al. Estrogen effects on fetal penile and urethral development in organotypic mouse genital tubercle culture. J. Urol. 182, 2511–2517 (2009).
Kalfa, N. et al. Genomic variants of ATF3 in patients with hypospadias. J. Urol. 180, 2183–2188 (2008).
Wang, H. et al. The stress response mediator ATF3 represses androgen signaling by binding the androgen receptor. Mol. Cell. Biol. 32, 3190–3202 (2012).
Tanaka, Y. et al. Systems analysis of ATF3 in stress response and cancer reveals opposing effects on pro-apoptotic genes in p53 pathway. PLoS ONE 6, e26848 (2011).
Tamura, K. et al. Stress response gene ATF3 is a target of c-myc in serum-induced cell proliferation. EMBO J. 24, 2590–2601 (2005).
Li, J., Willingham, E. & Baskin, L. S. Gene expression profiles in mouse urethral development. BJU Int. 98, 880–885 (2006).
Kang, Y., Chen, C. R. & Massagué, J. A self-enabling TGFbeta response coupled to stress signaling: Smad engages stress response factor ATF3 for Id1 repression in epithelial cells. Mol. Cell 11, 915–926 (2003).
Willingham, E. & Baskin, L. S. Candidate genes and their response to environmental agents in the etiology of hypospadias. Nat. Clin. Pract. Urol. 4, 270–279 (2007).
Tannour-Louet, M. et al. Increased gene copy number of VAMP7 disrupts human male urogenital development through altered estrogen action. Nat. Med. 20, 715–724 (2014).
Dahlman-Wright, K. et al. Interplay between AP-1 and estrogen receptor α in regulating gene expression and proliferation networks in breast cancer cells. Carcinogenesis 33, 1684–1691 (2012).
Reutter, H., Hilger, A. C., Hildebrandt, F. & Ludwig, M. Underlying genetic factors of the VATER/VACTERL association with special emphasis on the “Renal” phenotype. Pediatr. Nephrol. 31, 2025–2033 (2016).
Niederreither, K. & Dollé, P. Retinoic acid in development: towards an integrated view. Nat. Rev. Genet. 9, 541–553 (2008).
Jiang, J., Ma, L., Yuan, L., Wang, X. & Zhang, W. Study on developmental abnormalities in hypospadiac male rats induced by maternal exposure to di-n-butyl phthalate (DBP). Toxicology 232, 286–293 (2007).
Ogino, Y. et al. External genitalia formation: role of fibroblast growth factor, retinoic acid signaling, and distal urethral epithelium. Ann. NY Acad. Sci. 948, 13–31 (2001).
Liu, L. et al. Retinoic acid signaling regulates sonic hedgehog and bone morphogenetic protein signalings during genital tubercle development. Birth Defects Res. B Dev. Reprod. Toxicol. 95, 79–88 (2012).
Fukami, M. et al. Anorectal and urinary anomalies and aberrant retinoic acid metabolism in cytochrome P450 oxidoreductase deficiency. Mol. Genet. Metab. 100, 269–273 (2010).
Udhane, S. S., Pandey, A. V., Hofer, G., Mullis, P. E. & Flück, C. E. Retinoic acid receptor beta and angiopoietin-like protein 1 are involved in the regulation of human androgen biosynthesis. Sci. Rep. 5, 10132 (2015).
Li, M. T., Richter, F., Chang, C., Irwin, R. J. & Huang, H. Androgen and retinoic acid interaction in LNCaP cells, effects on cell proliferation and expression of retinoic acid receptors and epidermal growth factor receptor. BMC Cancer 2, 16 (2002).
Rivera-Gonzalez, G. C. et al. Retinoic acid and androgen receptors combine to achieve tissue specific control of human prostatic transglutaminase expression: a novel regulatory network with broader significance. Nucleic Acids Res. 40, 4825–4840 (2012).
Acknowledgements
The authors thank A. Thomson, L. Baskin, J. Cunha, R. Nishinakamura, S. Takahashi, G. Prins, K.-I. Matsumoto, H. Reutter, and T. DeFalco for their encouragement and discussion points. The authors also thank T. I. Iba and all laboratory colleagues for their assistance. This work was supported by the Japan Society for the Promotion of Science grants 18K06938, 18K06837, 17K18024, 15H04300, 15K15403, 15K10647, 15K19013, and 15J11033.
Author information
Authors and Affiliations
Contributions
S.Ma., K.S., and G.Y. discussed the content, wrote the manuscript, and reviewed and edited the manuscript before submission. A.M., D.K., A.R.A., S.Mi., R.H., and Y.O. discussed the content and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Matsushita, S., Suzuki, K., Murashima, A. et al. Regulation of masculinization: androgen signalling for external genitalia development. Nat Rev Urol 15, 358–368 (2018). https://doi.org/10.1038/s41585-018-0008-y
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41585-018-0008-y
This article is cited by
-
Periconceptional maternal folate supplementation impacts a diverse range of congenital malformations
Pediatric Research (2024)
-
Role of epigenetics in the etiology of hypospadias through penile foreskin DNA methylation alterations
Scientific Reports (2023)
-
Hypospadias: lessons learned. An overview of incidence, epidemiology, surgery, research, complications, and outcomes
International Journal of Impotence Research (2023)
-
hsa_circ_0000417 downregulation suppresses androgen receptor expression and apoptotic signals in human foreskin fibroblasts via sponging miR-6756-5p
Molecular Biology Reports (2023)
-
Improved biomarker discovery through a plot twist in transcriptomic data analysis
BMC Biology (2022)