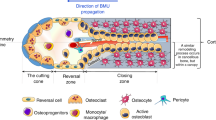
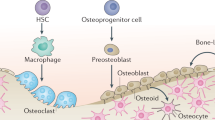
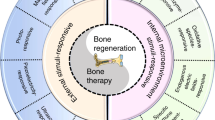
Abstract
Bone has a remarkable endogenous regenerative capacity that enables scarless healing and restoration of its prior mechanical function, even under challenging conditions such as advanced age and metabolic or immunological degenerative diseases. However — despite much progress — a high number of bone injuries still heal with unsatisfactory outcomes. The mechanisms leading to impaired healing are heterogeneous, and involve exuberant and non-resolving immune reactions or overstrained mechanical conditions that affect the delicate regulation of the early initiation of scar-free healing. Every healing process begins phylogenetically with an inflammatory reaction, but its spatial and temporal intensity must be tightly controlled. Dysregulation of this inflammatory cascade directly affects the subsequent healing phases and hinders the healing progression. This Review discusses the complex processes underlying bone regeneration, focusing on the early healing phase and its highly dynamic environment, where vibrant changes in cellular and tissue composition alter the mechanical environment and thus affect the signalling pathways that orchestrate the healing process. Essential to scar-free healing is the interplay of various dynamic cascades that control timely resolution of local inflammation and tissue self-organization, while also providing sufficient local stability to initiate endogenous restoration. Various immunotherapy and mechanobiology-based therapy options are under investigation for promoting bone regeneration.
Key points
-
Bone healing is a dynamic yet stable process that occurs throughout an individuals’ lifespan; patient-specific factors can increase the risk of healing disorders but the causal relationship is incompletely understood.
-
The bone healing process is tightly regulated and very well orchestrated but might easily become disrupted; a relevant percentage of patients with a fracture experience unsatisfactory healing outcomes.
-
Emerging evidence highlights the patients’ immune competence as a decisive factor in the healing process; various components of the immune system have beneficial or detrimental effects.
-
Mechanical conditions promote bone regeneration, but this knowledge has not yet been exploited in daily clinical practice or current surgical treatment strategies.
-
The initial stages of bone healing are characterized by dynamic self-organization of the tissue that governs the healing outcome; invading immune and/or matrix-generating cells define the signalling pattern that guides healing.
-
New patient-specific treatment approaches are on the horizon that target immunomodulatory and biomechanical aspects.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
United States Bone and Joint Initiative. The Burden of Musculoskeletal Diseases in the United States, 3rd edn (United States Bone and Joint Initiative, 2014).
Storm, A. Gesundheitsreport 2018. Analyse der Arbeitsunfähigkeitsdaten, Band 21 (DAK-Gesundheit, 2018).
Brennan, S. L. et al. Rheumatoid arthritis and incident fracture in women: a case–control study. BMC Musculoskelet. Disord. 15, 13 (2014).
Claes, L., Recknagel, S. & Ignatius, A. Fracture healing under healthy and inflammatory conditions. Nat. Rev. Rheumatol. 8, 133–143 (2012).
GBD 2019 Fracture Collaborators Global, regional, and national burden of bone fractures in 204 countries and territories, 1990–2019: a systematic analysis from the Global Burden of Disease Study 2019 Lancet Healthy Longev. 2 e580–e592 (2021).
Borgstrom, F. et al. Fragility fractures in Europe: burden, management and opportunities. Arch. Osteoporos. 15, 59 (2020).
Tompkins, B. A. et al. IMPACT: preclinical studies of cell therapy for human disease. Circ. Res. 122, 1006–1020 (2018).
Grigorian-Shamagian, L. et al. Insights into therapeutic products, preclinical research models, and clinical trials in cardiac regenerative and reparative medicine: where are we now and the way ahead. Current opinion paper of the ESC Working Group on Cardiovascular Regenerative and Reparative Medicine. Cardiovasc. Res. 117, 1428–1433 (2021).
Shigeto, J. et al. Preclinical toxicity studies for regenerative medicine in Japan. Clin. Ther. 40, 1813–1822 (2018).
Greenhill, C. Metabolism: role of bone in glucose metabolism. Nat. Rev. Endocrinol. 14, 191 (2018).
Langdahl, B., Ferrari, S. & Dempster, D. W. Bone modeling and remodeling: potential as therapeutic targets for the treatment of osteoporosis. Ther. Adv. Musculoskelet. Dis. 8, 225–235 (2016).
Rolvien, T. & Amling, M. Disuse osteoporosis: clinical and mechanistic insights. Calcif. Tissue Int. 110, 592–604 (2022).
Dello Russo, C. et al. Physiological adaptations affecting drug pharmacokinetics in space: what do we really know? A critical review of the literature. Br. J. Pharmacol. 179, 2538–2557 (2022).
Lombardi, G., Ziemann, E. & Banfi, G. Physical activity and bone health: what is the role of immune system? A narrative review of the third way. Front. Endocrinol. 10, 60 (2019).
Arron, J. R. & Choi, Y. Bone versus immune system. Nature 408, 535–536 (2000).
Knecht, R. S. et al. Mechanobiological principles influence the immune response in regeneration: implications for bone healing. Front. Bioeng. Biotechnol. 9, 614508 (2021).
Shen, B. et al. A mechanosensitive peri-arteriolar niche for osteogenesis and lymphopoiesis. Nature 591, 438–444 (2021).
Bucher, C. H., Lei, H., Duda, G. N., Volk, H.-D. & Schmidt-Bleek, K. The role of immune reactivity in bone regeneration Adv. Tech. Bone Regen. 18 3697–3707 (2016).
Bucher, C. H. et al. Experience in the adaptive immunity impacts bone homeostasis, remodeling, and healing. Front. Immunol. 10, 797 (2019).
Schmidt-Bleek, K., Kwee, B. J., Mooney, D. J. & Duda, G. N. Boon and bane of inflammation in bone tissue regeneration and its link with angiogenesis. Tissue Eng. Part B Rev. 21, 354–364 (2015).
Schell, H. et al. The haematoma and its role in bone healing. J. Exp. Orthop. 4, 5 (2017).
Gaber, T., Dziurla, R., Tripmacher, R., Burmester, G. R. & Buttgereit, F. Hypoxia inducible factor (HIF) in rheumatology: low O2! See what HIF can do! Ann. Rheum. Dis. 64, 971–980 (2005).
Lang, A. et al. MIF does only marginally enhance the pro-regenerative capacities of DFO in a mouse-osteotomy-model of compromised bone healing conditions. Bone 154, 116247 (2022).
Loeffler, J., Duda, G. N., Sass, F. A. & Dienelt, A. The metabolic microenvironment steers bone tissue regeneration. Trends Endocrinol. Metab. 29, 99–110 (2018).
Street, J. et al. Is human fracture hematoma inherently angiogenic? Clin. Orthop. Relat. Res. https://doi.org/10.1097/00003086-200009000-00033 (2000).
Berkmann, J. C. et al. Early pH changes in musculoskeletal tissues upon injury – aerobic catabolic pathway activity linked to inter-individual differences in local pH. Int. J. Mol. Sci. https://doi.org/10.3390/ijms21072513 (2020).
Gerstenfeld, L. C., Cullinane, D. M., Barnes, G. L., Graves, D. T. & Einhorn, T. A. Fracture healing as a post-natal developmental process: molecular, spatial, and temporal aspects of its regulation. J. Cell. Biochem. 88, 873–884 (2003).
Bogeska, R. et al. Inflammatory exposure drives long-lived impairment of hematopoietic stem cell self-renewal activity and accelerated aging. Cell Stem Cell 29, 1273–1284 (2022).
Schlundt, C. et al. Clinical and research approaches to treat non-union fracture. Curr. Osteoporos. Rep. 16, 155–168 (2018).
Steward, S. K. Fracture non-union: a review of clinical challenges and future research needs. Malays. Orthop. J. 13, 1–10 (2019).
Sass, F. A. et al. Immunology guides skeletal muscle regeneration. Int. J. Mol. Sci. https://doi.org/10.3390/ijms19030835 (2018).
Schmidt-Bleek, K. et al. Cellular composition of the initial fracture hematoma compared to a muscle hematoma: a study in sheep. J. Orthop. Res. 27, 1147–1151 (2009).
Jeyaraman, M. et al. Osteogenic and chondrogenic potential of periosteum-derived mesenchymal stromal cells: do they hold the key to the future? Pharmaceuticals 14, 1133 (2021).
Matsushita, Y. et al. A Wnt-mediated transformation of the bone marrow stromal cell identity orchestrates skeletal regeneration. Nat. Commun. 11, 332 (2020).
Julien, A. et al. Direct contribution of skeletal muscle mesenchymal progenitors to bone repair. Nat. Commun. 12, 2860 (2021).
Ambrosi, T. H. et al. Aged skeletal stem cells generate an inflammatory degenerative niche. Nature 597, 256–262 (2021).
Colnot, C. Skeletal cell fate decisions within periosteum and bone marrow during bone regeneration. J. Bone Miner. Res. 24, 274–282 (2009).
Moore, S. R. et al. Translating periosteum’s regenerative power: insights from quantitative analysis of tissue genesis with a periosteum substitute implant. Stem Cell Transl. Med. 5, 1739–1749 (2016).
Debnath, S. et al. Discovery of a periosteal stem cell mediating intramembranous bone formation. Nature 562, 133–139 (2018).
van Gastel, N. et al. Lipid availability determines fate of skeletal progenitor cells via SOX9. Nature 579, 111–117 (2020).
Tsukasaki, M. et al. Periosteal stem cells control growth plate stem cells during postnatal skeletal growth. Nat. Commun. 13, 4166 (2022).
Sass, F. A. et al. CD31+ cells from peripheral blood facilitate bone regeneration in biologically impaired conditions through combined effects on immunomodulation and angiogenesis. J. Bone Miner. Res. 32, 902–912 (2016).
Schmidt-Bleek, K. et al. Initial immune reaction and angiogenesis in bone healing. J. Tissue Eng. Regen. Med. 8, 120–130 (2012).
Maruyama, M. et al. Modulation of the inflammatory response and bone healing. Front. Endocrinol. 11, 386 (2020).
Weitzmann, M. N. Bone and the immune system. Toxicol. Pathol. 45, 911–924 (2017).
Guder, C., Gravius, S., Burger, C., Wirtz, D. C. & Schildberg, F. A. Osteoimmunology: a current update of the interplay between bone and the immune system. Front. Immunol. 11, 58 (2020).
Ono, T. & Takayanagi, H. Osteoimmunology in bone fracture healing. Curr. Osteoporos. Rep. 15, 367–375 (2017).
Muire, P. J., Mangum, L. H. & Wenke, J. C. Time course of immune response and immunomodulation during normal and delayed healing of musculoskeletal wounds. Front. Immunol. 11, 1056 (2020).
Yang, N. & Liu, Y. The role of the immune microenvironment in bone regeneration. Int. J. Med. Sci. 18, 3697–3707 (2021).
Tsukasaki, M. & Takayanagi, H. Osteoimmunology: evolving concepts in bone-immune interactions in health and disease. Nat. Rev. Immunol. 19, 626–642 (2019).
Takayanagi, H. Osteoimmunology: shared mechanisms and crosstalk between the immune and bone systems. Nat. Rev. Immunol. 7, 292–304 (2007).
Walsh, M. C., Takegahara, N., Kim, H. & Choi, Y. Updating osteoimmunology: regulation of bone cells by innate and adaptive immunity. Nat. Rev. Rheumatol. 14, 146–156 (2018).
Weitzmann, M. N. & Ofotokun, I. Physiological and pathophysiological bone turnover – role of the immune system. Nat. Rev. Endocrinol. 12, 518–532 (2016).
Alexander, K. A. et al. Osteal macrophages promote in vivo intramembranous bone healing in a mouse tibial injury model. J. Bone Miner. Res. 26, 1517–1532 (2011).
Ono, T. et al. IL-17-producing γδ T cells enhance bone regeneration. Nat. Commun. 7, 10928 (2016).
Ehnert, S. et al. Effects of immune cells on mesenchymal stem cells during fracture healing. World J. Stem Cell 13, 1667–1695 (2021).
Woloszyk, A. et al. Fracture hematoma micro-architecture influences transcriptional profile and plays a crucial role in determining bone healing outcomes. Biomater. Adv. 139, 213027 (2022).
Bastian, O. W., Koenderman, L., Alblas, J., Leenen, L. P. & Blokhuis, T. J. Neutrophils contribute to fracture healing by synthesizing fibronectin+ extracellular matrix rapidly after injury. Clin. Immunol. 164, 78–84 (2016).
Gaber, T. et al. Adaptation of human CD4+ T cells to pathophysiological hypoxia: a transcriptome analysis. J. Rheumatol. 36, 2655–2669 (2009).
Reinke, S. et al. Terminally differentiated CD8+ T cells negatively affect bone regeneration in humans. Sci. Transl. Med. 5, 177ra136 (2013).
Schlundt, C. et al. The multifaceted roles of macrophages in bone regeneration: a story of polarization, activation and time. Acta Biomater. 133, 46–57 (2021).
Bahney, C. S. et al. Cellular biology of fracture healing. J. Orthop. Res. 37, 35–50 (2019).
Mountziaris, P. M., Spicer, P. P., Kasper, F. K. & Mikos, A. G. Harnessing and modulating inflammation in strategies for bone regeneration. Tissue Eng. Part B, Rev. 17, 393–402 (2011).
Mountziaris, P. M. & Mikos, A. G. Modulation of the inflammatory response for enhanced bone tissue regeneration. Tissue Eng. Part B Rev. 14, 179–186 (2008).
Aizawa, T., Kon, T., Einhorn, T. A. & Gerstenfeld, L. C. Induction of apoptosis in chondrocytes by tumor necrosis factor-α. J. Orthop. Res. 19, 785–796 (2001).
Gerstenfeld, L. C. et al. Impaired fracture healing in the absence of TNF-α signaling: the role of TNF-α in endochondral cartilage resorption. J. Bone Miner. Res. 18, 1584–1592 (2003).
Gerstenfeld, L. C. et al. Impaired intramembranous bone formation during bone repair in the absence of tumor necrosis factor-α signaling. Cell Tissues Organs 169, 285–294 (2001).
Hashimoto, J. et al. Inhibitory effects of tumor necrosis factor alpha on fracture healing in rats. Bone 10, 453–457 (1989).
Kumar, B. V., Connors, T. J. & Farber, D. L. Human T cell development, localization, and function throughout life. Immunity 48, 202–213 (2018).
Epari, D. R., Lienau, J., Schell, H., Witt, F. & Duda, G. N. Pressure, oxygen tension and temperature in the periosteal callus during bone healing – an in vivo study in sheep. Bone 43, 734–739 (2008).
Stefanowski, J. et al. Limbostomy: longitudinal intravital microendoscopy in murine osteotomies. Cytometry A 97, 483–495 (2020).
Neve, A., Cantatore, F. P., Maruotti, N., Corrado, A. & Ribatti, D. Extracellular matrix modulates angiogenesis in physiological and pathological conditions. Biomed. Res. Int. 2014, 756078 (2014).
Newman, A. C., Nakatsu, M. N., Chou, W., Gershon, P. D. & Hughes, C. C. The requirement for fibroblasts in angiogenesis: fibroblast-derived matrix proteins are essential for endothelial cell lumen formation. Mol. Biol. Cell 22, 3791–3800 (2011).
Shiu, Y. T. et al. The role of mechanical stresses in angiogenesis. Crit. Rev. Biomed. Eng. 33, 431–510 (2005).
Grosso, A. et al. It takes two to tango: coupling of angiogenesis and osteogenesis for bone regeneration. Front. Bioeng. Biotechnol. 5, 68 (2017).
Chandurkar, M. K. & Han, S. J. Subcellular force quantification of endothelial cells using silicone pillar arrays. Methods Mol. Biol. 2375, 229–245 (2022).
Korff, T. & Augustin, H. G. Tensional forces in fibrillar extracellular matrices control directional capillary sprouting. J. Cell Sci. 112, 3249–3258 (1999).
Ceccarelli, J., Cheng, A. & Putnam, A. J. Mechanical strain controls endothelial patterning during angiogenic sprouting. Cell Mol. Bioeng. 5, 463–473 (2012).
Ouyang, M. et al. Sensing traction force on the matrix induces cell–cell distant mechanical communications for self-assembly. ACS Biomater. Sci. Eng. 6, 5833–5848 (2020).
Checa, S., Rausch, M. K., Petersen, A., Kuhl, E. & Duda, G. N. The emergence of extracellular matrix mechanics and cell traction forces as important regulators of cellular self-organization. Biomech. Model. Mechanobiol. 14, 1–13 (2015).
Huebsch, N. et al. Matrix elasticity of void-forming hydrogels controls transplanted-stem-cell-mediated bone formation. Nat. Mater. 14, 1269–1277 (2015).
Chaudhuri, O. et al. Hydrogels with tunable stress relaxation regulate stem cell fate and activity. Nat. Mater. 15, 326–334 (2016).
Borgiani, E. et al. Age-related changes in the mechanical regulation of bone healing are explained by altered cellular mechanoresponse. J. Bone Miner. Res. 34, 1923–1937 (2019).
Perier-Metz, C., Duda, G. N. & Checa, S. Initial mechanical conditions within an optimized bone scaffold do not ensure bone regeneration – an in silico analysis. Biomech. Model. Mechanobiol. 20, 1723–1731 (2021).
Claes, L. E. & Meyers, N. The direction of tissue strain affects the neovascularization in the fracture-healing zone. Med. Hypotheses 137, 109537 (2020).
Lienau, J. et al. Initial vascularization and tissue differentiation are influenced by fixation stability. J. Orthop. Res. 23, 639–645 (2005).
Cardwell, R. D. et al. Static and cyclic mechanical loading of mesenchymal stem cells on elastomeric, electrospun polyurethane meshes. J. Biomech. Eng. https://doi.org/10.1115/1.4030404 (2015).
Wang, Q., Huang, H., Wei, K. & Zhao, Y. Time-dependent combinatory effects of active mechanical loading and passive topographical cues on cell orientation. Biotechnol. Bioeng. 113, 2191–2201 (2016).
Schreivogel, S., Kuchibhotla, V., Knaus, P., Duda, G. N. & Petersen, A. Load-induced osteogenic differentiation of mesenchymal stromal cells is caused by mechano-regulated autocrine signaling. J. Tissue Eng. Regen. Med. 13, 1992–2008 (2019).
Kreja, L. et al. Effects of mechanical strain on human mesenchymal stem cells and ligament fibroblasts in a textured poly(L-lactide) scaffold for ligament tissue engineering. J. Mater. Sci. Mater. Med. 23, 2575–2582 (2012).
Petersen, A., Joly, P., Bergmann, C., Korus, G. & Duda, G. N. The impact of substrate stiffness and mechanical loading on fibroblast-induced scaffold remodeling. Tissue Eng. Part A 18, 1804–1817 (2012).
Kurpinski, K., Chu, J., Wang, D. & Li, S. Proteomic profiling of mesenchymal stem cell responses to mechanical strain and TGF-β1. Cell Mol. Bioeng. 2, 606–614 (2009).
Kopf, J., Petersen, A., Duda, G. N. & Knaus, P. BMP2 and mechanical loading cooperatively regulate immediate early signalling events in the BMP pathway. BMC Biol. 10, 37 (2012).
K. S Kang et al. Flexure-based device for cyclic strain-mediated osteogenic differentiation J. Biomech. Eng. 135 114501 (2013).
Delaine-Smith, R. M. & Reilly, G. C. The effects of mechanical loading on mesenchymal stem cell differentiation and matrix production. Vitam. Horm. 87, 417–480 (2011).
Legant, W. R., Chen, C. S. & Vogel, V. Force-induced fibronectin assembly and matrix remodeling in a 3D microtissue model of tissue morphogenesis. Integr. Biol. 4, 1164–1174 (2012).
Brauer, E. et al. Collagen fibrils mechanically contribute to tissue contraction in an in vitro wound healing scenario. Adv. Sci. 6, 1801780 (2019).
Huang, J. et al. Dermal extracellular matrix molecules in skin development, homeostasis, wound regeneration and diseases. Semin. Cell Dev. Biol. 128, 137–144 (2022).
Nikoloudaki, G., Snider, P., Simmons, O., Conway, S. J. & Hamilton, D. W. Periostin and matrix stiffness combine to regulate myofibroblast differentiation and fibronectin synthesis during palatal healing. Matrix Biol. 94, 31–56 (2020).
Pountos, I., Georgouli, T., Pneumaticos, S. & Giannoudis, P. V. Fracture non-union: can biomarkers predict outcome? Injury 44, 1725–1732 (2013).
Ryaby, J. T. Clinical effects of electromagnetic and electric fields on fracture healing. Clin. Orthop. Relat. Res. https://doi.org/10.1097/00003086-199810001-00021 (1998).
Mills, L. A., Aitken, S. A. & Simpson, A. The risk of non-union per fracture: current myths and revised figures from a population of over 4 million adults. Acta Orthop. 88, 434–439 (2017).
Leow, J. M., Clement, N. D. & Simpson, A. Application of the radiographic union scale for tibial fractures (RUST): assessment of healing rate and time of tibial fractures managed with intramedullary nailing. Orthop. Traumatol. Surg. Res. 106, 89–93 (2020).
Alt, V. et al. A health economic analysis of the use of rhBMP-2 in Gustilo-Anderson grade III open tibial fractures for the UK, Germany, and France. Injury 40, 1269–1275 (2009).
Kanakaris, N. K. et al. Application of bone morphogenetic proteins to femoral non-unions: a 4-year multicentre experience. Injury 40, S54–S61 (2009).
Kanakaris, N. K. et al. Application of BMP-7 to tibial non-unions: a 3-year multicenter experience. Injury 39, S83–S90 (2008).
Gelalis, I. D. et al. Diagnostic and treatment modalities in nonunions of the femoral shaft: a review. Injury 43, 980–988 (2012).
Corrales, L. A., Morshed, S., Bhandari, M. & Miclau, T. 3rd Variability in the assessment of fracture-healing in orthopaedic trauma studies. J. Bone Jt. Surg. Am. 90, 1862–1868 (2008).
Simpson, A. The forgotten phase of fracture healing: the need to predict nonunion. Bone Jt. Res. 6, 610–611 (2017).
Nicholson, J. A., Yapp, L. Z., Keating, J. F. & Simpson, A. Monitoring of fracture healing. Update on current and future imaging modalities to predict union. Injury 52, S29–S34 (2021).
Zura, R. et al. Epidemiology of fracture nonunion in 18 human bones. JAMA Surg. 151, e162775 (2016).
Schneider, E. et al. Loads acting in an intramedullary nail during fracture healing in the human femur. J. Biomech. 34, 849–857 (2001).
Claes, L. E. & Cunningham, J. L. Monitoring the mechanical properties of healing bone. Clin. Orthop. Relat. Res. 467, 1964–1971 (2009).
Calori, G. M. et al. Non-unions. Clin. Cases Min. Bone Metab. 14, 186–188 (2017).
Wittauer, M. et al. Definition of long-bone nonunion: a scoping review of prospective clinical trials to evaluate current practice. Injury 52, 3200–3205 (2021).
Whelan, D. B. et al. Development of the radiographic union score for tibial fractures for the assessment of tibial fracture healing after intramedullary fixation. J. Trauma. 68, 629–632 (2010).
Chiavaras, M. M. et al. The Radiographic Union Score for Hip (RUSH): the use of a checklist to evaluate hip fracture healing improves agreement between radiologists and orthopedic surgeons. Skelet. Radiol. 42, 1079–1088 (2013).
Frank, T. et al. The Radiographic Union Score for Hip (RUSH) identifies radiographic nonunion of femoral neck fractures. Clin. Orthop. Relat. Res. 474, 1396–1404 (2016).
Bhandari, M. et al. Radiographic Union Score for Hip substantially improves agreement between surgeons and radiologists. BMC Musculoskelet. Disord. 14, 70 (2013).
Leow, J. M., Clement, N. D., Tawonsawatruk, T., Simpson, C. J. & Simpson, A. H. The radiographic union scale in tibial (RUST) fractures: reliability of the outcome measure at an independent centre. Bone Jt. Res. 5, 116–121 (2016).
Sun, G. et al. Regulatory B cell is critical in bone union process through suppressing proinflammatory cytokines and stimulating Foxp3 in Treg cells. Clin. Exp. Pharmacol. Physiol. 44, 455–462 (2017).
Chitwood, J. R. et al. Predicting fracture healing with blood biomarkers: the potential to assess patient risk of fracture nonunion. Biomarkers 26, 703–717 (2021).
Working, Z. M. et al. A quantitative serum biomarker of circulating collagen X effectively correlates with endochondral fracture healing. J. Orthop. Res. 39, 53–62 (2021).
Jiang, H. et al. Downregulation of regulatory T cell function in patients with delayed fracture healing. Clin. Exp. Pharmacol. Physiol. 45, 430–436 (2018).
Schlundt, C. et al. Individual effector/regulator T cell ratios impact bone regeneration. Front. Immunol. 10, 1954 (2019).
Konnecke, I. et al. T and B cells participate in bone repair by infiltrating the fracture callus in a two-wave fashion. Bone 64, 155–165 (2014).
Horwitz, E. M. Advancing regenerative medicine the translational way. Sci. Transl. Med. 5, 177fs179 (2013).
Helfet, D. L. et al. AO philosophy and principles of fracture management – its evolution and evaluation. J. Bone Jt. Surg. Am. 85, 1156–1160 (2003).
Duda, G. N., Haas, N. P. & Bergmann, G. Founding of the Julius Wolff Institut Charite – Universitatsmedizin Berlin: editorial comment. Clin. Orthop. Relat. Res. 468, 1050–1051 (2010).
Palomares, K. T. et al. Mechanical stimulation alters tissue differentiation and molecular expression during bone healing. J. Orthop. Res. 27, 1123–1132 (2009).
Duda, G. N. et al. Does partial weight bearing unload a healing bone in external ring fixation. Langenbecks Arch. Surg. 388, 298–304 (2003).
Vetter, A. et al. Temporal tissue patterns in bone healing of sheep. J. Orthop. Res. 28, 1440–1447 (2010).
Braun, B. J. et al. Weight-bearing recommendations after operative fracture treatment – fact or fiction? Gait results with and feasibility of a dynamic, continuous pedobarography insole. Int. Orthop. 41, 1507–1512 (2017).
Claes, L., Eckert-Hubner, K. & Augat, P. The fracture gap size influences the local vascularization and tissue differentiation in callus healing. Langenbecks Arch. Surg. 388, 316–322 (2003).
Elliott, D. S. et al. A unified theory of bone healing and nonunion: BHN theory. Bone Jt. J. 98-B, 884–891 (2016).
Schell, H. et al. The course of bone healing is influenced by the initial shear fixation stability. J. Orthop. Res. 23, 1022–1028 (2005).
Epari, D. R., Schell, H., Bail, H. J. & Duda, G. N. Instability prolongs the chondral phase during bone healing in sheep. Bone 38, 864–870 (2006).
Epari, D. R., Taylor, W. R., Heller, M. O. & Duda, G. N. Mechanical conditions in the initial phase of bone healing. Clin. Biomech. 21, 646–655 (2006).
Kaspar, K. et al. Angle stable locking reduces interfragmentary movements and promotes healing after unreamed nailing. Study of a displaced osteotomy model in sheep tibiae. J. Bone Jt. Surg. Am. 87, 2028–2037 (2005).
Heyland, M. et al. Semi-rigid screws provide an auxiliary option to plate working length to control interfragmentary movement in locking plate fixation at the distal femur. Injury 46, S24–S32 (2015).
Mardian, S., Schaser, K. D., Duda, G. N. & Heyland, M. Working length of locking plates determines interfragmentary movement in distal femur fractures under physiological loading. Clin. Biomech. 30, 391–396 (2015).
Mardian, S. et al. What constitutes a good osteosynthesis? [German]. Chirurg 92, 863–872 (2021).
Dreyer, M. J. et al. European Society of Biomechanics S.M. Perren Award 2022: standardized tibio-femoral implant loads and kinematics. J. Biomech. 141, 111171 (2022).
Virzì, A. et al. Comprehensive review of 3D segmentation software tools for MRI usable for pelvic surgery planning. J. Digit. Imaging 33, 99–110 (2020).
Reichert, J. C. et al. A tissue engineering solution for segmental defect regeneration in load-bearing long bones. Sci. Transl. Med. 4, 141ra193 (2012).
Cipitria, A. et al. Porous scaffold architecture guides tissue formation. J. Bone Miner. Res. 27, 1275–1288 (2012).
Strong, A. L., Neumeister, M. W. & Levi, B. Stem cells and tissue engineering: regeneration of the skin and its contents. Clin. Plast. Surg. 44, 635–650 (2017).
Srivastava, A. K. & Bulte, J. W. Seeing stem cells at work in vivo. Stem Cell Rev. Rep. 10, 127–144 (2014).
Chikate, T. R. & Tang, L. Tracking and imaging of transplanted stem cells in animals. Methods Mol. Biol. 2150, 45–56 (2020).
Challen, G. A., Boles, N., Lin, K. K. & Goodell, M. A. Mouse hematopoietic stem cell identification and analysis. Cytometry A 75, 14–24 (2009).
Bhat, S., Viswanathan, P., Chandanala, S., Prasanna, S. J. & Seetharam, R. N. Expansion and characterization of bone marrow derived human mesenchymal stromal cells in serum-free conditions. Sci. Rep. 11, 3403 (2021).
Wang, Y., Yi, H. & Song, Y. The safety of MSC therapy over the past 15 years: a meta-analysis. Stem Cell Res. Ther. 12, 545 (2021).
Thum, T., Bauersachs, J., Poole-Wilson, P. A., Volk, H. D. & Anker, S. D. The dying stem cell hypothesis: immune modulation as a novel mechanism for progenitor cell therapy in cardiac muscle. J. Am. Coll. Cardiol. 46, 1799–1802 (2005).
Jiang, W. & Xu, J. Immune modulation by mesenchymal stem cells. Cell Prolif. 53, e12712 (2020).
Cai, Y. et al. Stroke treatment: is exosome therapy superior to stem cell therapy. Biochimie 179, 190–204 (2020).
Denu, R. A. et al. Fibroblasts and mesenchymal stromal/stem cells are phenotypically indistinguishable. Acta Haematol. 136, 85–97 (2016).
Hematti, P. Mesenchymal stromal cells and fibroblasts: a case of mistaken identity. Cytotherapy 14, 516–521 (2012).
Haniffa, M. A., Collin, M. P., Buckley, C. D. & Dazzi, F. Mesenchymal stem cells: the fibroblasts’ new clothes. Haematologica 94, 258–263 (2009).
Bautista-Hernandez, L. A., Gomez-Olivares, J. L., Buentello-Volante, B. & Bautista-de Lucio, V. M. Fibroblasts: the unknown sentinels eliciting immune responses against microorganisms. Eur. J. Microbiol. Immunol. 7, 151–157 (2017).
Geissler, S. et al. In serum veritas–in serum sanitas? Cell non-autonomous aging compromises differentiation and survival of mesenchymal stromal cells via the oxidative stress pathway. Cell Death Dis. 4, e970 (2013).
Wendler, S. et al. Immune modulation to enhance bone healing – a new concept to induce bone using prostacyclin to locally modulate immunity. Front. Immunol. 10, 713 (2019).
Schlundt, C. et al. Macrophages in bone fracture healing: their essential role in endochondral ossification. Bone 106, 78–89 (2018).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04543682 (2022).
Wan, C. et al. Activation of the hypoxia-inducible factor-1α pathway accelerates bone regeneration. Proc. Natl Acad. Sci. USA 105, 686–691 (2008).
Komatsu, D. E., Bosch-Marce, M., Semenza, G. L. & Hadjiargyrou, M. Enhanced bone regeneration associated with decreased apoptosis in mice with partial HIF-1α deficiency. J. Bone Miner. Res. 22, 366–374 (2007).
Wang, Y. et al. The hypoxia-inducible factor α pathway couples angiogenesis to osteogenesis during skeletal development. J. Clin. Invest. 117, 1616–1626 (2007).
Schmidt, A. H. Autologous bone graft: is it still the gold standard? Injury 52, S18–S22 (2021).
Yang, M. et al. Ophiopogonin D promotes bone regeneration by stimulating CD31(hi) EMCN(hi) vessel formation. Cell Prolif. 53, e12784 (2020).
Kim, S. W., Kim, H. & Yoon, Y. S. Advances in bone marrow-derived cell therapy: CD31-expressing cells as next generation cardiovascular cell therapy. Regen. Med. 6, 335–349 (2011).
Ernst, M. Smart implants in fracture care – only buzzword or real opportunity? Injury 52, S101–S105 (2021).
Tanzer, M., Laverdière, C., Barimani, B. & Hart, A. Augmented reality in arthroplasty: an overview of clinical applications, benefits, and limitations. J. Am. Acad. Orthop. Surg. 15, e760–e768 (2022).
Petersen, A. et al. A biomaterial with a channel-like pore architecture induces endochondral healing of bone defects. Nat. Commun. 9, 4430 (2018).
Paris, M. et al. Scaffold curvature-mediated novel biomineralization process originates a continuous soft tissue-to-bone interface. Acta Biomater. 60, 64–80 (2017).
Pobloth, A. M. et al. Mechanobiologically optimized 3D titanium-mesh scaffolds enhance bone regeneration in critical segmental defects in sheep. Sci. Transl. Med. 10, eaam8828 (2018).
Koh, A., Guerado, E. & Giannoudis, P. V. Atypical femoral fractures related to bisphosphonate treatment: issues and controversies related to their surgical management. Bone Jt. J. 99-B, 295–302 (2017).
Andrzejowski, P. & Giannoudis, P. V. The ‘diamond concept’ for long bone non-union management. J. Orthop. Traumatol. 20, 21 (2019).
Kroner, J. et al. Mast cells are critical regulators of bone fracture-induced inflammation and osteoclast formation and activity. J. Bone Miner. Res. 32, 2431–2444 (2017).
Kovtun, A. et al. The crucial role of neutrophil granulocytes in bone fracture healing. Eur. Cell Mater. 32, 152–162 (2016).
Schwarz, C. S. et al. Spatio-temporal bone remodeling after hematopoietic stem cell transplantation. Int. J. Mol. Sci. 22, 267 (2020).
Ambrosi, T. H. et al. Adipocyte accumulation in the bone marrow during obesity and aging impairs stem cell-based hematopoietic and bone regeneration. Cell Stem Cell https://doi.org/10.1016/j.stem.2017.02.009 (2017).
Acknowledgements
The authors acknowledge funding from the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) (SFB 1444).
Author information
Authors and Affiliations
Contributions
G.N.D., S.G., S.C., A.P. and K.S.-B. researched data for the article and contributed substantially to discussion of the content. All authors wrote the article and reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Rheumatology thanks H. Takayanagi, M. Haffner-Luntzer and L. Gerstenfeld for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Callus
-
Cartilaginous (soft callus) or bony (hard callus) material forming a connecting bridge across a bone fracture during repair.
- Cartilage hypertrophy
-
Cartilaginous tissue that is highly active and undergoing remodelling and calcification
- Cortical bone
-
A dense bone layer that surrounds the bone marrow cavity.
- Dynamization
-
A decrease in fixation stability to stimulate callus formation and bone formation.
- Endochondral ossification
-
The process in which bone is formed indirectly through a cartilage intermediate.
- Haematoma
-
A mass of mostly clotted blood that forms within an organ or tissue following blood vessel disruption.
- Intramembranous ossification
-
The process in which bone is formed directly from mesenchymal connective tissue.
- Lamellar bone
-
A mature form of bone consisting of collagen fibres organized in a regular and parallel fashion to form sheets (lamellae), which in turn form osteons to generate mechanically strong bone.
- Matrix mineralization
-
The process by which organic bone matrix is enriched with calcium phosphate, the main inorganic component of bone.
- Mechanotransduction
-
The mechanisms by which mechanical stimuli are converted into biological signals.
- Mechanical loading
-
An external force (such as a weight), either constant or variable, that imposes physical stress on a system or component.
- Mechanobiology
-
The study of how cells sense and respond to mechanical stimuli.
- Mechanosensation
-
The transduction of mechanical stimuli into a cellular reaction.
- Sprouting angiogenesis
-
The formation of new blood vessels (angiogenesis) from pre-existing vessels.
- Stress relaxation
-
A time-dependent decrease in stress under a constant strain.
- Trabecular bone
-
A honeycomb-like network (75–95% porosity) of interconnected bone rods and plates.
- Woven bone
-
A primitive form of bone, consisting of haphazardly organized collagen fibres that are mechanically weak.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Duda, G.N., Geissler, S., Checa, S. et al. The decisive early phase of bone regeneration. Nat Rev Rheumatol 19, 78–95 (2023). https://doi.org/10.1038/s41584-022-00887-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41584-022-00887-0
This article is cited by
-
Fixators dynamization for delayed union and non-union of femur and tibial fractures: a review of techniques, timing and influence factors
Journal of Orthopaedic Surgery and Research (2023)
-
Early tissue and healing responses after maxillary sinus augmentation using horizontal platelet rich fibrin bone blocks
BMC Oral Health (2023)
-
Gut T cells help mediate fracture healing
Nature Reviews Rheumatology (2023)
-
The ratio of alpha-calcitonin gene-related peptide to substance P is associated with the transition of bone metabolic states during aging and healing
Journal of Molecular Histology (2023)