Abstract
Glycosylation has a profound influence on protein activity and cell biology through a variety of mechanisms, such as protein stability, receptor interactions and signal transduction. In many rheumatic diseases, a shift in protein glycosylation occurs, and is associated with inflammatory processes and disease progression. For example, the Fc-glycan composition on (auto)antibodies is associated with disease activity, and the presence of additional glycans in the antigen-binding domains of some autoreactive B cell receptors can affect B cell activation. In addition, changes in synovial fibroblast cell-surface glycosylation can alter the synovial microenvironment and are associated with an altered inflammatory state and disease activity in rheumatoid arthritis. The development of our understanding of the role of glycosylation of plasma proteins (particularly (auto)antibodies), cells and tissues in rheumatic pathological conditions suggests that glycosylation-based interventions could be used in the treatment of these diseases.
Key points
-
Autoantigen-specific IgG in patients with rheumatic diseases has a distinct N-glycosylation signature in the fragment crystallizable (Fc) domain, characterized by fucosylation without sialylation or galactosylation.
-
In rheumatoid arthritis (RA) and anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis, autoantigen-specific IgG, as well as autoreactive B cell receptors, are extensively N-glycosylated in the fragment antigen-binding (Fab) domain.
-
Specific Fc and Fab IgG glycan signatures are associated with RA disease activity and remission.
-
Mechanistic evidence is lacking on how fucosylated, agalactosylated IgG Fc glycans possibly cause a pro-inflammatory phenotype in humans.
-
RA is associated with reduction of cell-surface sialylation of synovial fibroblasts, affecting their interactions with galectin-3 and resulting in a cytokine-induced switch towards a pro-inflammatory phenotype.
-
Glycan-based therapies could intervene in inflammatory processes by alteration of glycosylation, or by specific targeting and depletion of autoreactive B cells and autoantibodies.
Similar content being viewed by others
Introduction
Glycobiology is the study of the structure and biological function of oligosaccharides and polysaccharides (also known as glycans), which are often linked to lipids or amino acid side chains in proteins. Considering that about 50% of genes encode proteins that are glycosylated1, glycans are fundamental to biology. Although glycans are key players in many biological processes, and are implicated in most known diseases, study of glycans is lagging behind that of other classes of biomolecules, mainly because of their enormous complexity and the lack of appropriate analytical tools, which are only now becoming available2. Glycans are assembled sequentially by the concerted actions of multiple enzymes, resulting in a heterogeneous array of often structurally related, but functionally distinct carbohydrates, making their full structural and functional analysis a challenge. Glycans are not only key for many intracellular processes but also for cellular communication, as cell surfaces are covered with an often dense layer of glycans termed the glycocalyx, which dominates and modulates interactions between cells. These glycans can occur in the form of polysaccharides such as glycosaminoglycans, as well as in lipid-conjugated and protein-conjugated forms.
In this Review, we summarize the current information relating to the glycan signatures in rheumatic diseases, and their associated functional implications. We highlight the specific glycan characteristics of plasma proteins, (auto)antibodies, immune cells and inflamed tissues, and assess how alterations can affect immune recognition and disease development. Finally, we provide a perspective on how glycosylation-based interventions could be used in the treatment of rheumatic diseases.
Glycosylation of proteins
Proteins can have N-linked and/or O-linked glycosylation (Fig. 1a). N-linked glycans are attached to an asparagine (N) residue of an N-glycosylation consensus sequence, consisting of asparagine-X-serine/threonine (N-X-S/T, where ‘X’ can be any amino acid except for proline)3, or less commonly to an asparagine-X-cysteine (N-X-C) motif4,5. In the presence of such a consensus sequence, a precursor glycan is co-translationally or post-translationally attached to the asparagine residue in the endoplasmic reticulum (ER). Removal of glucose residues functions as a folding quality control, and only then does the protein enter the Golgi apparatus. Here, high-mannose structures (glycans with five to nine mannose residues) (Fig. 1a) may be trimmed down and subsequently extended to form complex N-glycans by an interplay of glycosidases and glycosyltransferases. By contrast, O-linked glycans are exclusively attached post-translationally in the Golgi apparatus to serine (S) or threonine (T) residues within more complex sequence motifs. Most commonly, N-acetylgalactosamine (GalNAc) residues (in the formation of O-GalNAc glycans) are transferred to the S/T amino acids, and can then be added to by a broad range of glycosyltransferases6. The final glycan composition can thus be influenced by multiple factors, including the availability of nucleotide sugars, the expression and localization of glycosyltransferases, glycan transporters and glycosidases, and the accessibility of the specific consensus sequences. The metabolic state of the cell also has a role in determining the composition of glycans, as it can affect the passage of the glycoproteins in the ER and the Golgi apparatus. Nearly all secreted, membrane or other proteins that pass through the ER are glycoproteins, including cytokines, chemokines and their receptors, as well as T cell receptors (TCRs), B cell receptors (BCRs), their secreted immunoglobulins, and MHC and MHC-like molecules. These proteins carry individual glycan modifications, which can have substantial effects on the structure and function of the proteins (for example, on half-life) and/or their interactions with surrounding molecules7. Glycans are involved in many immunological processes and are therefore likely to have important roles in regulation of the immune responses underlying rheumatic diseases. As early as 1985, the common rheumatic disease rheumatoid arthritis (RA) was characterized as being associated with defined alteration of the glycosylation of IgG8.
a, N-linked glycans are attached to asparagine (N) residues in N-X-S/T motifs, where ‘X’ is any amino acid except for proline. A precursor N-glycan is co-translationally or post-translationally attached in the endoplasmic reticulum (ER). After the removal of terminal glucose and mannose residues (folding control) by α-glucosidase (α-Glc) and α-mannosidase (α-Man) enzymes, and addition of N-acetylglucosamine (GlcNAc) by GlcNAc transferase (GlcNAcT), the protein enters the Golgi apparatus, where the high-mannose structure is trimmed down and subsequently built up to complex-type di-antennary (A2), tri-antennary (A3) and tetra-antennary (A4) N-glycans that may carry sialyl-Lewis X terminal motifs. O-linked glycans are attached post-translationally in the Golgi apparatus to serine (S) or threonine (T) amino acids, and can be further extended. b, Schematic representation of an IgG molecule. The fragment crystallizable (Fc) domain is 100% N-glycosylated at N297, and the fragment antigen-binding (Fab) domain is 15–25% N-glycosylated at N-X-S/T consensus motifs. Fab glycosylation is increased on anti-citrullinated protein antibody (ACPA) IgG from patients with rheumatoid arthritis (RA), on anti-myeloperoxidase (MPO) and anti-proteinase 3 (PR3) IgG from patients with anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV), on anti-muscle-specific kinase (MuSK) receptor antibodies from patients with myasthenia gravis (MG) and on total IgG from patients with primary Sjögren syndrome (pSS), multiple sclerosis (MS) or systemic lupus erythematosus (SLE). Rheumatic-disease-specific IgG Fc glycans are characterized by the presence of fucose but not galactose (G0F), and G0F is also observed on antigen-specific ACPA and anti-MPO IgG. c, The glycan signatures of ACPA IgG in RA. Fc galactosylation decreases and processed Fab glycosylation (represented by ‘G2FBS2’, where G is galactose, F is fucose, B is bisecting GlcNAc and S is sialic acid) increases towards disease onset and is associated with disease severity. FucT, fucosyltransferase; GalT, galactosyltransferase; PsA, psoriatic arthritis; SialylT, sialyltransferase; SpA, spondyloarthritis.
Glycosylation of total serum proteins
Glycosylation can have a fundamental effect on protein characteristics, and disease-associated changes have been reported for liver-derived acute-phase proteins and plasma cell-derived antibodies. A detailed characterization of total serum-protein N-glycans in patients with RA compared with healthy individuals demonstrated elevation of fucosylation of tri-antennary (A3) and tetra-antennary (A4) glycans, particularly in the presence of α2,3-linked sialylation9. This increase in A3 and A4 fucosylation and sialylation suggests the presence of branched N-linked glycans with sialyl-Lewis X antennary motifs, a tetrasaccharide composed of N-acetylglucosamine (GlcNAc), fucose, galactose and terminal α2,3-linked sialic acid (Fig. 1a). Elevation of the expression of sialyl-Lewis X motifs occurs in several acute-phase proteins, such as α1-acid glycoprotein, haptoglobin, α1-antichymotrypsin and transferrin, in various inflammatory conditions10, and sialyl-Lewis X is known to bind to the endothelial leukocyte adhesion molecule (E-selectin), a receptor on endothelial cells, thereby having a role in the homing of immune cells at sites of inflammation. The serum-protein N-glycan analysis of patients with RA further revealed a positive association between disease activity and the presence of sialylated and fucosylated A3 glycans, and a negative association with galactosylation of di-antennary (A2) N-glycans. The latter finding is likely related to the characteristic glycosylation changes observed for the abundant serum protein IgG9, but could also reflect disease-specific glycosylation changes affecting other proteins. Further studies are needed to determine the proteins that carry the RA-specific N-glycan signatures, and to characterize their functional effects and biomarker potential. The characteristic glycan signatures of immunoglobulins in rheumatic diseases and their potential biological implications are highlighted in the next section.
Glycosylation of antibodies
Fc domain glycosylation of (auto)antibodies in rheumatic diseases
IgG, secreted by class-switched B cells, is a highly abundant glycoprotein in human serum, and all human IgGs in the circulation carry N297-linked glycans in the CH2 domain of the fragment crystallizable (Fc) region11 (Fig. 1b). These N-linked glycans are mainly complex type, di-antennary glycans (A2), and they commonly contain core fucose, along with varying content of antennary galactose (with a total of zero, one or two galactose residues represented as G0, G1 or G2, respectively) and, to a minor extent, bisecting GlcNAc (GlcNAc attached to the core β-mannose residue) or terminal sialic acids12,13. A high prevalence of A2 Fc N-glycans that are fucosylated and that lack terminal sialic acids and galactose (known as G0F glycans) is present in serum IgG from patients with osteoarthritis (OA) or, particularly, RA8,14. However, in OA the apparent prevalence of G0F Fc glycans might have resulted from the age difference between patients and healthy controls, and so might not be specific for the disease. As IgG Fc glycosylation is associated with basic human population descriptors such as age and sex12,15,16,17, it is important to include age-matched and sex-matched healthy individuals as controls in studies of disease glycosylation signatures. Disease-specific elevation of the prevalence of G0F IgG Fc glycans has, in addition to RA, been found in patients with psoriatic arthritis18, juvenile idiopathic arthritis19, systemic lupus erythematosus (SLE)20, active spondyloarthritis21, anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV)22 or SLE complicated by Sjögren syndrome23, suggesting that it is a common feature of rheumatic diseases. Other inflammatory diseases such as inflammatory bowel disease (IBD)24,25, multiple sclerosis26 and Lambert–Eaton myasthenic syndrome27 are associated with a similar shift towards G0F Fc glycans.
Intriguingly, results from several studies have shown that agalactosylation of IgG Fc N-glycans is associated with disease activity as well as disease progression and relapse, and thus could complement the currently available biomarkers for rheumatic diseases20,21,24,25,26,28 (Fig. 1c). Consistently, galactosylation levels of total IgG from patients with RA increase during pregnancy (which is often associated with temporary disease remission) and rapidly decrease postpartum29,30. An inverse correlation between Fc N-glycan sialylation and disease severity also occurs in patients with RA, granulomatosis with polyangiitis (GPA), antiphospholipid syndrome, AAV or SLE, with reduction of sialylation preceding disease onset20,31,32,33,34. Contrarily, elevation of IgG Fc N-glycan galactosylation and sialylation (determined by lectin-based enzyme-linked immunosorbent assays) correlates with reduction of disease activity in patients with RA35,36. However, these lectin-derived results should be interpreted cautiously, as no specific Fc glycan analysis was performed, so the findings could have been influenced by N-glycans attached to the fragment antigen-binding (Fab) domains of the immunoglobulins. In contrast to the observed changes in IgG Fc galactosylation and sialylation, core fucosylation was not affected by disease activity or inflammation31,37,38, whereas conflicting data exist in relation to changes to bisecting GlcNAc. Upregulation of Fc bisecting GlcNAc occurs in IgG from patients with SLE20, whereas little or no effect is associated with RA31, and downregulation occurs in patients with GPA33.
Glycosylation of IgA can be affected by pregnancy in patients with RA39,40, with upregulation of N-glycan bisection and O-glycan sialylation relative to healthy individuals. However, in contrast to IgG Fc glycans, IgA glycan profiles are not associated with disease activity, suggesting differential regulation and function of immunoglobulin isotypes in RA. IgA subclass-specific differences also occur in RA, with the sialic acid content of the IgA1 subclass being greater than that of IgA2. This elevated sialylation may contribute to the lesser inflammatory properties of IgA1. Notably, disease-specific antibodies in patients with RA show a shift towards the pro-inflammatory IgA2 subclass that is associated with disease activity41.
Despite detailed analyses of Fc glycans in total IgG, the Fc glycan profile has only been resolved for a few rheumatic-disease-specific autoantibodies. Results from studies of anti-citrullinated protein antibodies (ACPAs) of patients with RA suggest that (as in total IgG) ACPA IgG galactosylation is reduced compared with healthy individuals, whereas fucosylation is not affected (or possibly even increases)31,42. In line with the observations presented above, agalactosylated Fc glycans of ACPA IgG are associated with the progression of the disease and systemic inflammation31. Furthermore, agalactosylated Fc glycans are observed for the myeloperoxidase antigen-specific antibodies in patients with AAV compared with total IgG glycan profiles of healthy individuals43. Evidence relating to the glycosylation of proteinase 3-specific ANCA IgG in GPA varies according to the study design. Fc galactosylation and sialylation of proteinase 3-ANCA IgG were not lower than those of total IgG when patients were sampled in disease remission, and no association with GPA relapse was identified38. However, sampling of patients with active GPA showed less Fc galactosylation and sialylation of total IgG1 or IgG2 than in healthy individuals33. In addition, galactosylation, sialylation and bisection of proteinase 3-ANCA IgG were lower than those in total IgG1, and proteinase 3-ANCA Fc galactosylation correlated with concentrations of inflammatory cytokines, and therefore with disease activity33. Consequently, not only total IgG, but also antigen-specific IgG, has Fc N-glycosylation that relates to the disease state, with an increase in G0F glycans at the peak of the disease (Fig. 1c).
Functional effects of G0F Fc glycosylation
Although the available evidence clearly shows that IgG G0F Fc glycans are associated with inflammation and disease progression in rheumatic diseases, the functional relevance of agalactosylated Fc glycans on IgG is not yet known. For example, it needs to be clarified whether the glycosylation changes are ‘only’ a consequence of the inflammatory milieu or may themselves influence inflammation. Fc N-glycans are known to have important effects on the structure of the Fc domain, and can thereby modulate IgG effector functions. For instance, Fc N-glycans can sterically interact with N-glycans expressed on Fcγ receptors (FcγRs), thereby modulating FcγR-mediated phagocytosis and antibody-dependent cellular cytotoxicity (ADCC)44,45. In particular, fucosylated Fc glycans sterically hinder FcγRIIIa activation by decreasing its binding to IgG via glycan–glycan interference (Fig. 2a), explaining the up-to-20-fold increased binding affinity and ADCC of afucosylated immune-complexed IgG46,47. These findings emphasize the importance of studying, in addition to IgG glycosylation patterns, those of FcɣRIII, which presumably affect antibody binding. The biological implications of the changes in IgG Fc N-glycan fucosylation have been highlighted by the observation that titres of afucosylated anti-spike-protein IgG in patients with COVID-19 correlate closely with disease severity48,49. This observation was explained by the enhanced ability of afucosylated anti-spike IgG to elicit inflammatory responses through the activation of FcγRIII-expressing immune cells.
a, Fragment crystallizable (Fc) region N-glycan galactosylation increases Fcγ receptor FcγRIIIa binding and antibody-dependent cellular cytotoxicity (ADCC) in the absence of core fucosylation (represented by ‘G2F0’, where G is galactose and F is fucose). ADCC is diminished by G0F1-modified IgG immune complexes because of repulsion between the IgG and FcγR N-glycan fucose residues (fucose clashing). Galactosylation of Fc glycans enhances hexamerization and subsequent C1q binding and complement-dependent cytotoxicity (CDC). b, Processed disialylated IgG Fab glycans can influence antigen binding via steric or charge-induced repulsion or by competing with the antigen for the binding pocket, as evidenced by dynamic simulations on Fab crystal structures105. Fab glycans can enhance B cell receptor (BCR) signalling while downregulating BCR internalization and antigen uptake. B cell activation is potentially influenced by differences in BCR downregulation, clustering (steric or charge-induced repulsion) or interactions with membrane-bound or soluble lectins. These effects on B cells may foster a breach of tolerance by mediating the escape from important tolerance checkpoints. Gal-9, galectin-9; Siglec, sialic acid-binding lectin. Part b is adapted from ref.105, CC BY-NC 4.0 (https://creativecommons.org/licenses/by-nc/4.0/).
Fc galactosylation and sialylation can also influence the effector functions of IgG. However, the precise contribution of Fc-glycan galactosylation is under debate. Although it has been reported, predominantly in murine models, that Fc glycans with high levels of terminal galactose and sialic acid residues exhibit anti-inflammatory behaviour50,51,52,53,54,55, other results indicate that galactosylation of Fc glycans is associated with affinity for FcɣRIIIa, as well as with the ability to activate complement56,57,58. The causes of these apparent discrepancies are not known, but they may be related to the need to study the immunological consequences of human IgG Fc glycosylation in more ‘natural’ settings, such as in immune complexes, or in the context of antigens localized on cell membranes. The discrepancies might also result from differences in the biology of the interactions between murine and human IgG Fc and complement. For example, results from studies with mice demonstrate anti-inflammatory characteristics of IgG with sialylated Fc N-glycans, including ACPA IgG, as evidenced by their ability to suppress the development of collagen-induced arthritis52. These studies suggest that the anti-inflammatory properties are mediated by enhanced binding of sialylated IgG to dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin (DC-SIGN), identified in humanized DC-SIGN mice53, or to the IgE receptor CD23 (ref.54), triggering the expression of immunosuppressive cytokines and receptors. These data are, however, not supported by results from other in vitro studies, which have failed to demonstrate the postulated interaction of DC-SIGN with sialylated Fc domains, or by the analysis of sialic acid-enriched intravenous immunoglobulins59,60,61,62,63,64,65,66. Enhanced anti-inflammatory effects have also been reported for hypergalactosylated IgG when present in immune complexes in mice, suggesting that Fc galactosylation promotes the association between FcɣRIIb and the C-type lectin receptor dectin-1, which in turn inhibits C5a-mediated inflammatory responses50,51. Thus, the terminal galactosylation of the IgG Fc glycan could be seen as a component of a feedback loop that controls complement-mediated and chemokine-mediated inflammation in mice. Consistent with the anti-inflammatory properties of galactosylated IgG, pro-inflammatory effects are reported for IgG with agalactosylated Fc N-glycans, as evidenced by the ability to bind to the mannose-binding lectin (MBL), enabling activation of the lectin complement pathway67. However, data from experiments in MBL-deficient mice show that IgG with G0 Fc glycans can mediate inflammation independent of the MBL pathway68. The notion that agalactosylated IgG does not exert pro-inflammatory activities by binding to MBL is also consistent with results showing that polymorphisms affecting MBL expression are not associated with disease activity in RA69,70.
In contrast to the data from studies in mice, results from human studies indicate higher affinity of galactosylated IgG Fc N-glycans for FcγRIIIa, particularly in the absence of core fucose56,71 (Fig. 2a), suggesting a pro-inflammatory effect. For human IgG Fc glycan bisection and sialylation, only minor effects on FcγR binding occur56. With respect to the modulation of complement activation, although conflicting data are available for IgG Fc sialylation (with results indicating either a positive or a negative association with binding to C1q)56,58,72, examination of various human IgG glycoforms has revealed enhanced binding of hypergalactosylated Fc to C1q (the initiator of the classic complement pathway), independent of the levels of fucosylation56,57. Elucidation of the mechanism by which Fc galactosylation affects complement activation indicates that it enhances the potential of IgG to form stable hexameric structures, which is a prerequisite for efficient C1q binding and subsequent complement-dependent cytotoxicity58,73 (Fig. 2a).
As the evidence suggests a pro-inflammatory phenotype for human galactosylated Fc glycans, it does not support the notion that G0 glycosylation traits found on IgG from patients with rheumatic diseases represent pro-inflammatory IgG features. Similarly, the in vitro data do not justify the classification of human IgG with increased galactosylation and sialylation (as found in association with disease remission) as anti-inflammatory, but rather suggest enhanced IgG-facilitated immune effector functions. Thus, as mechanistic evidence is currently lacking, the question remains as to whether IgG Fc glycans have pro-inflammatory or anti-inflammatory effects rather than being mere consequences of inflammation.
Regulation of Fc glycosylation
To better understand the function of Fc N-glycans and whether antibody glycan changes are a consequence or a cause of inflammation, it is important to understand how Fc glycosylation is regulated. To date, the main insights into the regulation of Fc glycans derive from studies in mice52,74,75. Findings from these studies suggest a B cell-intrinsic regulation of IgG Fc glycosylation by, for example, upregulation of the β-galactoside α-2,6-sialyltransferase 1 (ST6Gal1)52. An important role of the IL-23–type 17 T helper (TH17) cell axis in the regulation of sialyltransferase expression by B cells was revealed by the observation that IL-23-activated TH17 cells accumulate in the germinal centre, resulting in a low-sialylation phenotype in subsequent autoantibody responses75. On the basis of these findings, IL-23 secretion by, for example, myeloid cells, is proposed to trigger the production of IL-21 and IL-22 by TH17 cells, which in turn leads to downregulation of ST6Gal1 in differentiating antibody-producing cells, thereby determining the IgG glycosylation profile. Consistent with this effect of T cell cytokines on the regulation of IgG glycosylation, findings from other studies have demonstrated that immunizing mice using different adjuvants induces distinct IgG sialylation profiles programmed by different TH cell subsets74. Reduction of sialylation depends on T follicular helper (TFH) cells secreting IL-6. Furthermore, the induction of IL-27-receptor-dependent IFNγ-positive TFH1 cells and IL-6–IL-23-dependent IL-17-producing TFH17 cells downregulates the expression of ST6Gal1 in germinal centre B cells74.
In addition to B cell-intrinsic IgG glycan regulation, there are also B cell-extrinsic effects. For example, terminal IgG sialylation can occur via glycosyltransferases and glycosidases in the extracellular environment, and mice with ST6Gal1-deficient B cells are able to express sialylated IgG, possibly through liver-derived ST6Gal1 and platelet-derived cytidine-5′-monophosphate-sialic acid76. Providing further evidence of a role in mice of B cell-extrinsic factors in IgG sialylation, IgG produced ex vivo by B cells has less sialylation than IgG isolated from plasma of the same animals77. Results from confocal microscopy indicate that IgG in B cells has close contact with α-(1,6)-fucosyltransferase, but limited contact with ST6Gal1, suggesting that B cell intracellular IgG trafficking affects sialylation77.
Also, data from human studies suggest a B cell-intrinsic effect on the regulation of IgG N-glycans, as lower galactosyltransferase activity was found in B cells from patients with RA than in unaffected individuals, suggesting that the low level of IgG Fc galactosylation in patients with RA may be the result of specific enzyme activity78. Furthermore, the T cell-derived cytokine IL-21 and the Toll-like receptor 9 ligand CpG (cytosine-phosphate-guanine dideoxynucleotide) upregulate Fc galactosylation and sialylation and reduce bisection on IgG secreted by primary human B cells79. By contrast, all-trans retinoic acid, a natural metabolite of vitamin A, promotes reduction of IgG Fc galactosylation and sialylation79. Hormones have important roles in the regulation of glycosyltransferase expression, specifically in B cells, and thereby affect IgG Fc glycosylation. For example, oestrogen treatment can increase Fc sialylation and ST6Gal1 expression in antibody-producing cells in mice and in humans80. Oestrogen treatment (especially oestradiol) can also increase Fc galactosylation via upregulation of expression of β-1,4-galactosyltransferase 1 (refs.81,82). These findings may provide a mechanistic explanation for the elevation of the risk of rheumatic disease among menopausal women, and possibly define a pathway by which age and sex modulate immunity83. However, even with these important insights into the regulation of antibody Fc glycosylation, the precise pathways regulating N-glycosylation of IgG in health and disease remain largely unexplored, and further studies are needed to improve our understanding of how glycosylation changes occur and whether they are relevant to the disease course.
Fab glycosylation of (auto)antibodies and BCRs in rheumatic diseases
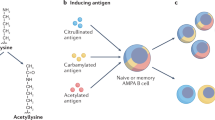
In addition to the evolutionarily conserved Fc N-glycans, 15–25% of human IgG Fab regions can carry N-glycosylation consensus sequences13,84 (Fig. 1b). Relative to Fc glycans, complex-type Fab N-glycans have higher levels of galactosylation, sialylation and bisection, whereas fucosylation is reduced13,85. In addition, low levels of high-mannose-type structures occur13,85, potentially depending on the location of the N-glycosylation sites and thus the accessibility for glycosyltransferases86. The naive human B cell antibody repertoire is almost devoid of such sites, as only IGHV1-8, IGHV4-34, IGHV5-10-1, IGLV3-12 and IGLV5-37 encode N-glycosylation motifs84. Therefore, Fab N-glycosylation sequences are mainly introduced during antigen-specific immune responses following somatic hypermutation87, in a process that sequence analyses of (auto)antibody responses indicate is likely to be selective. The introduced N-linked glycosylation sites present in the Fab domain are, unlike those in the Fc region, not necessarily occupied with a glycan, as the accessibility of Fab N-glycosylation sites may be constrained88. The Fab N-glycosylation sites primarily emerge near antigen-binding pockets (in framework or complementarity-determining regions) in both heavy and light chains89. Similar to Fc glycans, Fab glycans can have an effect on the stability of antibodies90, might affect the propensity of IgG to form immune complexes or aggregates91, and are thus potentially able to modulate IgG effector functions (such as complement activation). Along with the influence on antibody stability and aggregation, Fab glycans have differential effects on antigen binding depending on the number and the location of glycosylation consensus sequences89,92,93,94 (Fig. 2b). Results from studies in mice have demonstrated that Fab glycans can prevent binding to self-antigens, while maintaining cross-reactivity to foreign antigens, and that this ‘antigenic redemption’ might enable BCRs to move away from autoreactivity and to escape negative selection95.
In contrast to the 15–25% Fab glycosylation in serum IgG, N-glycans in the variable domains of the heavy and light chains are found on >90% of RA-specific ACPA IgG autoantibodies96,97. Moreover, autoantibodies isolated from the site of inflammation, the synovial fluid, can have a Fab glycan prevalence of >100%, indicating that multiple glycans are attached to the variable region of one ACPA molecule96. Evidence indicates that B cells that express ACPA IgG selectively introduce N-linked glycosylation sites into ACPA Fab domains following somatic hypermutation98,99. Structural analysis demonstrates that ACPA IgG Fab glycans have high a prevalence of bisection and galactosylation and are mainly disialylated96. ACPA Fab glycans are not only abundant in established RA, as their presence increases towards the onset of disease100, but they are predictive for the development of RA101 (Fig. 1c). Intriguingly, a lower ACPA IgG Fab glycan prevalence at RA onset is associated with a higher likelihood of subsequently achieving sustained drug-free remission100. In addition, the presence of Fab-glycosylated ACPA IgG is associated with the most prominent genetic risk factor for ACPA-positive RA, the human leukocyte antigen shared epitope alleles102,103. Together with data showing that ACPA undergo limited avidity maturation104, these findings suggest that the selection of autoreactive ACPA-expressing B cells is driven not only by affinity to the antigen but also by the introduction of N-glycans into the Fab domains. Thus, Fab glycans could facilitate the escape of B cells from important checkpoints that control their activation and their ability to expand. Consistent with the idea of antigenic redemption, ACPA IgG Fab glycans were found to reduce binding to certain lower affinity (auto)antigens, while maintaining binding to other, higher affinity antigens, compared with ACPA IgG lacking Fab glycans105. Structural and crystallographic analyses suggest that antigen binding is probably affected by steric repulsion between the spatially demanding Fab glycans terminating with negatively charged sialic acids and the cognate antigens, and/or by competition between the antigen and the Fab glycan for the ACPA binding pocket (Fig. 2b).
In experiments involving the expression of membrane IgG BCRs derived from patients with RA on a human Burkitt lymphoma-derived (Ramos) model B cell line, knocked out for its endogenous BCR and the enzyme activation-induced cytidine deaminase, BCRs with Fab glycosylation had longer surface expression after antigenic triggering and more intense activation than their non-Fab-glycosylated counterparts105. These observations substantiate the hypothesis that N-glycans in the Fab region provide a selective advantage for autoreactive B cells by affecting antigen binding and the threshold of B cell activation (Fig. 2b). This activation advantage might be conveyed by the interaction of hypersialylated Fab glycans with glycan-binding proteins (lectins). In particular, sialic acid-binding lectins (Siglecs) might be instrumental in providing a competitive advantage to autoreactive cells, as glycan–Siglec interactions are known to control the activity of B cells. For example, sialylated trophoblast glycans can engage CD22 inhibitory signalling in antigen-specific follicular B cells in mice, and as B cells present trophoblast antigens to CD4+ T cells, this B cell suppression in turn suppresses T cell responses, promoting fetomaternal tolerance106. However, in ACPA-expressing B cells, no influence of the negative regulator CD22 on the Fab glycan-mediated effects on BCR signalling is apparent105. Therefore, a precise mechanistic understanding of how Fab glycans affect B cell activation and biology is presently lacking. The mechanism might include interaction of terminal sialic acid-containing Fab glycans with other membrane-bound or soluble lectins, such as Siglec-10 or galectin-9 (refs.107,108). However, it is also conceivable that Fab glycans alter the nanoscale organization of BCRs and thereby affect signalling (Fig. 2b). Dimerization and localization of BCRs could be influenced by steric or charge-induced repulsion of the bulky, negatively charged Fab glycans or by interactions with lectins. Notably, deficiency of mannoside acetylglucosaminyltransferase 5, an enzyme that increases GlcNAc β1,6 branching on N-glycans, reduces TCR N-glycan branching and lowers the threshold for T cell activation by directly promoting TCR clustering109. By contrast, the presence of branching N-glycans on TCRs enables interaction with surrounding galectins and thus decreases TCR clustering and localization with other co-receptors110. Enhanced branching of TCR N-glycans thus decreases the activation status of T cells and consequently susceptibility to autoimmune diseases such as ulcerative colitis111. Fab glycans may also contribute to the selection of dysregulated B cells in follicular lymphoma, in which N-glycan sites are abundant in BCR Fab domains112. Highly mannosylated Fab glycans on B cells in follicular lymphoma may interact with mannose-binding lectins, causing B cell stimulation without the need for antigenic stimulation113,114,115.
In addition to ACPA, other autoantibodies associated with rheumatic diseases also have a high prevalence of Fab glycans. For example, an increased binding of autoantibodies to the sialic acid-binding Sambucus nigra agglutinin and thus most probably the presence of sialic acid-containing Fab glycans was observed for ANCA directed against myeloperoxidase116. Mass spectrometry confirmed that a major fraction of anti-myeloperoxidase-specific IgG is highly glycosylated in the Fab domain, with disialylated and bisected glycan traits43. IgG enriched for S. nigra agglutinin-binding also showed enhanced binding to proteinase 3, suggesting that proteinase 3-specific IgG has a higher prevalence of sialic acid-containing Fab glycans116. Additionally, patients with primary Sjögren syndrome, multiple sclerosis or SLE have a higher prevalence of IgG sequences with acquired N-linked glycosylation sites than healthy individuals117,118, although the presence of Fab glycans on autoantibodies associated with these diseases has not yet been shown. N-linked glycosylation motifs are also present in the variable domains of anti-muscle-specific kinase receptor autoantibodies, suggesting a role for Fab glycans in other autoimmune diseases, such as myasthenia gravis119,120. Thus, Fab glycosylation seems to be a feature of several autoreactive B cell responses and secreted autoantibodies (such as ACPA, anti-myeloperoxidase and anti-proteinase 3 antibodies), with potentially important immunomodulatory functions. Notably, anti-drug antibodies that emerge in patients treated with adalimumab or infliximab also have a high prevalence of Fab N-glycans. These data are intriguing and suggest that the introduction of N-linked glycosylation sites into the immunoglobulin variable domain is triggered by chronic and systemic antigen exposure in conjunction with the corresponding helper activity of CD4+ T cells, which is required to enable somatic hypermutation in autoreactive B cells89.
Thus, accumulating evidence of the role of Fab glycans in autoreactive B cell survival underscores the importance of glycan-based pathways in the modulation of immunity, and emphasizes their broad relevance.
Glycosylation of cells and tissues
The surfaces of cells are covered with carbohydrates, and this sugar coating, or glycocalyx, can act as a master regulator of inflammation, either by direct alteration of immune-cell activity and function or through recognition via glycan-binding receptors expressed by other (immune) cells121. For example, modified cell-surface glycosylation can be a hallmark of chronic inflammatory conditions, and about 95% of cancer cells have a cytokine-induced modification of the glycocalyx that leads to local inflammation and cancer progression122. The glycocalyx can affect the shape of the cell membrane123 and receptor organization, ultimately leading to a change of fundamental cell processes such as cell adhesion, migration and signal transduction124. Consequently, changes in glycocalyx composition could have important roles in the loss of immunotolerance, and so in the processes underlying rheumatic diseases125.
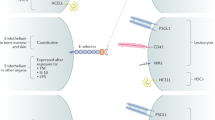
Results from a study of synovial fibroblasts indicate that the cytokine milieu in the inflamed joint can alter synovial fibroblast glycosylation, which in turn regulates immune-cell recruitment and inflammatory responses126. Specifically, the synovial fibroblast N-glycome is rich in high-mannose glycans and di-antennary complex glycans, extended by N-acetyllactosamines (LacNAcs), either core-fucosylated or non-fucosylated, and terminated with sialic acids. The extent of synovial fibroblast terminal sialylation differs between health and disease and also between cells derived from the less-inflammatory joint disease OA and those derived from RA. TNF stimulation downregulates ST6Gal1 expression, leading to reduction of synovial fibroblast sialylation (Fig. 3a). A similar connection between TNF treatment and alteration of synovial fibroblast glycosylation was also described in a study that used lectin-binding assays127. The ‘remodelled’, desialylated glycocalyx is likely to contribute to a shift towards a more pro-inflammatory synovial fibroblast phenotype in RA. Consistently, elevation of synovial fibroblast sialylation in the synovium of patients with RA is associated with disease remission after treatment with TNF inhibitors126. In the O-glycome, reduction of the prevalence of sialylated O-glycans occurs under inflammatory conditions in the collagen-induced arthritis mouse model126. Although causality has not yet been demonstrated, together these data provide the first evidence that, in rheumatic disease, sialylation can potentially act as a ‘molecular switch’, controlling the synovial fibroblast inflammatory or resting state. In this respect, synovial fibroblast desialylation in RA might enable galectin-3, a protein that binds to glycans with terminal galactosylation, to induce the secretion of pro-inflammatory cytokines and mononuclear-cell-recruiting chemokines (such as IL-6 and CCL2)128. This activity would, in turn, increase TNF levels and thereby further downregulate sialylation, triggering a vicious cycle (Fig. 3a). This feedback loop might contribute to the persistence of the disease, and is consistent with results showing that galectins can modulate synovial inflammation, and that they are upregulated in patients diagnosed with RA128,129,130,131. Similarly, galectins produced by chondrocytes could contribute to cartilage breakdown and inflammation, as indicated by studies on galectin-4-mediated activation of OA chondrocytes132. In addition, the absence of interactions with sialic-acid-binding lectins, such as Siglecs and selectins, could have a key role in determining the pro-inflammatory phenotype of desialylated synovial fibroblasts in RA126.
a, The N-glycome of the synovial fibroblast is rich in high-mannose and short di-antennary glycans with differential terminal sialylation in both health and disease states. In rheumatoid arthritis (RA), TNF stimulates the downregulation of β-galactoside α-2,6-sialyltransferase 1 (ST6Gal1), resulting in reduction of surface sialylation and a molecular switch to a more pro-inflammatory synovial fibroblast phenotype. Desialylated synovial fibroblast surface glycans may interact with galectin-3 (Gal-3), inducing the secretion of pro-inflammatory cytokines (IL-6, CCL2), which in turn upregulate TNF expression and thus the desialylation of the synovial fibroblast surface (in a vicious cycle). b, Abnormal glomerulus tissue glycosylation in systemic lupus erythematosus. Accumulation of mannose N-glycans is the result of a deficient complex-N-glycosylation pathway characterized by downregulation of expression of α-mannosidase II (α-Man II) and promotion of O-mannosylation by upregulation of expression of protein O-mannosyl-transferase 1 (POMT1). Mannose-enriched N-glycans are typically found on the surface of pathogens and can therefore trigger activation of antigen-presenting cells (APCs) by mannose glycan-binding receptors such as dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin (DC-SIGN), ultimately leading to a loss of self-tolerance. GlcNAcT, N-acetylglucosamine transferase.
Among the glycosyltransferases, fucosyltransferase 1 (FUT1) is upregulated in synovial tissue (in both synovial fibroblasts and macrophages) of patients with RA, leading to production of α(1,2)-linked fucosylated glycans133. FUT1 expression is thought to be important for synovial fibroblast angiogenesis, leucocyte–synovial fibroblast cell adhesion and synovial fibroblast proliferation, which are key processes in inflammation and the pathogenesis of RA133. The expression of other glycosyltransferases can also be affected by the inflammatory milieu, and TNF can induce the expression of β(1,4)-galactosyltransferase I in synovial tissue of patients with RA, where it might promote cell–cell or cell–matrix adhesion134. Similarly, alterations in fucosylation and sialylation have been reported for synovial and plasma fibronectin in RA in relation to disease activity135. These studies highlight the importance of cytokine-induced alteration of tissue and cell-surface glycosylation in the progression and persistence of rheumatic diseases such as RA.
Glycosylation of proteoglycan 4 (also known as lubricin) differs between patients with OA and those with RA. Lubricin is a heavily glycosylated boundary lubricant that covers the surface of cartilage and contributes to joint function and mobility136. Lubricin, a mucin-type O-linked proteoglycan, is synthesized by fibroblast-like synoviocytes and chondrocytes, and is abundant in the synovial fluid and synovial membrane. A higher proportion of disialylated O-linked glycans occurs on lubricin in RA than in OA137, and this sialylation potentially enhances lubrication of the joints in RA, and may be a protective response against disease progression. By contrast, the truncated O-glycans in OA have an altered ability to bind to galectin-3, which might contribute to the destabilization of boundary lubrication and to joint degradation137.
Modification of tissue glycosylation occurs in SLE. A comprehensive characterization of cellular glycosylation in kidney tissue samples from patients with SLE and its severe clinical manifestation lupus nephritis revealed a unique mannose-enriched glycan signature138. The ‘abnormal’ glycosylation resulted from a deficiency in the pathway of complex N-glycosylation characterized by downregulation of expression of α-mannosidase 2, and upregulation of O-mannosylation through expression of mannosyl-transferase (Fig. 3b). The abundant cellular mannosylation was not only a marker for lupus nephritis but also predicted the development of chronic kidney disease with high specificity, and so might have potential for prognostic applications. Notably, a case report has described two sisters with α-mannosidosis (a disorder characterized by an intra-lysosomal accumulation of low-complexity, high-mannose glycans) who both developed SLE139. The underlying pathways leading to disease in these patients are not known, but these observations suggest that aberrant mannosylation contributes to the risk of developing SLE. In this regard, mutation in mice of the gene encoding α-mannosidase 2, which is involved in regulation of the branching of N-linked glycans, results in a systemic autoimmune disease that resembles SLE140. Loss of α-mannosidase 2 alters N-glycan branching and attenuates the ability of the immune system to maintain self-tolerance, as evidenced by the production of autoantibodies and failure of kidney function. The molecular details underlying such transformations are not known. However, as an accumulation of high-mannose N-glycans can typically be found on the surface of several pathogens, it is reasonable to speculate that these pathogens can trigger the activation of antigen-presenting cells by mannose-glycan-binding receptors such as DC-SIGN141 (Fig. 3b), which is notable by its presence in the glomeruli of patients with SLE142.
Alteration of tissue glycosylation occurs in autoimmune diseases such as idiopathic inflammatory myopathy, in which low levels of sialic acid in muscle cells result from mutations in GNE, which encodes a bifunctional enzyme that is responsible for the synthesis of the sialic acid precursor N-acetylneuraminic acid143. Supplementation with sialic acid precursors prevents muscle atrophy in a mouse model of inflammatory myopathy144, and was described as a safe and effective treatment in a phase II study of patients with GNE myopathy145. In IBD, polymorphisms in FUT2 are linked to disease susceptibility146 and, in mice, commensal gut microorganisms promote Fut2 expression in intestinal epithelial cells through activation of innate lymphoid cells147. In addition, in biopsy-derived colon tissue samples from patients with IBD, expression of ST6GAL1 is upregulated, leading to inhibition of galectin-1 binding to T cells, suggesting that this galectin-1 binding is important for maintenance of gut homeostasis148.
The available evidence suggests that glycobiology has an important role in immune regulation, and that studying the glycome (the complex glycan structures expressed on tissues and cells) in greater detail might enable us to better understand the development and progression of rheumatic diseases. Progress in this area could open up new avenues for treatment strategies, and identify novel disease biomarkers.
Clinical potential of glycosylation
The IgG-specific glycan signatures that are associated with rheumatic diseases can potentially be used as prognostic markers to improve the identification of homogeneous patient groups. However, glycosylation-based approaches have not yet been integrated into clinical practice, partly because of the challenging and costly nature of glycan analyses. More accessible diagnostic tools and high-throughput screening methods, such as lectin-based assays, might enable the measurement of specific IgG glycosylation profiles in patients in the near future. Furthermore, specifically modifying the glycosylation traits of proteins (in particular antibodies), tissues and cells might provide a new avenue for glycan-based therapeutics, mirroring recent developments in cancer therapy149,150. For example, results from studies in mice indicate that alteration of the Fc glycosylation of antibodies might be a strategy for therapeutic interference with autoimmune processes. Findings from preclinical models suggest that the administration of enzymes that deglycosylate the IgG Fc domain can affect arthritis intensity in mice151,152. Endoglycosidase S treatment of mice results in efficient IgG N-glycan removal and a subsequent anti-inflammatory phenotype, including reduction of joint swelling and recruitment of inflammatory effector cells152. Endoglycosidase S has also shown therapeutic potential against IgG-mediated disease by hydrolysis of IgG glycans following intravenous administration in rabbits, resulting in modulation of IgG effector functions153. It cannot yet be excluded that the anti-inflammatory effect of endoglycosidase S is caused by deglycosylation of other (serum) proteins, cell surfaces or tissues, beyond IgG effector mechanisms154, although structural analyses have revealed distinct binding sites within endoglycosidase S that are specific for complex-type N-linked glycans in the context of IgG Fc domains155. In addition to the therapeutic potential of glycosidase administration, the transfer of sialylated, anti-inflammatory immune complexes can attenuate the development of arthritis and diminish numbers of pathogenic TH17 cells and autoantibody responses156. Thus, influencing the glycosylation profiles of autoantigen-specific IgGs could represent a promising approach for intercepting pathogenic T cell and B cell responses. However, as there is no evidence yet of direct effects of IgG Fc sialylation on FcγR or complement interactions in humans, this concept requires further experimental and mechanistic validation.
In addition to manipulation of the glycosylation of antibodies, alteration of the surface glycosylation of synovial fibroblast or other cell types could represent a promising therapeutic intervention to reduce inflammation. This modification could potentially be accomplished by cytokine-mediated modulation of the enzyme machinery that determines levels of sialylation or galactosylation. This theoretical concept of using TNF inhibitors to regulate glycosyltransferase expression in synovial fibroblast and thus cell-surface glycosylation is illustrated in Fig. 4a. Another cost-effective and non-toxic therapeutic intervention could be the direct administration of glycans or alteration of the metabolite supply for glycan biosynthesis157,158. For example, it is well documented that enhancement of the hexosamine pathway by administration of GlcNAc reduces chronic inflammation and autoimmunity111,158,159,160. The oral and enematic administration of GlcNAc both reduce the progression and severity of colitis in mice111. GlcNAc supplementation increases N-glycan branching of the TCR and thereby reduces hyperactivation of T cells. Given the immunoregulatory effect of GlcNAc in IBD, it is currently being tested in two clinical trials (a phase II/III trial (NCT01893606) and a phase III trial (NCT02504060)), following on from a pilot study in which children with IBD who received GlcNAc achieved clinical remission with no reported side effects159. GlcNAc supplementation could also be a therapeutic approach for the suppression of multiple sclerosis by rescue of the N-glycan branching defect of T cells. In mice, reduction of N-glycan branching promotes neurodegeneration, spontaneous inflammatory demyelination, TCR clustering and signalling and loss of the autoimmune inhibitor cytotoxic T lymphocyte antigen 4 (ref.160), thereby increasing disease severity. In addition, serum levels of endogenous GlcNAc are lower in patients with multiple sclerosis than in unaffected individuals161, and oral administration can increase serum GlcNAc levels and subsequent N-glycan branching on T cells159. The administration of GlcNAc as a dietary supplement could therefore represent a safe and cost-effective therapeutic approach in patients with autoimmune diseases, and possibly also in patients with rheumatic diseases.
Glycan-based therapies that intervene in inflammatory processes include the administration of enzymes such as endoglycosidase S, which alters the fragment crystallizable (Fc)-domain glycosylation of antibodies. One cost-effective and non-toxic intervention is the direct administration of glycans to promote alteration of glycan biosynthesis. Other glycosylation-related therapeutic approaches include specific glycoengineering of proteins such as antibodies, alteration of glycosylation by TNF inhibition, lectin-mediated inhibition of autoreactive B cells and targeted autoantibody degradation. a, Hypothetical method to change the pro-inflammatory phenotype of synovial fibroblasts in rheumatic diseases by manipulating glycosylation by treatment with TNF inhibitors. Abrogation of TNF-mediated downregulation of β-galactoside α-2,6-sialyltransferase 1 (ST6Gal1) results in an anti-inflammatory hypersialylated synovial fibroblast glycan coat. Sialylation prevents interaction with galectin-3 (Gal-3), which further breaks the vicious cycle in which TNF promotes desialylation. b, Sialic-acid-binding immunoglobulin-type lectin (Siglec)-engaging tolerance-inducing antigenic liposomes (STALs) carry autoantigens and high-affinity ligands for CD22 (also known as Siglec-2) or Siglec-10, negative regulators of B cell receptor (BCR) signalling, and can thus enforce an association between the BCR and the negative regulators. Inhibited BCR signalling results in pro-apoptotic downstream signalling events and the apoptosis of antigen-specific B cells. c, Targeted autoantibody degradation by MoDE-As (molecular degraders of extracellular proteins through the asialoglycoprotein receptor (ASGPR)), small molecules carrying an N-acetylgalactosamine (GalNAc) ASGPR binding motif and an autoantigen. MoDE-As target autoantibodies and facilitate target-specific internalization and subsequent lysosomal degradation through ASGPR expressed on liver cells. SHP1, SRC homology 2 domain-containing protein tyrosine phosphatase-1.
The great therapeutic potential of glycosylation is further demonstrated by the development of glycoengineered monoclonal antibodies for use as biologic agents. For example, the anti-CD20 antibody obinutuzumab was glycoengineered to reduce its fucosylation, resulting in improved therapeutic efficacy. The absence of core fucose increased the binding affinity for FcγRIIIa, thereby promoting ADCC and improving therapeutic efficacy in patients with cancer, resulting in prolonged progression-free survival and increased response rates162.
Glycan-based interventions could be used for targeted degradation of autoreactive B cells or the antibodies they secrete. For example, Siglec-engaging tolerance-inducing antigenic liposomes (STALs) can recruit Siglecs, which are negative regulators of B cell receptor signalling, to the immunological synapses of autoantigen-specific B cells163 (Fig. 4b). To this end, STALs contain an antigen and a high-affinity ligand for a Siglec (CD22 or Siglec-10), and are thus able to bring the BCR in close proximity to the negative regulators, which in turn inhibits B cell activation and eventually leads to a downstream apoptotic signal. The functionality of this system was demonstrated by specifically targeting and inhibiting immortalized ACPA-expressing B cell lines using multivalent scaffolds that carried citrullinated antigens and ligands for the immunomodulatory receptor CD22 (ref.164). In addition to STALs, other glycosylation-based protein-degradation platforms could be harnessed to modulate the pathogenic effects of immunoglobulins. The bifunctional small molecules MoDE-As (molecular degraders of extracellular proteins through the asialoglycoprotein receptor (ASGPR))165 could bring autoantibodies into proximity with ASGPR, a liver-specific lysosome-targeting receptor, thereby resulting in degradation of the target protein in a specific manner (Fig. 4c). MODE-As can be constructed by conjugation of autoantigens to a tri-antennary GalNAc motif, which is recognized by ASGPR. In this way, specific autoantibodies can be targeted for endocytosis and rapid degradation by lysosomal proteases, thereby potentially alleviating the pathological symptoms of rheumatic diseases.
Conclusions
Glycosylation is a common modification on proteins and cells, and changes in glycan composition are directly linked to many diseases. Almost 40 years ago, RA was defined as a disease with specific glycosylation patterns, and great progress has been made in the understanding of this characteristic. Patients with rheumatic diseases have immunoglobulins (notably IgG) with aberrant Fc glycan forms that lack terminal galactoses and sialic acids, whereas fucosylation remains stable. This specific glycan signature is associated with the initiation and resolution of inflammation, and could complement current clinical biomarkers for diagnostic purposes. Although considered as pro-inflammatory, no clear mechanistic evidence is yet available to confirm that agalactosylated Fc glycans aggravate antibody effector functions in humans. The observed glycosylation changes could be caused by the inflammatory milieu, as a result of differential expression of glycosyltransferases, without any direct effect on the pathogenicity of IgG. In addition to unusual Fc glycosylation, several autoantibodies harbour N-glycans in their antigen-binding domains. Notably, the ACPA IgG autoantibodies that characterize RA contain an abundance of bisected and disialylated Fab glycans. The findings that Fab glycans affect antigen binding and the activation threshold of autoreactive B cells have advanced our understanding of the involvement of immunoglobulin N-glycans in disease development. Fab glycosylation might not only be a potential biomarker for disease prediction but might also help autoreactive B cells, which have key roles in disease, to overcome the tight control mechanisms that are usually in place to prevent the development of autoimmunity. Tissues and cells also have characteristic disease-related glycosylation patterns that can affect immunity. For example, cytokine-induced desialylation can reprogram synovial fibroblast into an inflammatory phenotype, causing the progression and persistence of RA. In SLE a unique high-mannose tissue glycan signature has been observed, which is potentially associated with a loss of tolerance. As the immune system routinely responds to high-mannose glycans presented on the surface of foreign pathogens, the overlap in the glycosylation patterns between host and pathogen might lead to the loss of self-tolerance and the development of SLE.
Collectively, although we are beginning to define rheumatic disease-specific glycosylation, and to understand its importance, we lack clear understanding of the molecular and cellular pathways responsible for these changes and their effects on the immune system. Greater knowledge of the factors that regulate glycosylation, and of their mechanistic consequences, is required to identify which glycosylation pathways can be targeted to intervene in disease progression. Therefore, decisive progress in this exciting field of glycobiology is eagerly awaited.
Change history
23 February 2023
A Correction to this paper has been published: https://doi.org/10.1038/s41584-023-00922-8
References
Jefferis, R. Recombinant proteins and monoclonal antibodies. Adv. Biochem. Eng. Biotechnol. 175, 281–318 (2021).
Haan, N. et al. Developments and perspectives in high-throughput protein glycomics: enabling the analysis of thousands of samples. Glycobiology 32, 651–663 (2022).
Marshall, R. D. Glycoproteins. Ann. Rev. Biochem. 41, 673–702 (1972).
Zielinska, D. F., Gnad, F., Wisniewski, J. R. & Mann, M. Precision mapping of an in vivo N-glycoproteome reveals rigid topological and sequence constraints. Cell 141, 897–907 (2010).
Bause, E. & Legler, G. The role of the hydroxy amino acid in the triplet sequence Asn-Xaa-Thr(Ser) for the N-glycosylation step during glycoprotein biosynthesis. Biochem. J. 195, 639–644 (1981).
Varki, A. Essentials of Glycobiology 4th edn (ed. Inglis, J.) (Cold Spring Harbor Laboratory Press, 2022).
Lis, H. & Sharon, N. Protein glycosylation. Struct. Funct. Asp. Eur. J. Biochem. 218, 1–27 (1993).
Parekh, R. B. et al. Association of rheumatoid arthritis and primary osteoarthritis with changes in the glycosylation pattern of total serum IgG. Nature 316, 452–457 (1985).
Reiding, K. R. et al. Serum protein N-glycosylation changes with rheumatoid arthritis disease activity during and after pregnancy. Front. Med. 4, 241 (2017).
Albrecht, S., Unwin, L., Muniyappa, M. & Rudd, P. M. Glycosylation as a marker for inflammatory arthritis. Cancer Biomark. 14, 17–28 (2014).
de Haan, N., Falck, D. & Wuhrer, M. Monitoring of immunoglobulin N- and O-glycosylation in health and disease. Glycobiology 30, 226–240 (2020).
Bakovic, M. P. et al. High-throughput IgG Fc N-glycosylation profiling by mass spectrometry of glycopeptides. J. Proteome Res. 12, 821–831 (2013).
Bondt, A. et al. Immunoglobulin G (IgG) Fab glycosylation analysis using a new mass spectrometric high-throughput profiling method reveals pregnancy-associated changes. Mol. Cell. Proteom. 13, 3029–3039 (2014).
Bond, A. et al. A detailed lectin analysis of IgG glycosylation, demonstrating disease specific changes in terminal galactose and N-acetylglucosamine. J. Autoimmun. 10, 77–85 (1997).
Kristic, J. et al. Glycans are a novel biomarker of chronological and biological ages. J. Gerontol. A Biol. Sci. Med. Sci. 69, 779–789 (2014).
Keusch, J., Levy, Y., Shoenfeld, Y. & Youinou, P. Analysis of different glycosylation states in IgG subclasses. Clin. Chim. Acta 252, 147–158 (1996).
Chen, G. et al. Human IgG Fc-glycosylation profiling reveals associations with age, sex, female sex hormones and thyroid cancer. J. Proteom. 75, 2824–2834 (2012).
Watson, M., Rudd, P. M., Bland, M., Dwek, R. A. & Axford, J. S. Sugar printing rheumatic diseases: a potential method for disease differentiation using immunoglobulin G oligosaccharides. Arthritis Rheum. 42, 1682–1690 (1999).
Ercan, A. et al. Multiple juvenile idiopathic arthritis subtypes demonstrate proinflammatory IgG glycosylation. Arthritis Rheum. 64, 3025–3033 (2012).
Vuckovic, F. et al. Association of systemic lupus erythematosus with decreased immunosuppressive potential of the IgG glycome. Arthritis Rheumatol. 67, 2978–2989 (2015).
Leirisalo-Repo, M., Hernandez-Munoz, H. E. & Rook, G. A. Agalactosyl IgG is elevated in patients with active spondyloarthropathy. Rheumatol. Int. 18, 171–176 (1999).
Holland, M. et al. Hypogalactosylation of serum IgG in patients with ANCA-associated systemic vasculitis. Clin. Exp. Immunol. 129, 183–190 (2002).
Parekh, R. et al. A comparative analysis of disease-associated changes in the galactosylation of serum IgG. J. Autoimmun. 2, 101–114 (1989).
Trbojevic Akmacic, I. et al. Inflammatory bowel disease associates with proinflammatory potential of the immunoglobulin G glycome. Inflamm. Bowel Dis. 21, 1237–1247 (2015).
Tomana, M., Schrohenloher, R. E., Koopman, W. J., Alarcon, G. S. & Paul, W. A. Abnormal glycosylation of serum IgG from patients with chronic inflammatory diseases. Arthritis Rheum. 31, 333–338 (1988).
Wuhrer, M. et al. Pro-inflammatory pattern of IgG1 Fc glycosylation in multiple sclerosis cerebrospinal fluid. J. Neuroinflammation 12, 235 (2015).
Selman, M. H. et al. IgG fc N-glycosylation changes in Lambert-Eaton myasthenic syndrome and myasthenia gravis. J. Proteome Res. 10, 143–152 (2011).
Ercan, A. et al. Aberrant IgG galactosylation precedes disease onset, correlates with disease activity, and is prevalent in autoantibodies in rheumatoid arthritis. Arthritis Rheum. 62, 2239–2248 (2010).
Rook, G. A. et al. Changes in IgG glycoform levels are associated with remission of arthritis during pregnancy. J. Autoimmun. 4, 779–794 (1991).
van de Geijn, F. E. et al. Immunoglobulin G galactosylation and sialylation are associated with pregnancy-induced improvement of rheumatoid arthritis and the postpartum flare: results from a large prospective cohort study. Arthritis Res. Ther. 11, R193 (2009).
Rombouts, Y. et al. Anti-citrullinated protein antibodies acquire a pro-inflammatory Fc glycosylation phenotype prior to the onset of rheumatoid arthritis. Ann. Rheum. Dis. 74, 234–241 (2015).
Espy, C. et al. Sialylation levels of anti-proteinase 3 antibodies are associated with the activity of granulomatosis with polyangiitis (Wegener’s). Arthritis Rheum. 63, 2105–2115 (2011).
Wuhrer, M. et al. Skewed Fc glycosylation profiles of anti-proteinase 3 immunoglobulin G1 autoantibodies from granulomatosis with polyangiitis patients show low levels of bisection, galactosylation, and sialylation. J. Proteome Res. 14, 1657–1665 (2015).
Fickentscher, C. et al. The pathogenicity of anti-beta2GP1-IgG autoantibodies depends on Fc glycosylation. J. Immunol. Res. 2015, 638129 (2015).
Pasek, M. et al. Galactosylation of IgG from rheumatoid arthritis (RA) patients–changes during therapy. Glycoconj. J. 23, 463–471 (2006).
Gindzienska-Sieskiewicz, E. et al. Changes of glycosylation of IgG in rheumatoid arthritis patients treated with methotrexate. Adv. Med. Sci. 61, 193–197 (2016).
Selman, M. H. et al. Changes in antigen-specific IgG1 Fc N-glycosylation upon influenza and tetanus vaccination. Mol. Cell. Proteom. 11, M111 014563 (2012).
Kemna, M. J. et al. Galactosylation and sialylation levels of IgG predict relapse in patients with PR3-ANCA associated vasculitis. EBioMedicine 17, 108–118 (2017).
Bondt, A. et al. IgA N- and O-glycosylation profiling reveals no association with the pregnancy-related improvement in rheumatoid arthritis. Arthritis Res. Ther. 19, 160 (2017).
Bondt, A. et al. Longitudinal monitoring of immunoglobulin A glycosylation during pregnancy by simultaneous MALDI-FTICR-MS analysis of N- and O-glycopeptides. Sci. Rep. 6, 27955 (2016).
Steffen, U. et al. IgA subclasses have different effector functions associated with distinct glycosylation profiles. Nat. Commun. 11, 120 (2020).
Scherer, H. U. et al. Glycan profiling of anti-citrullinated protein antibodies isolated from human serum and synovial fluid. Arthritis Rheum. 62, 1620–1629 (2010).
Lardinois, O. M. et al. Immunoglobulins G from patients with ANCA-associated vasculitis are atypically glycosylated in both the Fc and Fab regions and the relation to disease activity. PLoS ONE 14, e0213215 (2019).
Kiyoshi, M., Tsumoto, K., Ishii-Watabe, A. & Caaveiro, J. M. M. Glycosylation of IgG-Fc: a molecular perspective. Int. Immunol. 29, 311–317 (2017).
Shinkawa, T. et al. The absence of fucose but not the presence of galactose or bisecting N-acetylglucosamine of human IgG1 complex-type oligosaccharides shows the critical role of enhancing antibody-dependent cellular cytotoxicity. J. Biol. Chem. 278, 3466–3473 (2003).
Ferrara, C., Stuart, F., Sondermann, P., Brunker, P. & Umana, P. The carbohydrate at FcγRIIIa Asn-162. An element required for high affinity binding to non-fucosylated IgG glycoforms. J. Biol. Chem. 281, 5032–5036 (2006).
Shields, R. L. et al. Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human FcγRIII and antibody-dependent cellular toxicity. J. Biol. Chem. 277, 26733–26740 (2002).
Larsen, M. D. et al. Afucosylated IgG characterizes enveloped viral responses and correlates with COVID-19 severity. Science 371, eabc8378 (2021).
Pongracz, T., Vidarsson, G. & Wuhrer, M. Antibody glycosylation in COVID-19. Glycoconj. J. 39, 335–344 (2022).
Karsten, C. M. et al. Anti-inflammatory activity of IgG1 mediated by Fc galactosylation and association of FcγRIIB and dectin-1. Nat. Med. 18, 1401–1406 (2012).
Heyl, K. A., Karsten, C. M. & Slevogt, H. Galectin-3 binds highly galactosylated IgG1 and is crucial for the IgG1 complex mediated inhibition of C5aReceptor induced immune responses. Biochem. Biophys. Res. Commun. 479, 86–90 (2016).
Ohmi, Y. et al. Sialylation converts arthritogenic IgG into inhibitors of collagen-induced arthritis. Nat. Commun. 7, 11205 (2016).
Anthony, R. M., Kobayashi, T., Wermeling, F. & Ravetch, J. V. Intravenous gammaglobulin suppresses inflammation through a novel TH2 pathway. Nature 475, 110–113 (2011).
Sondermann, P., Pincetic, A., Maamary, J., Lammens, K. & Ravetch, J. V. General mechanism for modulating immunoglobulin effector function. Proc. Natl Acad. Sci. USA 110, 9868–9872 (2013).
Rademacher, T. W., Williams, P. & Dwek, R. A. Agalactosyl glycoforms of IgG autoantibodies are pathogenic. Proc. Natl Acad. Sci. USA 91, 6123–6127 (1994).
Dekkers, G. et al. Decoding the human immunoglobulin G-glycan repertoire reveals a spectrum of Fc-receptor- and complement-mediated-effector activities. Front. Immunol. 8, 877 (2017).
Peschke, B., Keller, C. W., Weber, P., Quast, I. & Lunemann, J. D. Fc-galactosylation of human immunoglobulin gamma isotypes improves C1q binding and enhances complement-dependent cytotoxicity. Front. Immunol. 8, 646 (2017).
van Osch, T. L. J. et al. Fc galactosylation promotes hexamerization of human IgG1, leading to enhanced classical complement activation. J. Immunol. 207, 1545–1554 (2021).
Yu, X., Vasiljevic, S., Mitchell, D. A., Crispin, M. & Scanlan, C. N. Dissecting the molecular mechanism of IVIg therapy: the interaction between serum IgG and DC-SIGN is independent of antibody glycoform or Fc domain. J. Mol. Biol. 425, 1253–1258 (2013).
Crispin, M., Yu, X. & Bowden, T. A. Crystal structure of sialylated IgG Fc: implications for the mechanism of intravenous immunoglobulin therapy. Proc. Natl Acad. Sci. USA 110, E3544–E3546 (2013).
Ahmed, A. A. et al. Structural characterization of anti-inflammatory immunoglobulin G Fc proteins. J. Mol. Biol. 426, 3166–3179 (2014).
Campbell, I. K. et al. Therapeutic effect of IVIG on inflammatory arthritis in mice is dependent on the Fc portion and independent of sialylation or basophils. J. Immunol. 192, 5031–5038 (2014).
Leontyev, D. et al. Sialylation-independent mechanism involved in the amelioration of murine immune thrombocytopenia using intravenous gammaglobulin. Transfusion 52, 1799–1805 (2012).
Tjon, A. S. et al. Intravenous immunoglobulin treatment in humans suppresses dendritic cell function via stimulation of IL-4 and IL-13 production. J. Immunol. 192, 5625–5634 (2014).
Bayry, J., Bansal, K., Kazatchkine, M. D. & Kaveri, S. V. DC-SIGN and alpha2,6-sialylated IgG Fc interaction is dispensable for the anti-inflammatory activity of IVIg on human dendritic cells. Proc. Natl Acad. Sci. USA 106, E24 (2009).
Guhr, T. et al. Enrichment of sialylated IgG by lectin fractionation does not enhance the efficacy of immunoglobulin G in a murine model of immune thrombocytopenia. PLoS ONE 6, e21246 (2011).
Malhotra, R. et al. Glycosylation changes of IgG associated with rheumatoid arthritis can activate complement via the mannose-binding protein. Nat. Med. 1, 237–243 (1995).
Nimmerjahn, F., Anthony, R. M. & Ravetch, J. V. Agalactosylated IgG antibodies depend on cellular Fc receptors for in vivo activity. Proc. Natl Acad. Sci. USA 104, 8433–8437 (2007).
van de Geijn, F. E. et al. Mannose-binding lectin does not explain the course and outcome of pregnancy in rheumatoid arthritis. Arthritis Res. Ther. 13, R10 (2011).
van de Geijn, F. E. et al. Mannose-binding lectin polymorphisms are not associated with rheumatoid arthritis–confirmation in two large cohorts. Rheumatology 47, 1168–1171 (2008).
Lippold, S., Nicolardi, S., Wuhrer, M. & Falck, D. Proteoform-resolved FcRIIIa binding assay for Fab glycosylated monoclonal antibodies achieved by affinity chromatography mass spectrometry of Fc moieties. Front. Chem. 7, 698 (2019).
Quast, I. et al. Sialylation of IgG Fc domain impairs complement-dependent cytotoxicity. J. Clin. Invest. 125, 4160–4170 (2015).
Wei, B. et al. Fc galactosylation follows consecutive reaction kinetics and enhances immunoglobulin G hexamerization for complement activation. mAbs 13, 1893427 (2021).
Bartsch, Y. C. et al. IgG Fc sialylation is regulated during the germinal center reaction following immunization with different adjuvants. J. Allergy Clin. Immunol. 146, 652–666.e11 (2020).
Pfeifle, R. et al. Regulation of autoantibody activity by the IL-23-TH17 axis determines the onset of autoimmune disease. Nat. Immunol. 18, 104–113 (2017).
Jones, M. B. et al. B-cell-independent sialylation of IgG. Proc. Natl Acad. Sci. USA 113, 7207–7212 (2016).
Glendenning, L. M., Zhou, J. Y., Reynero, K. M. & Cobb, B. A. Divergent Golgi trafficking limits B cell-mediated IgG sialylation. J. Leukoc. Biol. https://doi.org/10.1002/JLB.3MA0522-731R (2022).
Axford, J. S. et al. Reduced B-cell galactosyltransferase activity in rheumatoid arthritis. Lancet 2, 1486–1488 (1987).
Wang, J. et al. Fc-glycosylation of IgG1 is modulated by B-cell stimuli. Mol. Cell. Proteom. 10, M110 004655 (2011).
Engdahl, C. et al. Estrogen induces St6gal1 expression and increases IgG sialylation in mice and patients with rheumatoid arthritis: a potential explanation for the increased risk of rheumatoid arthritis in postmenopausal women. Arthritis Res. Ther. 20, 84 (2018).
Mijakovac, A. et al. Effects of estradiol on immunoglobulin G glycosylation: mapping of the downstream signaling mechanism. Front. Immunol. 12, 680227 (2021).
Ercan, A. et al. Estrogens regulate glycosylation of IgG in women and men. JCI Insight 2, e89703 (2017).
Lagattuta, K. A. & Nigrovic, P. A. Estrogen-driven changes in immunoglobulin G Fc glycosylation. Exp. Suppl. 112, 341–361 (2021).
van de Bovenkamp, F. S., Hafkenscheid, L., Rispens, T. & Rombouts, Y. The emerging importance of IgG Fab glycosylation in immunity. J. Immunol. 196, 1435–1441 (2016).
Anumula, K. R. Quantitative glycan profiling of normal human plasma derived immunoglobulin and its fragments Fab and Fc. J. Immunol. Methods 382, 167–176 (2012).
Endo, T., Wright, A., Morrison, S. L. & Kobata, A. Glycosylation of the variable region of immunoglobulin G — site specific maturation of the sugar chains. Mol. Immunol. 32, 931–940 (1995).
Dunn-Walters, D., Boursier, L. & Spencer, J. Effect of somatic hypermutation on potential N-glycosylation sites in human immunoglobulin heavy chain variable regions. Mol. Immunol. 37, 107–113 (2000).
Koers, J. et al. Biased N-glycosylation site distribution and acquisition across the antibody V region during B cell maturation. J. Immunol. 202, 2220–2228 (2019).
van de Bovenkamp, F. S. et al. Adaptive antibody diversification through N-linked glycosylation of the immunoglobulin variable region. Proc. Natl Acad. Sci. USA 115, 1901–1906 (2018).
van de Bovenkamp, F. S. et al. Variable domain N-linked glycans acquired during antigen-specific immune responses can contribute to Immunoglobulin G antibody stability. Front. Immunol. 9, 740 (2018).
Courtois, F., Agrawal, N. J., Lauer, T. M. & Trout, B. L. Rational design of therapeutic mAbs against aggregation through protein engineering and incorporation of glycosylation motifs applied to bevacizumab. mAbs 8, 99–112 (2016).
Leibiger, H., Wustner, D., Stigler, R. D. & Marx, U. Variable domain-linked oligosaccharides of a human monoclonal IgG: structure and influence on antigen binding. Biochem. J. 338, 529–538 (1999).
Tachibana, H., Kim, J. Y. & Shirahata, S. Building high affinity human antibodies by altering the glycosylation on the light chain variable region in N-acetylglucosamine-supplemented hybridoma cultures. Cytotechnology 23, 151–159 (1997).
Coloma, M. J., Trinh, R. K., Martinez, A. R. & Morrison, S. L. Position effects of variable region carbohydrate on the affinity and in vivo behavior of an anti-(1→6) dextran antibody. J. Immunol. 162, 2162–2170 (1999).
Sabouri, Z. et al. Redemption of autoantibodies on anergic B cells by variable-region glycosylation and mutation away from self-reactivity. Proc. Natl Acad. Sci. USA 111, E2567–E2575 (2014).
Hafkenscheid, L. et al. Structural analysis of variable domain glycosylation of anti-citrullinated protein antibodies in rheumatoid arthritis reveals the presence of highly sialylated glycans. Mol. Cell. Proteom. 16, 278–287 (2017).
Rombouts, Y. et al. Extensive glycosylation of ACPA-IgG variable domains modulates binding to citrullinated antigens in rheumatoid arthritis. Ann. Rheum. Dis. 75, 578–585 (2016).
Vergroesen, R. D. et al. B-cell receptor sequencing of anti-citrullinated protein antibody (ACPA) IgG-expressing B cells indicates a selective advantage for the introduction of N-glycosylation sites during somatic hypermutation. Ann. Rheum. Dis. 77, 956–958 (2018).
Vergroesen, R. D. et al. N-Glycosylation site analysis of citrullinated antigen-specific B-cell receptors indicates alternative selection pathways during autoreactive B-cell development. Front. Immunol. 10, 2092 (2019).
Kissel, T. et al. IgG anti-citrullinated protein antibody variable domain glycosylation increases before the onset of rheumatoid arthritis and stabilizes thereafter: a cross-sectional study encompassing ~1,500 samples. Arthritis Rheumatol. 74, 1147–1158 (2022).
Hafkenscheid, L. et al. N-Linked glycans in the variable domain of IgG anti-citrullinated protein antibodies predict the development of rheumatoid arthritis. Arthritis Rheumatol. 71, 1626–1633 (2019).
Kissel, T. et al. On the presence of HLA-SE alleles and ACPA-IgG variable domain glycosylation in the phase preceding the development of rheumatoid arthritis. Ann. Rheum. Dis. 78, 1616–1620 (2019).
Kissel, T. et al. Genetic predisposition (HLA-SE) is associated with ACPA-IgG variable domain glycosylation in the predisease phase of RA. Ann. Rheum. Dis. 81, 141–143 (2022).
Suwannalai, P. et al. Anti-citrullinated protein antibodies have a low avidity compared with antibodies against recall antigens. Ann. Rheum. Dis. 70, 373–379 (2011).
Kissel, T. et al. Surface Ig variable domain glycosylation affects autoantigen binding and acts as threshold for human autoreactive B cell activation. Sci. Adv. 8, eabm1759 (2022).
Rizzuto, G. et al. Establishment of fetomaternal tolerance through glycan-mediated B cell suppression. Nature 603, 497–502 (2022).
Cao, A. et al. Galectin-9 binds IgM-BCR to regulate B cell signaling. Nat. Commun. 9, 3288 (2018).
Meyer, S. J., Linder, A. T., Brandl, C. & Nitschke, L. B cell Siglecs — news on signaling and its interplay with ligand binding. Front. Immunol. 9, 2820 (2018).
Demetriou, M., Granovsky, M., Quaggin, S. & Dennis, J. W. Negative regulation of T-cell activation and autoimmunity by Mgat5 N-glycosylation. Nature 409, 733–739 (2001).
Marth, J. D. & Grewal, P. K. Mammalian glycosylation in immunity. Nat. Rev. Immunol. 8, 874–887 (2008).
Dias, A. M. et al. Metabolic control of T cell immune response through glycans in inflammatory bowel disease. Proc. Natl Acad. Sci. USA 115, E4651–E4660 (2018).
Zhu, D. et al. Acquisition of potential N-glycosylation sites in the immunoglobulin variable region by somatic mutation is a distinctive feature of follicular lymphoma. Blood 99, 2562–2568 (2002).
Linley, A. et al. Lectin binding to surface Ig variable regions provides a universal persistent activating signal for follicular lymphoma cells. Blood 126, 1902–1910 (2015).
Radcliffe, C. M. et al. Human follicular lymphoma cells contain oligomannose glycans in the antigen-binding site of the B-cell receptor. J. Biol. Chem. 282, 7405–7415 (2007).
Coelho, V. et al. Glycosylation of surface Ig creates a functional bridge between human follicular lymphoma and microenvironmental lectins. Proc. Natl Acad. Sci. USA 107, 18587–18592 (2010).
Xu, P. C. et al. Influence of variable domain glycosylation on anti-neutrophil cytoplasmic autoantibodies and anti-glomerular basement membrane autoantibodies. BMC Immunol. 13, 10 (2012).
Hamza, N. et al. Ig gene analysis reveals altered selective pressures on Ig-producing cells in parotid glands of primary Sjogren’s syndrome patients. J. Immunol. 194, 514–521 (2015).
Visser, A., Hamza, N., Kroese, F. G. M. & Bos, N. A. Acquiring new N-glycosylation sites in variable regions of immunoglobulin genes by somatic hypermutation is a common feature of autoimmune diseases. Ann. Rheum. Dis. 77, e69 (2018).
Huijbers, M. G. et al. MuSK myasthenia gravis monoclonal antibodies: valency dictates pathogenicity. Neurol. Neuroimmunol. Neuroinflamm. 6, e547 (2019).
Mandel-Brehm, C. et al. Elevated N-linked glycosylation of IgG V regions in myasthenia gravis disease subtypes. J. Immunol. 207, 2005–2014 (2021).
Pereira, M. S. et al. Glycans as key checkpoints of T cell activity and function. Front. Immunol. 9, 2754 (2018).
Kanyo, N. et al. Glycocalyx regulates the strength and kinetics of cancer cell adhesion revealed by biophysical models based on high resolution label-free optical data. Sci. Rep. 10, 22422 (2020).
Shurer, C. R. et al. Physical principles of membrane shape regulation by the glycocalyx. Cell 177, 1757–1770.e21 (2019).
Paszek, M. J. et al. The cancer glycocalyx mechanically primes integrin-mediated growth and survival. Nature 511, 319–325 (2014).
Alves, I., Fernandes, A., Santos-Pereira, B., Azevedo, C. M. & Pinho, S. S. Glycans as a key factor in self and nonself discrimination: impact on the breach of immune tolerance. FEBS Lett. 596, 1485–1502 (2022).
Wang, Y. et al. Loss of α2-6 sialylation promotes the transformation of synovial fibroblasts into a pro-inflammatory phenotype in arthritis. Nat. Commun. 12, 2343 (2021).
Yang, X., Lehotay, M., Anastassiades, T., Harrison, M. & Brockhausen, I. The effect of TNF-α on glycosylation pathways in bovine synoviocytes. Biochem. Cell Biol. 82, 559–568 (2004).
Filer, A. et al. Galectin 3 induces a distinctive pattern of cytokine and chemokine production in rheumatoid synovial fibroblasts via selective signaling pathways. Arthritis Rheum. 60, 1604–1614 (2009).
Pearson, M. J. et al. Endogenous galectin-9 suppresses apoptosis in human rheumatoid arthritis synovial fibroblasts. Sci. Rep. 8, 12887 (2018).
Forsman, H. et al. Galectin 3 aggravates joint inflammation and destruction in antigen-induced arthritis. Arthritis Rheum. 63, 445–454 (2011).
Ohshima, S. et al. Galectin 3 and its binding protein in rheumatoid arthritis. Arthritis Rheum. 48, 2788–2795 (2003).
Pichler, K. M. et al. Galectin network in osteoarthritis: galectin-4 programs a pathogenic signature of gene and effector expression in human chondrocytes in vitro. Histochem. Cell Biol. 157, 139–151 (2022).
Isozaki, T. et al. Fucosyltransferase 1 mediates angiogenesis, cell adhesion and rheumatoid arthritis synovial tissue fibroblast proliferation. Arthritis Res. Ther. 16, R28 (2014).
Xu, D. et al. Tumor necrosis factor-alpha up-regulates the expression of beta1,4-galactosyltransferase-I in human fibroblast-like synoviocytes. Inflammation 34, 531–538 (2011).
Przybysz, M., Maszczak, D., Borysewicz, K., Szechinski, J. & Katnik-Prastowska, I. Relative sialylation and fucosylation of synovial and plasma fibronectins in relation to the progression and activity of rheumatoid arthritis. Glycoconj. J. 24, 543–550 (2007).
Flowers, S. A. et al. Lubricin binds cartilage proteins, cartilage oligomeric matrix protein, fibronectin and collagen II at the cartilage surface. Sci. Rep. 7, 13149 (2017).
Estrella, R. P., Whitelock, J. M., Packer, N. H. & Karlsson, N. G. The glycosylation of human synovial lubricin: implications for its role in inflammation. Biochem. J. 429, 359–367 (2010).
Alves, I. et al. Protein mannosylation as a diagnostic and prognostic biomarker of lupus nephritis: an unusual glycan neoepitope in systemic lupus erythematosus. Arthritis Rheumatol. 73, 2069–2077 (2021).
Urushihara, M. et al. Sisters with α-mannosidosis and systemic lupus erythematosus. Eur. J. Pediatr. 163, 192–195 (2004).
Chui, D. et al. Genetic remodeling of protein glycosylation in vivo induces autoimmune disease. Proc. Natl Acad. Sci. USA 98, 1142–1147 (2001).
van Kooyk, Y. & Rabinovich, G. A. Protein-glycan interactions in the control of innate and adaptive immune responses. Nat. Immunol. 9, 593–601 (2008).
Cai, M. et al. DC-SIGN expression on podocytes and its role in inflammatory immune response of lupus nephritis. Clin. Exp. Immunol. 183, 317–325 (2016).
Noguchi, S. et al. Reduction of UDP-N-acetylglucosamine 2-epimerase/N-acetylmannosamine kinase activity and sialylation in distal myopathy with rimmed vacuoles. J. Biol. Chem. 279, 11402–11407 (2004).
Malicdan, M. C., Noguchi, S., Hayashi, Y. K., Nonaka, I. & Nishino, I. Prophylactic treatment with sialic acid metabolites precludes the development of the myopathic phenotype in the DMRV-hIBM mouse model. Nat. Med. 15, 690–695 (2009).
Carrillo, N. et al. Safety and efficacy of N-acetylmannosamine (ManNAc) in patients with GNE myopathy: an open-label phase 2 study. Genet. Med. 23, 2067–2075 (2021).
McGovern, D. P. et al. Fucosyltransferase 2 (FUT2) non-secretor status is associated with Crohn’s disease. Hum. Mol. Genet. 19, 3468–3476 (2010).
Goto, Y. et al. Innate lymphoid cells regulate intestinal epithelial cell glycosylation. Science 345, 1254009 (2014).
Morosi, L. G. et al. Control of intestinal inflammation by glycosylation-dependent lectin-driven immunoregulatory circuits. Sci. Adv. 7, eabf8630 (2021).
Costa, A. F., Campos, D., Reis, C. A. & Gomes, C. Targeting glycosylation: a new road for cancer drug discovery. Trends Cancer 6, 757–766 (2020).
Dube, D. H. & Bertozzi, C. R. Glycans in cancer and inflammation — potential for therapeutics and diagnostics. Nat. Rev. Drug Discov. 4, 477–488 (2005).
Nandakumar, K. S. et al. Endoglycosidase treatment abrogates IgG arthritogenicity: importance of IgG glycosylation in arthritis. Eur. J. Immunol. 37, 2973–2982 (2007).
Albert, H., Collin, M., Dudziak, D., Ravetch, J. V. & Nimmerjahn, F. In vivo enzymatic modulation of IgG glycosylation inhibits autoimmune disease in an IgG subclass-dependent manner. Proc. Natl Acad. Sci. USA 105, 15005–15009 (2008).
Collin, M., Shannon, O. & Bjorck, L. IgG glycan hydrolysis by a bacterial enzyme as a therapy against autoimmune conditions. Proc. Natl Acad. Sci. USA 105, 4265–4270 (2008).
Nandakumar, K. S. et al. Dominant suppression of inflammation by glycan-hydrolyzed IgG. Proc. Natl Acad. Sci. USA 111, 15851 (2014).
Trastoy, B. et al. Structural basis for the recognition of complex-type N-glycans by Endoglycosidase S. Nat. Commun. 9, 1874 (2018).
Bartsch, Y. C. et al. Sialylated autoantigen-reactive IgG antibodies attenuate disease development in autoimmune mouse models of lupus nephritis and rheumatoid arthritis. Front. Immunol. 9, 1183 (2018).
Verhelst, X. et al. Protein glycosylation as a diagnostic and prognostic marker of chronic inflammatory gastrointestinal and liver diseases. Gastroenterology 158, 95–110 (2020).
Araujo, L., Khim, P., Mkhikian, H., Mortales, C. L. & Demetriou, M. Glycolysis and glutaminolysis cooperatively control T cell function by limiting metabolite supply to N-glycosylation. eLife 6, e21330 (2017).
Salvatore, S. et al. A pilot study of N-acetyl glucosamine, a nutritional substrate for glycosaminoglycan synthesis, in paediatric chronic inflammatory bowel disease. Aliment. Pharmacol. Ther. 14, 1567–1579 (2000).
Mkhikian, H. et al. Genetics and the environment converge to dysregulate N-glycosylation in multiple sclerosis. Nat. Commun. 2, 334 (2011).
Brandt, A. U. et al. Association of a marker of N-acetylglucosamine with progressive multiple sclerosis and neurodegeneration. JAMA Neurol. 78, 842–852 (2021).
Pereira, N. A., Chan, K. F., Lin, P. C. & Song, Z. The “less-is-more” in therapeutic antibodies: afucosylated anti-cancer antibodies with enhanced antibody-dependent cellular cytotoxicity. mAbs 10, 693–711 (2018).
Macauley, M. S., Crocker, P. R. & Paulson, J. C. Siglec-mediated regulation of immune cell function in disease. Nat. Rev. Immunol. 14, 653–666 (2014).
Kristyanto, H. et al. Multifunctional, multivalent PIC polymer scaffolds for targeting antigen-specific, autoreactive B cells. ACS Biomater. Sci. Eng. 8, 1486–1493 (2022).
Caianiello, D. F. et al. Bifunctional small molecules that mediate the degradation of extracellular proteins. Nat. Chem. Biol. 17, 947–953 (2021).
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
R.E.M.T. and T.W.J.H. are mentioned inventors on a patent application on ACPA-IgG V-domain glycosylation. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Rheumatology thanks Salomé Pinho, Mattias Collin and Peter Nigrovic for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kissel, T., Toes, R.E.M., Huizinga, T.W.J. et al. Glycobiology of rheumatic diseases. Nat Rev Rheumatol 19, 28–43 (2023). https://doi.org/10.1038/s41584-022-00867-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41584-022-00867-4
This article is cited by
-
N-linked Fc glycosylation is not required for IgG-B-cell receptor function in a GC-derived B-cell line
Nature Communications (2024)
-
A critical evaluation of ultrasensitive single-cell proteomics strategies
Analytical and Bioanalytical Chemistry (2024)
-
Mannosylation is a cause and target in lupus nephritis
Nature Reviews Rheumatology (2023)
-
Effect of posttranslational modifications and subclass on IgG activity: from immunity to immunotherapy
Nature Immunology (2023)