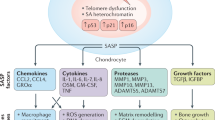
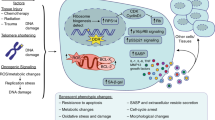
Abstract
Ageing is characterized by a progressive loss of cellular function that leads to a decline in tissue homeostasis, increased vulnerability and adverse health outcomes. Important advances in ageing research have now identified a set of nine candidate hallmarks that are generally considered to contribute to the ageing process and that together determine the ageing phenotype, which is the clinical manifestation of age-related dysfunction in chronic diseases. Although most rheumatic diseases are not yet considered to be age related, available evidence increasingly emphasizes the prevalence of ageing hallmarks in these chronic diseases. On the basis of the current evidence relating to the molecular and cellular ageing pathways involved in rheumatic diseases, we propose that these diseases share a number of features that are observed in ageing, and that they can therefore be considered to be diseases of premature or accelerated ageing. Although more data are needed to clarify whether accelerated ageing drives the development of rheumatic diseases or whether it results from the chronic inflammatory environment, central components of age-related pathways are currently being targeted in clinical trials and may provide a new avenue of therapeutic intervention for patients with rheumatic diseases.
Key points
-
Rheumatic disease prevalence is increased as a consequence of population ageing.
-
Rheumatic diseases share common ageing characteristics and molecular pathways, which enables their classification as premature-ageing or accelerated-ageing diseases.
-
Ageing and inflammation form a self-perpetuating, vicious cycle to advance rheumatic disease in patients and accelerate ageing phenotypes.
-
Anti-ageing drugs may have therapeutic potential for the management and treatment of patients with rheumatic disease.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Partridge, L., Deelen, J. & Slagboom, P. E. Facing up to the global challenges of ageing. Nature 561, 45–56 (2018).
Lopez-Otin, C., Blasco, M. A., Partridge, L., Serrano, M. & Kroemer, G. The hallmarks of aging. Cell 153, 1194–1217 (2013).
GBD 2017 DALYs and HALE Collaborators. Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 392, 1859–1922 (2018).
Shiels, P. G. et al. Manipulating the exposome to enable better ageing. Biochem. J. 478, 2889–2898 (2021).
Van Houtven, G., et al Costs of illness among older adults: an analysis of six major health conditions with significant environmental risk factors. RTI Press Publication No. RR-0002-0809. (RTI Press, 2008).
Atella, V. et al. Trends in age-related disease burden and healthcare utilization. Aging Cell 18, e12861 (2019).
Christensen, K., Doblhammer, G., Rau, R. & Vaupel, J. W. Ageing populations: the challenges ahead. Lancet 374, 1196–1208 (2009).
Frenk, S. & Houseley, J. Can aging be beneficial? Aging 9, 2016–2017 (2017).
Ferrucci, L., Levine, M. E., Kuo, P. L. & Simonsick, E. M. Time and the metrics of aging. Circ. Res. 123, 740–744 (2018).
Verstappen, S. M. M. & Carmona, L. Epidemiology of rheumatic and musculoskeletal diseases. Best. Pract. Res. Clin. Rheumatol. 32, 167–168 (2018).
Sangha, O. Epidemiology of rheumatic diseases. Rheumatology 39 (Suppl. 2), 3–12 (2000).
Branco, J. C. et al. Prevalence of rheumatic and musculoskeletal diseases and their impact on health-related quality of life, physical function and mental health in Portugal: results from EpiReumaPt — a national health survey. RMD Open 2, e000166 (2016).
Gabriel, S. E. & Michaud, K. Epidemiological studies in incidence, prevalence, mortality, and comorbidity of the rheumatic diseases. Arthritis Res. Ther. 11, 229 (2009).
Loeser, R. F., Collins, J. A. & Diekman, B. O. Ageing and the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 12, 412–420 (2016).
Boots, A. M. et al. The influence of ageing on the development and management of rheumatoid arthritis. Nat. Rev. Rheumatol. 9, 604–613 (2013).
Mahmoudi, S. & Brunet, A. Aging and reprogramming: a two-way street. Curr. Opin. Cell Biol. 24, 744–756 (2012).
Melzer, D., Pilling, L. C. & Ferrucci, L. The genetics of human ageing. Nat. Rev. Genet. 21, 88–101 (2020).
Sebastiani, P. & Perls, T. T. The genetics of extreme longevity: lessons from the New England centenarian study. Front. Genet. 3, 277 (2012).
Dorman, J. B., Albinder, B., Shroyer, T. & Kenyon, C. The age-1 and daf-2 genes function in a common pathway to control the lifespan of Caenorhabditis elegans. Genetics 141, 1399–1406 (1995).
Friedman, D. B. & Johnson, T. E. A mutation in the age-1 gene in Caenorhabditis elegans lengthens life and reduces hermaphrodite fertility. Genetics 118, 75–86 (1988).
Cagan, A. et al. Somatic mutation rates scale with lifespan across mammals. Nature 604, 517–524 (2022).
Amoretti, M. et al. Production and detection of cold antihydrogen atoms. Nature 419, 456–459 (2002).
Henis-Korenblit, S. et al. Insulin/IGF-1 signaling mutants reprogram ER stress response regulators to promote longevity. Proc. Natl Acad. Sci. USA 107, 9730–9735 (2010).
Martin, G. M. & Oshima, J. Lessons from human progeroid syndromes. Nature 408, 263–266 (2000).
Kazak, L., Reyes, A. & Holt, I. J. Minimizing the damage: repair pathways keep mitochondrial DNA intact. Nat. Rev. Mol. Cell Biol. 13, 659–671 (2012).
Blackburn, E. H., Greider, C. W. & Szostak, J. W. Telomeres and telomerase: the path from maize, Tetrahymena and yeast to human cancer and aging. Nat. Med. 12, 1133–1138 (2006).
Chalan, P., van den Berg, A., Kroesen, B. J., Brouwer, L. & Boots, A. Rheumatoid arthritis, immunosenescence and the hallmarks of aging. Curr. Aging Sci. 8, 131–146 (2015).
Souliotis, V. L. et al. DNA damage response and oxidative stress in systemic autoimmunity. Int. J. Mol. Sci. 21, 55 (2019).
Souliotis, V. L., Vlachogiannis, N. I., Pappa, M., Argyriou, A. & Sfikakis, P. P. DNA damage accumulation, defective chromatin organization and deficient DNA repair capacity in patients with rheumatoid arthritis. Clin. Immunol. 203, 28–36 (2019).
Shao, L. et al. Deficiency of the DNA repair enzyme ATM in rheumatoid arthritis. J. Exp. Med. 206, 1435–1449 (2009).
Micheli, C. et al. UCTD and SLE patients show increased levels of oxidative and DNA damage together with an altered kinetics of DSB repair. Mutagenesis 36, 429–436 (2021).
Mireles-Canales, M. P., Gonzalez-Chavez, S. A., Quinonez-Flores, C. M., Leon-Lopez, E. A. & Pacheco-Tena, C. DNA damage and deficiencies in the mechanisms of its repair: implications in the pathogenesis of systemic lupus erythematosus. J. Immunol. Res. 2018, 8214379 (2018).
Noble, P. W. et al. DNA-damaging autoantibodies and cancer: the lupus butterfly theory. Nat. Rev. Rheumatol. 12, 429–434 (2016).
McConnell, J. R., Crockard, A. D., Cairns, A. P. & Bell, A. L. Neutrophils from systemic lupus erythematosus patients demonstrate increased nuclear DNA damage. Clin. Exp. Rheumatol. 20, 653–660 (2002).
Vlachogiannis, N. I. et al. Association between DNA damage response, fibrosis and type I interferon signature in systemic sclerosis. Front. Immunol. 11, 582401 (2020).
Palomino, G. M. et al. Patients with systemic sclerosis present increased DNA damage differentially associated with DNA repair gene polymorphisms. J. Rheumatol. 41, 458–465 (2014).
Li, Y. et al. Deficient activity of the nuclease MRE11A induces T cell aging and promotes arthritogenic effector functions in patients with rheumatoid arthritis. Immunity 45, 903–916 (2016).
Rose, J. et al. DNA damage, discoordinated gene expression and cellular senescence in osteoarthritic chondrocytes. Osteoarthritis Cartilage 20, 1020–1028 (2012).
La Rubia, M., Rus, A., Molina, F. & Del Moral, M. L. Is fibromyalgia-related oxidative stress implicated in the decline of physical and mental health status? Clin. Exp. Rheumatol. 31, S121–S127 (2013).
Talens, R. P. et al. Epigenetic variation during the adult lifespan: cross-sectional and longitudinal data on monozygotic twin pairs. Aging Cell 11, 694–703 (2012).
Fraga, M. F. & Esteller, M. Epigenetics and aging: the targets and the marks. Trends Genet. 23, 413–418 (2007).
Ballestar, E. & Li, T. New insights into the epigenetics of inflammatory rheumatic diseases. Nat. Rev. Rheumatol. 13, 593–605 (2017).
Nygaard, G. & Firestein, G. S. Restoring synovial homeostasis in rheumatoid arthritis by targeting fibroblast-like synoviocytes. Nat. Rev. Rheumatol. 16, 316–333 (2020).
Karouzakis, E. et al. DNA methylation regulates the expression of CXCL12 in rheumatoid arthritis synovial fibroblasts. Genes Immun. 12, 643–652 (2011).
Alsaleh, G. et al. Reduced DICER1 expression bestows rheumatoid arthritis synoviocytes proinflammatory properties and resistance to apoptotic stimuli. Arthritis Rheumatol. 68, 1839–1848 (2016).
Philippe, L. et al. MiR-20a regulates ASK1 expression and TLR4-dependent cytokine release in rheumatoid fibroblast-like synoviocytes. Ann. Rheum. Dis. 72, 1071–1079 (2013).
Grabiec, A. M. & Reedquist, K. A. Histone deacetylases in RA: epigenetics and epiphenomena. Arthritis Res. Ther. 12, 142 (2010).
Huber, L. C. et al. Histone deacetylase/acetylase activity in total synovial tissue derived from rheumatoid arthritis and osteoarthritis patients. Arthritis Rheum. 56, 1087–1093 (2007).
Wang, Y. et al. Aberrant histone modification in peripheral blood B cells from patients with systemic sclerosis. Clin. Immunol. 149, 46–54 (2013).
Thabet, Y. et al. Epigenetic dysregulation in salivary glands from patients with primary Sjogren’s syndrome may be ascribed to infiltrating B cells. J. Autoimmun. 41, 175–181 (2013).
Yu, X. et al. DNA hypermethylation leads to lower FOXP3 expression in CD4+ T cells of patients with primary Sjogren’s syndrome. Clin. Immunol. 148, 254–257 (2013).
Huck, S. & Zouali, M. DNA methylation: a potential pathway to abnormal autoreactive lupus B cells. Clin. Immunol. Immunopathol. 80, 1–8 (1996).
Hu, N. et al. Abnormal histone modification patterns in lupus CD4+ T cells. J. Rheumatol. 35, 804–810 (2008).
Javierre, B. M. et al. Changes in the pattern of DNA methylation associate with twin discordance in systemic lupus erythematosus. Genome Res. 20, 170–179 (2010).
Deng, C. et al. Decreased Ras-mitogen-activated protein kinase signaling may cause DNA hypomethylation in T lymphocytes from lupus patients. Arthritis Rheum. 44, 397–407 (2001).
Li, Y., Gorelik, G., Strickland, F. M. & Richardson, B. C. Oxidative stress, T cell DNA methylation, and lupus. Arthritis Rheumatol. 66, 1574–1582 (2014).
Ugalde, A. P., Espanol, Y. & Lopez-Otin, C. Micromanaging aging with miRNAs: new messages from the nuclear envelope. Nucleus 2, 549–555 (2011).
Maurer, B. et al. MicroRNA-29, a key regulator of collagen expression in systemic sclerosis. Arthritis Rheum. 62, 1733–1743 (2010).
Alsaleh, G. et al. MiR-30a-3p negatively regulates BAFF synthesis in systemic sclerosis and rheumatoid arthritis fibroblasts. PLoS One 9, e111266 (2014).
Pauley, K. M. et al. Altered miR-146a expression in Sjogren’s syndrome and its functional role in innate immunity. Eur. J. Immunol. 41, 2029–2039 (2011).
Shi, H., Zheng, L. Y., Zhang, P. & Yu, C. Q. miR-146a and miR-155 expression in PBMCs from patients with Sjogren’s syndrome. J. Oral. Pathol. Med. 43, 792–797 (2014).
Wang, X. et al. MicroRNA-146a-5p enhances T helper 17 cell differentiation via decreasing a disintegrin and metalloprotease 17 level in primary Sjogren’s syndrome. Bioengineered 12, 310–324 (2021).
Qin, H. et al. MicroRNA-29b contributes to DNA hypomethylation of CD4+ T cells in systemic lupus erythematosus by indirectly targeting DNA methyltransferase 1. J. Dermatol. Sci. 69, 61–67 (2013).
Pan, W. et al. MicroRNA-21 and microRNA-148a contribute to DNA hypomethylation in lupus CD4+ T cells by directly and indirectly targeting DNA methyltransferase 1. J. Immunol. 184, 6773–6781 (2010).
Zhu, J. et al. Stem cell-homing hydrogel-based miR-29b-5p delivery promotes cartilage regeneration by suppressing senescence in an osteoarthritis rat model. Sci. Adv. 8, eabk0011 (2022).
De Cecco, M. et al. L1 drives IFN in senescent cells and promotes age-associated inflammation. Nature 566, 73–78 (2019).
Mavragani, C. P. et al. Expression of Long Interspersed Nuclear Element 1 retroelements and induction of type I interferon in patients with systemic autoimmune disease. Arthritis Rheumatol. 68, 2686–2696 (2016).
Simon, M. et al. LINE1 derepression in aged wild-type and SIRT6-deficient mice drives inflammation. Cell Metab. 29, 871–885 e875 (2019).
Gorbunova, V. et al. The role of retrotransposable elements in ageing and age-associated diseases. Nature 596, 43–53 (2021).
Blasco, M. A. Telomere length, stem cells and aging. Nat. Chem. Biol. 3, 640–649 (2007).
Armanios, M. et al. Short telomeres are sufficient to cause the degenerative defects associated with aging. Am. J. Hum. Genet. 85, 823–832 (2009).
Blasco, M. A. et al. Telomere shortening and tumor formation by mouse cells lacking telomerase RNA. Cell 91, 25–34 (1997).
Herrera, E. et al. Disease states associated with telomerase deficiency appear earlier in mice with short telomeres. EMBO J. 18, 2950–2960 (1999).
Steer, S. E. et al. Reduced telomere length in rheumatoid arthritis is independent of disease activity and duration. Ann. Rheum. Dis. 66, 476–480 (2007).
Koetz, K. et al. T cell homeostasis in patients with rheumatoid arthritis. Proc. Natl Acad. Sci. USA 97, 9203–9208 (2000).
Zeng, Z. et al. Association of telomere length with risk of rheumatoid arthritis: a meta-analysis and Mendelian randomization. Rheumatology 59, 940–947 (2020).
Tamayo, M. et al. Differing patterns of peripheral blood leukocyte telomere length in rheumatologic diseases. Mutat. Res. 683, 68–73 (2010).
Yudoh, K., Matsuno, H., Nezuka, T. & Kimura, T. Different mechanisms of synovial hyperplasia in rheumatoid arthritis and pigmented villonodular synovitis: the role of telomerase activity in synovial proliferation. Arthritis Rheum. 42, 669–677 (1999).
Haque, S. et al. Shortened telomere length in patients with systemic lupus erythematosus. Arthritis Rheum. 65, 1319–1323 (2013).
Lee, Y. H. et al. Association between shortened telomere length and systemic lupus erythematosus: a meta-analysis. Lupus 26, 282–288 (2017).
MacIntyre, A. et al. Association of increased telomere lengths in limited scleroderma, with a lack of age-related telomere erosion. Ann. Rheum. Dis. 67, 1780–1782 (2008).
Lakota, K. et al. Short lymphocyte, but not granulocyte, telomere length in a subset of patients with systemic sclerosis. Ann. Rheum. Dis. 78, 1142–1144 (2019).
Artlett, C. M., Black, C. M., Briggs, D. C., Stevens, C. O. & Welsh, K. I. Telomere reduction in scleroderma patients: a possible cause for chromosomal instability. Br. J. Rheumatol. 35, 732–737 (1996).
Tarhan, F. et al. Telomerase activity in connective tissue diseases: elevated in rheumatoid arthritis, but markedly decreased in systemic sclerosis. Rheumatol. Int. 28, 579–583 (2008).
Hassett, A. L. et al. Pain is associated with short leukocyte telomere length in women with fibromyalgia. J. Pain. 13, 959–969 (2012).
Mensa, E. et al. The telomere world and aging: analytical challenges and future perspectives. Ageing Res. Rev. 50, 27–42 (2019).
Heba, A. C. et al. Telomeres: new players in immune-mediated inflammatory diseases? J. Autoimmun. 123, 102699 (2021).
Hipp, M. S., Kasturi, P. & Hartl, F. U. The proteostasis network and its decline in ageing. Nat. Rev. Mol. Cell Biol. 20, 421–435 (2019).
Sasaki, H. et al. Autophagy modulates osteoarthritis-related gene expression in human chondrocytes. Arthritis Rheum. 64, 1920–1928 (2012).
Hui, W. et al. Oxidative changes and signalling pathways are pivotal in initiating age-related changes in articular cartilage. Ann. Rheum. Dis. 75, 449–458 (2016).
Dumit, V. I. et al. Altered MCM protein levels and autophagic flux in aged and systemic sclerosis dermal fibroblasts. J. Invest. Dermatol. 134, 2321–2330 (2014).
Zhang, Y. et al. Cartilage-specific deletion of mTOR upregulates autophagy and protects mice from osteoarthritis. Ann. Rheum. Dis. 74, 1432–1440 (2015).
Clarke, A. J. et al. Autophagy is activated in systemic lupus erythematosus and required for plasmablast development. Ann. Rheum. Dis. 74, 912–920 (2015).
Sorice, M. et al. Autophagy generates citrullinated peptides in human synoviocytes: a possible trigger for anti-citrullinated peptide antibodies. Rheumatology 55, 1374–1385 (2016).
Serrano, R. L., Chen, L. Y., Lotz, M. K., Liu-Bryan, R. & Terkeltaub, R. Impaired proteasomal function in human osteoarthritic chondrocytes can contribute to decreased levels of SOX9 and aggrecan. Arthritis Rheumatol. 70, 1030–1041 (2018).
Radwan, M. et al. Protection against murine osteoarthritis by inhibition of the 26 S proteasome and lysine-48 linked ubiquitination. Ann. Rheum. Dis. 74, 1580–1587 (2015).
Tan, L., Register, T. C. & Yammani, R. R. Age-related decline in expression of molecular chaperones induces endoplasmic reticulum stress and chondrocyte apoptosis in articular cartilage. Aging Dis. 11, 1091–1102 (2020).
Ariosa-Morejon, Y. et al. Age-dependent changes in protein incorporation into collagen-rich tissues of mice by in vivo pulsed SILAC labelling. Elife 10, e66635 (2021).
Edgar, D. et al. Random point mutations with major effects on protein-coding genes are the driving force behind premature aging in mtDNA mutator mice. Cell Metab. 10, 131–138 (2009).
Green, D. R., Galluzzi, L. & Kroemer, G. Mitochondria and the autophagy-inflammation-cell death axis in organismal aging. Science 333, 1109–1112 (2011).
Kujoth, G. C. et al. Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science 309, 481–484 (2005).
Trifunovic, A. et al. Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature 429, 417–423 (2004).
Blanco, F. J., Valdes, A. M. & Rego-Perez, I. Mitochondrial DNA variation and the pathogenesis of osteoarthritis phenotypes. Nat. Rev. Rheumatol. 14, 327–340 (2018).
Fearon, U., Canavan, M., Biniecka, M. & Veale, D. J. Hypoxia, mitochondrial dysfunction and synovial invasiveness in rheumatoid arthritis. Nat. Rev. Rheumatol. 12, 385–397 (2016).
Mancini, O. K. et al. Oxidative stress-induced senescence mediates inflammatory and fibrotic phenotypes in fibroblasts from systemic sclerosis patients. Rheumatology 61, 1265–1275 (2021).
Barrera, M. J. et al. Dysfunctional mitochondria as critical players in the inflammation of autoimmune diseases: potential role in Sjogren’s syndrome. Autoimmun. Rev. 20, 102867 (2021).
Vaamonde-Garcia, C. & Lopez-Armada, M. J. Role of mitochondrial dysfunction on rheumatic diseases. Biochem. Pharmacol. 165, 181–195 (2019).
Leishangthem, B. D., Sharma, A. & Bhatnagar, A. Role of altered mitochondria functions in the pathogenesis of systemic lupus erythematosus. Lupus 25, 272–281 (2016).
Da Sylva, T. R., Connor, A., Mburu, Y., Keystone, E. & Wu, G. E. Somatic mutations in the mitochondria of rheumatoid arthritis synoviocytes. Arthritis Res. Ther. 7, R844–R851 (2005).
Cordero, M. D. et al. Mutation in cytochrome b gene of mitochondrial DNA in a family with fibromyalgia is associated with NLRP3-inflammasome activation. J. Med. Genet. 53, 113–122 (2016).
Gazdhar, A. et al. Time-dependent and somatically acquired mitochondrial DNA mutagenesis and respiratory chain dysfunction in a scleroderma model of lung fibrosis. Sci. Rep. 4, 5336 (2014).
Fernandez-Moreno, M. et al. Mitochondrial DNA haplogroups influence the risk of incident knee osteoarthritis in OAI and CHECK cohorts. A meta-analysis and functional study. Ann. Rheum. Dis. 76, 1114–1122 (2017).
Chang, M. C. et al. Accumulation of mitochondrial DNA with 4977-bp deletion in knee cartilage — an association with idiopathic osteoarthritis. Osteoarthritis Cartilage 13, 1004–1011 (2005).
Onishi, M., Yamano, K., Sato, M., Matsuda, N. & Okamoto, K. Molecular mechanisms and physiological functions of mitophagy. EMBO J. 40, e104705 (2021).
Akamata, K. et al. SIRT3 is attenuated in systemic sclerosis skin and lungs, and its pharmacologic activation mitigates organ fibrosis. Oncotarget 7, 69321–69336 (2016).
Patel, A. S. et al. Epithelial cell mitochondrial dysfunction and PINK1 are induced by transforming growth factor-beta1 in pulmonary fibrosis. PLoS One 10, e0121246 (2015).
Cordero, M. D. et al. Mitochondrial dysfunction and mitophagy activation in blood mononuclear cells of fibromyalgia patients: implications in the pathogenesis of the disease. Arthritis Res. Ther. 12, R17 (2010).
Green, C. L., Lamming, D. W. & Fontana, L. Molecular mechanisms of dietary restriction promoting health and longevity. Nat. Rev. Mol. Cell Biol. 23, 56–73 (2022).
Harrison, D. E. et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 460, 392–395 (2009).
Selman, C. et al. Ribosomal protein S6 kinase 1 signaling regulates mammalian life span. Science 326, 140–144 (2009).
Laragione, T. & Gulko, P. S. mTOR regulates the invasive properties of synovial fibroblasts in rheumatoid arthritis. Mol. Med. 16, 352–358 (2010).
Saxena, A., Raychaudhuri, S. K. & Raychaudhuri, S. P. Interleukin-17-induced proliferation of fibroblast-like synovial cells is mTOR dependent. Arthritis Rheum. 63, 1465–1466 (2011).
Javier, A. F. et al. Rapamycin (sirolimus) inhibits proliferating cell nuclear antigen expression and blocks cell cycle in the G1 phase in human keratinocyte stem cells. J. Clin. Invest. 99, 2094–2099 (1997).
Chen, S. et al. mTOR Blockade by rapamycin in spondyloarthritis: impact on inflammation and new bone formation in vitro and in vivo. Front. Immunol. 10, 2344 (2019).
Buerger, C., Malisiewicz, B., Eiser, A., Hardt, K. & Boehncke, W. H. Mammalian target of rapamycin and its downstream signalling components are activated in psoriatic skin. Br. J. Dermatol. 169, 156–159 (2013).
Yoshizaki, A. et al. Treatment with rapamycin prevents fibrosis in tight-skin and bleomycin-induced mouse models of systemic sclerosis. Arthritis Rheum. 62, 2476–2487 (2010).
Tamaki, Z. et al. Effects of the immunosuppressant rapamycin on the expression of human alpha2(I) collagen and matrix metalloproteinase 1 genes in scleroderma dermal fibroblasts. J. Dermatol. Sci. 74, 251–259 (2014).
Zhang, M. et al. mTOR activation in CD8+ cells contributes to disease activity of rheumatoid arthritis and increases therapeutic response to TNF inhibitors. Rheumatology 61, 3010–3022 (2021).
Guan, Y., Yang, X., Yang, W., Charbonneau, C. & Chen, Q. Mechanical activation of mammalian target of rapamycin pathway is required for cartilage development. FASEB J. 28, 4470–4481 (2014).
Lopez de Figueroa, P., Lotz, M. K., Blanco, F. J. & Carames, B. Autophagy activation and protection from mitochondrial dysfunction in human chondrocytes. Arthritis Rheumatol. 67, 966–976 (2015).
Cejka, D. et al. Mammalian target of rapamycin signaling is crucial for joint destruction in experimental arthritis and is activated in osteoclasts from patients with rheumatoid arthritis. Arthritis Rheum. 62, 2294–2302 (2010).
Deng, Z. et al. The role of sirtuin 1 and its activator, resveratrol in osteoarthritis. Biosci. Rep. 39, BSR20190189 (2019).
Matsuzaki, T. et al. Disruption of Sirt1 in chondrocytes causes accelerated progression of osteoarthritis under mechanical stress and during ageing in mice. Ann. Rheum. Dis. 73, 1397–1404 (2014).
Sacitharan, P. K., Bou-Gharios, G. & Edwards, J. R. SIRT1 directly activates autophagy in human chondrocytes. Cell Death Discov. 6, 41 (2020).
Wendling, D. et al. Dysregulated serum IL-23 and SIRT1 activity in peripheral blood mononuclear cells of patients with rheumatoid arthritis. PLoS One 10, e0119981 (2015).
Hah, Y. S. et al. Myeloid deletion of SIRT1 aggravates serum transfer arthritis in mice via nuclear factor-κB activation. PLoS One 9, e87733 (2014).
Woo, S. J. et al. Myeloid deletion of SIRT1 suppresses collagen-induced arthritis in mice by modulating dendritic cell maturation. Exp. Mol. Med. 48, e221 (2016).
Niederer, F. et al. SIRT1 overexpression in the rheumatoid arthritis synovium contributes to proinflammatory cytokine production and apoptosis resistance. Ann. Rheum. Dis. 70, 1866–1873 (2011).
Li, G. et al. SIRT1 inhibits rheumatoid arthritis fibroblast-like synoviocyte aggressiveness and inflammatory response via suppressing NF-κB pathway. Biosci. Rep. 38, BSR20180541 (2018).
Wang, Z. L. et al. Resveratrol possesses protective effects in a pristane-induced lupus mouse model. PLoS One 9, e114792 (2014).
Zhang, J. et al. The type III histone deacetylase Sirt1 is essential for maintenance of T cell tolerance in mice. J. Clin. Invest. 119, 3048–3058 (2009).
Schett, G. et al. Diabetes is an independent predictor for severe osteoarthritis: results from a longitudinal cohort study. Diabetes Care 36, 403–409 (2013).
Quevedo-Abeledo, J. C. et al. Higher prevalence and degree of insulin resistance in patients with rheumatoid arthritis than in patients with systemic lupus erythematosus. J. Rheumatol. 48, 339–347 (2021).
Giles, J. T. et al. Insulin resistance in rheumatoid arthritis: disease-related indicators and associations with the presence and progression of subclinical atherosclerosis. Arthritis Rheumatol. 67, 626–636 (2015).
Sanchez-Perez, H. et al. Insulin resistance in systemic lupus erythematosus patients: contributing factors and relationship with subclinical atherosclerosis. Clin. Exp. Rheumatol. 35, 885–892 (2017).
Chen, H. H. et al. Ankylosing spondylitis and other inflammatory spondyloarthritis increase the risk of developing type 2 diabetes in an Asian population. Rheumatol. Int. 34, 265–270 (2014).
Ribeiro, M. et al. Diabetes-accelerated experimental osteoarthritis is prevented by autophagy activation. Osteoarthritis Cartilage 24, 2116–2125 (2016).
Richter, F. C., Obba, S. & Simon, A. K. Local exchange of metabolites shapes immunity. Immunology 155, 309–319 (2018).
Davan-Wetton, C. S. A., Pessolano, E., Perretti, M. & Montero-Melendez, T. Senescence under appraisal: hopes and challenges revisited. Cell. Mol. Life Sci. 78, 3333–3354 (2021).
Pawlik, A. et al. The expansion of CD4+CD28− T cells in patients with rheumatoid arthritis. Arthritis Res. Ther. 5, R210–R213 (2003).
Petersen, L. E. et al. Premature immunosenescence is associated with memory dysfunction in rheumatoid arthritis. Neuroimmunomodulation 22, 130–137 (2015).
Zabinska, M., Krajewska, M., Koscielska-Kasprzak, K. & Klinger, M. CD3+CD8+CD28− T lymphocytes in patients with lupus nephritis. J. Immunol. Res. 2016, 1058165 (2016).
Schirmer, M. et al. Circulating cytotoxic CD8+ CD28− T cells in ankylosing spondylitis. Arthritis Res. 4, 71–76 (2002).
Fessler, J. et al. Senescent T-cells promote bone loss in rheumatoid arthritis. Front. Immunol. 9, 95 (2018).
Sawai, H. et al. T cell costimulation by fractalkine-expressing synoviocytes in rheumatoid arthritis. Arthritis Rheum. 52, 1392–1401 (2005).
Fessler, J. et al. Novel senescent regulatory T-cell subset with impaired suppressive function in rheumatoid arthritis. Front. Immunol. 8, 300 (2017).
Stranks, A. J. et al. Autophagy controls acquisition of aging features in macrophages. J. Innate Immun. 7, 375–391 (2015).
Ong, S. M. et al. The pro-inflammatory phenotype of the human non-classical monocyte subset is attributed to senescence. Cell Death Dis. 9, 266 (2018).
Pai, S. & Thomas, R. Immune deficiency or hyperactivity-Nf-κb illuminates autoimmunity. J. Autoimmun. 31, 245–251 (2008).
Xu, M. et al. Transplanted senescent cells induce an osteoarthritis-like condition in mice. J. Gerontol. A Biol. Sci. Med. Sci. 72, 780–785 (2017).
Jeon, O. H. et al. Local clearance of senescent cells attenuates the development of post-traumatic osteoarthritis and creates a pro-regenerative environment. Nat. Med. 23, 775–781 (2017).
Del Rey, M. J. et al. Senescent synovial fibroblasts accumulate prematurely in rheumatoid arthritis tissues and display an enhanced inflammatory phenotype. Immun. Ageing 16, 29 (2019).
Taniguchi, K. et al. Induction of the p16INK4a senescence gene as a new therapeutic strategy for the treatment of rheumatoid arthritis. Nat. Med. 5, 760–767 (1999).
Nasu, K. et al. Adenoviral transfer of cyclin-dependent kinase inhibitor genes suppresses collagen-induced arthritis in mice. J. Immunol. 165, 7246–7252 (2000).
Murakami, Y., Mizoguchi, F., Saito, T., Miyasaka, N. & Kohsaka, H. p16INK4a exerts an anti-inflammatory effect through accelerated IRAK1 degradation in macrophages. J. Immunol. 189, 5066–5072 (2012).
Gu, Z. et al. Upregulation of p16INK4A promotes cellular senescence of bone marrow-derived mesenchymal stem cells from systemic lupus erythematosus patients. Cell. Signal. 24, 2307–2314 (2012).
Valencia, X., Yarboro, C., Illei, G. & Lipsky, P. E. Deficient CD4+CD25high T regulatory cell function in patients with active systemic lupus erythematosus. J. Immunol. 178, 2579–2588 (2007).
Camernik, K. et al. Increased exhaustion of the subchondral bone-derived mesenchymal stem/stromal cells in primary versus dysplastic osteoarthritis. Stem Cell Rev. Rep. 16, 742–754 (2020).
Murphy, J. M. et al. Reduced chondrogenic and adipogenic activity of mesenchymal stem cells from patients with advanced osteoarthritis. Arthritis Rheum. 46, 704–713 (2002).
Chia, S. L. et al. Fibroblast growth factor 2 is an intrinsic chondroprotective agent that suppresses ADAMTS-5 and delays cartilage degradation in murine osteoarthritis. Arthritis Rheum. 60, 2019–2027 (2009).
Lee, H. J. et al. Chronic inflammation-induced senescence impairs immunomodulatory properties of synovial fluid mesenchymal stem cells in rheumatoid arthritis. Stem Cell Res. Ther. 12, 502 (2021).
Cheng, R. J. et al. Mesenchymal stem cells: allogeneic MSC may be immunosuppressive but autologous MSC are dysfunctional in lupus patients. Front. Cell Dev. Biol. 7, 285 (2019).
Pang, W. W. et al. Human bone marrow hematopoietic stem cells are increased in frequency and myeloid-biased with age. Proc. Natl Acad. Sci. USA 108, 20012–20017 (2011).
Caiado, F., Pietras, E. M. & Manz, M. G. Inflammation as a regulator of hematopoietic stem cell function in disease, aging, and clonal selection. J. Exp. Med. 218, e20201541 (2021).
Challen, G. A. et al. Dnmt3a is essential for hematopoietic stem cell differentiation. Nat. Genet. 44, 23–31 (2011).
Solimando, A. G., Melaccio, A. & Ria, R. The bone marrow niche landscape: a journey through aging, extrinsic and intrinsic stressors in the haemopoietic milieu. J. Cancer Metastasis Treat. 8, 9 (2022).
Sikora, K. A., Wells, K., Bolek, E. C., Jones, A. I. & Grayson, P. C. Somatic mutations in rheumatologic diseases: VEXAS syndrome and beyond. Rheumatology 61, 3149–3160 (2022).
Abplanalp, W. T. et al. Clonal hematopoiesis-driver DNMT3A mutations alter immune cells in heart failure. Circ. Res. 128, 216–228 (2021).
Fuster, J. J. et al. Clonal hematopoiesis associated with TET2 deficiency accelerates atherosclerosis development in mice. Science 355, 842–847 (2017).
Savola, P. et al. Author correction: clonal hematopoiesis in patients with rheumatoid arthritis. Blood Cancer J. 11, 36 (2021).
Papadaki, H. A. et al. Bone marrow progenitor cell reserve and function and stromal cell function are defective in rheumatoid arthritis: evidence for a tumor necrosis factor alpha-mediated effect. Blood 99, 1610–1619 (2002).
Colmegna, I. et al. Defective proliferative capacity and accelerated telomeric loss of hematopoietic progenitor cells in rheumatoid arthritis. Arthritis Rheum. 58, 990–1000 (2008).
Colmegna, I., Pryshchep, S., Oishi, H., Goronzy, J. J. & Weyand, C. M. Dampened ERK signaling in hematopoietic progenitor cells in rheumatoid arthritis. Clin. Immunol. 143, 73–82 (2012).
Alvarado-de la Barrera, C., Alcocer-Varela, J., Richaud-Patin, Y., Alarcon-Segovia, D. & Llorente, L. Differential oncogene and TNF-α mRNA expression in bone marrow cells from systemic lupus erythematosus patients. Scand. J. Immunol. 48, 551–556 (1998).
Tiefenthaler, M. et al. Apoptosis of CD34 cells after incubation with sera of leukopenic patients with systemic lupus erythematosus. Lupus 12, 471–478 (2003).
Hernandez, G. et al. Pro-inflammatory cytokine blockade attenuates myeloid expansion in a murine model of rheumatoid arthritis. Haematologica 105, 585–597 (2020).
Papadaki, H. A., Kritikos, H. D., Valatas, V., Boumpas, D. T. & Eliopoulos, G. D. Anemia of chronic disease in rheumatoid arthritis is associated with increased apoptosis of bone marrow erythroid cells: improvement following anti-tumor necrosis factor-alpha antibody therapy. Blood 100, 474–482 (2002).
Corrado, A., Di Bello, V., d’Onofrio, F., Maruotti, N. & Cantatore, F. P. Anti-TNF-α effects on anemia in rheumatoid and psoriatic arthritis. Int. J. Immunopathol. Pharmacol. 30, 302–307 (2017).
Chen, Y. et al. Serum levels of hepcidin in rheumatoid arthritis and its correlation with disease activity and anemia: a meta-analysis. Immunol. Invest. 50, 243–258 (2021).
Beck, D. B. et al. Somatic mutations in UBA1 and severe adult-onset autoinflammatory disease. N. Engl. J. Med. 383, 2628–2638 (2020).
Acosta, J. C. et al. A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nat. Cell Biol. 15, 978–990 (2013).
Munoz-Espin, D. & Serrano, M. Cellular senescence: from physiology to pathology. Nat. Rev. Mol. Cell Biol. 15, 482–496 (2014).
Philipot, D. et al. p16INK4a and its regulator miR-24 link senescence and chondrocyte terminal differentiation-associated matrix remodeling in osteoarthritis. Arthritis Res. Ther. 16, R58 (2014).
Gu, Z. et al. Rapamycin reverses the senescent phenotype and improves immunoregulation of mesenchymal stem cells from MRL/lpr mice and systemic lupus erythematosus patients through inhibition of the mTOR signaling pathway. Aging 8, 1102–1114 (2016).
Yoshida, M. et al. Extracellular vesicle-contained eNAMPT delays aging and extends lifespan in mice. Cell Metab. 30, 329–342 e325 (2019).
Nederveen, J. P., Warnier, G., Di Carlo, A., Nilsson, M. I. & Tarnopolsky, M. A. Extracellular vesicles and exosomes: insights from exercise science. Front. Physiol. 11, 604274 (2020).
Basisty, N. et al. A proteomic atlas of senescence-associated secretomes for aging biomarker development. PLoS Biol. 18, e3000599 (2020).
Borghesan, M. et al. Small extracellular vesicles are key regulators of non-cell autonomous intercellular communication in senescence via the interferon protein IFITM3. Cell Rep. 27, 3956–3971 e3956 (2019).
Mensa, E. et al. Small extracellular vesicles deliver miR-21 and miR-217 as pro-senescence effectors to endothelial cells. J. Extracell. Vesicles 9, 1725285 (2020).
Khayrullin, A. et al. Very long-chain C24:1 ceramide is increased in serum extracellular vesicles with aging and can induce senescence in bone-derived mesenchymal stem cells. Cells 8, 37 (2019).
Ahmadi, M. & Rezaie, J. Ageing and mesenchymal stem cells derived exosomes: molecular insight and challenges. Cell Biochem. Funct. 39, 60–66 (2021).
Dong, C. et al. Circulating exosomes derived-miR-146a from systemic lupus erythematosus patients regulates senescence of mesenchymal stem cells. Biomed. Res. Int. 2019, 6071308 (2019).
Kolhe, R. et al. Gender-specific differential expression of exosomal miRNA in synovial fluid of patients with osteoarthritis. Sci. Rep. 7, 2029 (2017).
Kato, T. et al. Exosomes from IL-1β stimulated synovial fibroblasts induce osteoarthritic changes in articular chondrocytes. Arthritis Res. Ther. 16, R163 (2014).
Skriner, K., Adolph, K., Jungblut, P. R. & Burmester, G. R. Association of citrullinated proteins with synovial exosomes. Arthritis Rheum. 54, 3809–3814 (2006).
Cloutier, N. et al. The exposure of autoantigens by microparticles underlies the formation of potent inflammatory components: the microparticle-associated immune complexes. EMBO Mol. Med. 5, 235–249 (2013).
Sokolove, J., Zhao, X., Chandra, P. E. & Robinson, W. H. Immune complexes containing citrullinated fibrinogen costimulate macrophages via Toll-like receptor 4 and Fcγ receptor. Arthritis Rheum. 63, 53–62 (2011).
Zhang, H. G. et al. A membrane form of TNF-α presented by exosomes delays T cell activation-induced cell death. J. Immunol. 176, 7385–7393 (2006).
Cosenza, S., Ruiz, M., Toupet, K., Jorgensen, C. & Noel, D. Mesenchymal stem cells derived exosomes and microparticles protect cartilage and bone from degradation in osteoarthritis. Sci. Rep. 7, 16214 (2017).
Wang, Y. et al. Exosomes from embryonic mesenchymal stem cells alleviate osteoarthritis through balancing synthesis and degradation of cartilage extracellular matrix. Stem Cell Res. Ther. 8, 189 (2017).
You, D. G. et al. Metabolically engineered stem cell-derived exosomes to regulate macrophage heterogeneity in rheumatoid arthritis. Sci. Adv. 7, eabe0083 (2021).
Jin, J. et al. BMSC-derived extracellular vesicles intervened the pathogenic changes of scleroderma in mice through miRNAs. Stem Cell Res. Ther. 12, 327 (2021).
Clark, R. I. et al. Distinct shifts in microbiota composition during drosophila aging impair intestinal function and drive mortality. Cell Rep. 12, 1656–1667 (2015).
Barcena, C. et al. Healthspan and lifespan extension by fecal microbiota transplantation into progeroid mice. Nat. Med. 25, 1234–1242 (2019).
Parker, A. et al. Fecal microbiota transfer between young and aged mice reverses hallmarks of the aging gut, eye, and brain. Microbiome 10, 68 (2022).
Chan, M. M. et al. The microbial metabolite trimethylamine N-oxide links vascular dysfunctions and the autoimmune disease rheumatoid arthritis. Nutrients 11, 1821 (2019).
Boini, K. M., Hussain, T., Li, P. L. & Koka, S. Trimethylamine-N-oxide instigates NLRP3 inflammasome activation and endothelial dysfunction. Cell. Physiol. Biochem. 44, 152–162 (2017).
Seldin, M. M. et al. Trimethylamine N-oxide promotes vascular inflammation through signaling of mitogen-activated protein kinase and nuclear factor-κB. J. Am. Heart Assoc. 5, e002767 (2016).
Ctoi, A. F. et al. Gut microbiota and aging-a focus on centenarians. Biochim. Biophys. Acta Mol. Basis Dis. 1866, 165765 (2020).
DeJong, E. N., Surette, M. G. & Bowdish, D. M. E. The gut microbiota and unhealthy aging: disentangling cause from consequence. Cell Host Microbe 28, 180–189 (2020).
Evers, A. W., Zautra, A. & Thieme, K. Stress and resilience in rheumatic diseases: a review and glimpse into the future. Nat. Rev. Rheumatol. 7, 409–415 (2011).
Clegg, A., Young, J., Iliffe, S., Rikkert, M. O. & Rockwood, K. Frailty in elderly people. Lancet 381, 752–762 (2013).
Fried, L. P. et al. Frailty in older adults: evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 56, M146–M156 (2001).
Fried, L. P., Ferrucci, L., Darer, J., Williamson, J. D. & Anderson, G. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J. Gerontol. A Biol. Sci. Med. Sci. 59, 255–263 (2004).
Motta, F., Sica, A. & Selmi, C. Frailty in rheumatic diseases. Front. Immunol. 11, 576134 (2020).
Gijsen, R. et al. Causes and consequences of comorbidity: a review. J. Clin. Epidemiol. 54, 661–674 (2001).
Cacciatore, F. et al. Long-term mortality in frail elderly subjects with osteoarthritis. Rheumatology 53, 293–299 (2014).
Bergman, H. et al. Frailty: an emerging research and clinical paradigm–issues and controversies. J. Gerontol. A Biol. Sci. Med. Sci. 62, 731–737 (2007).
Hoogendijk, E. O. et al. Frailty: implications for clinical practice and public health. Lancet 394, 1365–1375 (2019).
Meessen, J. et al. Frailty in end-stage hip or knee osteoarthritis: validation of the Groningen Frailty Indicator (GFI) questionnaire. Rheumatol. Int. 38, 917–924 (2018).
Castell, M. V. et al. Osteoarthritis and frailty in elderly individuals across six European countries: results from the European Project on OSteoArthritis (EPOSA). BMC Musculoskelet. Disord. 16, 359 (2015).
Veronese, N. et al. Pain increases the risk of developing frailty in older adults with osteoarthritis. Pain. Med. 18, 414–427 (2017).
Salaffi, F., Di Carlo, M., Farah, S., Di Donato, E. & Carotti, M. Prevalence of frailty and its associated factors in patients with rheumatoid arthritis: a cross-sectional analysis. Clin. Rheumatol. 38, 1823–1830 (2019).
Katz, P. P. et al. Is frailty a relevant concept in SLE? Lupus Sci. Med. 4, e000186 (2017).
Haider, S. et al. Frailty in seropositive rheumatoid arthritis patients of working age: a cross-sectional study. Clin. Exp. Rheumatol. 37, 585–592 (2019).
Guler, S. A. et al. Severity and features of frailty in systemic sclerosis-associated interstitial lung disease. Respir. Med. 129, 1–7 (2017).
Nurmohamed, M. T., Heslinga, M. & Kitas, G. D. Cardiovascular comorbidity in rheumatic diseases. Nat. Rev. Rheumatol. 11, 693–704 (2015).
Radner, H., Yoshida, K., Smolen, J. S. & Solomon, D. H. Multimorbidity and rheumatic conditions-enhancing the concept of comorbidity. Nat. Rev. Rheumatol. 10, 252–256 (2014).
Conti, P., Gallenga, C. E., Caraffa, A., Ronconi, G. & Kritas, S. K. Impact of mast cells in fibromyalgia and low-grade chronic inflammation: can IL-37 play a role? Dermatol. Ther. 33, e13191 (2020).
Wang, W., Zhou, H. & Liu, L. Side effects of methotrexate therapy for rheumatoid arthritis: a systematic review. Eur. J. Med. Chem. 158, 502–516 (2018).
Silvagni, E. et al. One year in review 2020: novelties in the treatment of rheumatoid arthritis. Clin. Exp. Rheumatol. 38, 181–194 (2020).
Gomez-Garcia, L. et al. Reduced numbers of circulating CD28-negative CD4+ cells in patients with rheumatoid arthritis chronically treated with abatacept. Int. J. Rheum. Dis. 16, 469–471 (2013).
Scarsi, M., Ziglioli, T. & Airo, P. Decreased circulating CD28-negative T cells in patients with rheumatoid arthritis treated with abatacept are correlated with clinical response. J. Rheumatol. 37, 911–916 (2010).
Gerli, R. et al. CD4+CD28− T lymphocytes contribute to early atherosclerotic damage in rheumatoid arthritis patients. Circulation 109, 2744–2748 (2004).
Kageyama, Y., Takahashi, M., Ichikawa, T., Torikai, E. & Nagano, A. Reduction of oxidative stress marker levels by anti-TNF-α antibody, infliximab, in patients with rheumatoid arthritis. Clin. Exp. Rheumatol. 26, 73–80 (2008).
Kageyama, Y., Takahashi, M., Nagafusa, T., Torikai, E. & Nagano, A. Etanercept reduces the oxidative stress marker levels in patients with rheumatoid arthritis. Rheumatol. Int. 28, 245–251 (2008).
Hirao, M. et al. Serum level of oxidative stress marker is dramatically low in patients with rheumatoid arthritis treated with tocilizumab. Rheumatol. Int. 32, 4041–4045 (2012).
Harty, L. C. et al. Mitochondrial mutagenesis correlates with the local inflammatory environment in arthritis. Ann. Rheum. Dis. 71, 582–588 (2012).
Bruyn, G. A. et al. Everolimus in patients with rheumatoid arthritis receiving concomitant methotrexate: a 3-month, double-blind, randomised, placebo-controlled, parallel-group, proof-of-concept study. Ann. Rheum. Dis. 67, 1090–1095 (2008).
Lai, Z. W. et al. Sirolimus in patients with clinically active systemic lupus erythematosus resistant to, or intolerant of, conventional medications: a single-arm, open-label, phase 1/2 trial. Lancet 391, 1186–1196 (2018).
Kopf, H., de la Rosa, G. M., Howard, O. M. & Chen, X. Rapamycin inhibits differentiation of Th17 cells and promotes generation of FoxP3+ T regulatory cells. Int. Immunopharmacol. 7, 1819–1824 (2007).
Mo, C., Zeng, Z., Deng, Q., Ding, Y. & Xiao, R. Imbalance between T helper 17 and regulatory T cell subsets plays a significant role in the pathogenesis of systemic sclerosis. Biomed. Pharmacother. 108, 177–183 (2018).
Papotto, P. H., Reinhardt, A., Prinz, I. & Silva-Santos, B. Innately versatile: γδ17 T cells in inflammatory and autoimmune diseases. J. Autoimmun. 87, 26–37 (2018).
Radstake, T. R. et al. The pronounced Th17 profile in systemic sclerosis (SSc) together with intracellular expression of TGFβ and IFNγ distinguishes SSc phenotypes. PLoS One 4, e5903 (2009).
Moon, J. et al. Metformin ameliorates scleroderma via inhibiting Th17 cells and reducing mTOR-STAT3 signaling in skin fibroblasts. J. Transl. Med. 19, 192 (2021).
Kulkarni, A. S., Gubbi, S. & Barzilai, N. Benefits of metformin in attenuating the hallmarks of aging. Cell Metab. 32, 15–30 (2020).
American Federation for Aging Research. The TAME trial; targeting the biology of aging. Ushering a new era of interventions [online], https://www.afar.org/tame-trial (2022).
Wang, Y. et al. Association between metformin use and disease progression in obese people with knee osteoarthritis: data from the Osteoarthritis Initiative — a prospective cohort study. Arthritis Res. Ther. 21, 127 (2019).
Gharib, M., Elbaz, W., Darweesh, E., Sabri, N. A. & Shawki, M. A. Efficacy and safety of metformin use in rheumatoid arthritis: a randomized controlled study. Front. Pharmacol. 12, 726490 (2021).
Zhang, L. X. et al. Resveratrol (RV): a pharmacological review and call for further research. Biomed. Pharmacother. 143, 112164 (2021).
Rubinsztein, D. C., Marino, G. & Kroemer, G. Autophagy and aging. Cell 146, 682–695 (2011).
Carmona-Gutierrez, D., Hughes, A. L., Madeo, F. & Ruckenstuhl, C. The crucial impact of lysosomes in aging and longevity. Ageing Res. Rev. 32, 2–12 (2016).
Eisenberg, T. et al. Induction of autophagy by spermidine promotes longevity. Nat. Cell Biol. 11, 1305–1314 (2009).
Zhang, H. et al. Polyamines control eIF5A hypusination, TFEB translation, and autophagy to reverse B cell senescence. Mol. Cell 76, 110–125 e119 (2019).
Puleston, D. J. et al. Autophagy is a critical regulator of memory CD8+ T cell formation. Elife 3, e03706 (2014).
Sacitharan, P. K., Lwin, S., Gharios, G. B. & Edwards, J. R. Spermidine restores dysregulated autophagy and polyamine synthesis in aged and osteoarthritic chondrocytes via EP300. Exp. Mol. Med. 50, 123 (2018).
Zheng, W. et al. Fisetin inhibits IL-1β-induced inflammatory response in human osteoarthritis chondrocytes through activating SIRT1 and attenuates the progression of osteoarthritis in mice. Int. Immunopharmacol. 45, 135–147 (2017).
Lee, J. D. et al. Flavonol-rich RVHxR from Rhus verniciflua Stokes and its major compound fisetin inhibits inflammation-related cytokines and angiogenic factor in rheumatoid arthritic fibroblast-like synovial cells and in vivo models. Int. Immunopharmacol. 9, 268–276 (2009).
Xu, S. P. & Li, Y. S. Fisetin inhibits pristine-induced systemic lupus erythematosus in a murine model through CXCLs regulation. Int. J. Mol. Med. 42, 3220–3230 (2018).
Cribbs, A. P. et al. Methotrexate restores regulatory T cell function through demethylation of the FoxP3 upstream enhancer in patients with rheumatoid arthritis. Arthritis Rheumatol. 67, 1182–1192 (2015).
de Andres, M. C. et al. Assessment of global DNA methylation in peripheral blood cell subpopulations of early rheumatoid arthritis before and after methotrexate. Arthritis Res. Ther. 17, 233 (2015).
Garaud, S. et al. IL-6 modulates CD5 expression in B cells from patients with lupus by regulating DNA methylation. J. Immunol. 182, 5623–5632 (2009).
Chen, Y. M. et al. Association between autophagy and inflammation in patients with rheumatoid arthritis receiving biologic therapy. Arthritis Res. Ther. 20, 268 (2018).
Krasselt, M., Baerwald, C., Wagner, U. & Rossol, M. CD56+ monocytes have a dysregulated cytokine response to lipopolysaccharide and accumulate in rheumatoid arthritis and immunosenescence. Arthritis Res. Ther. 15, R139 (2013).
Smolen, J. S. et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann. Rheum. Dis. 76, 960–977 (2017).
Aletaha, D. & Smolen, J. S. Diagnosis and management of rheumatoid arthritis: a review. JAMA 320, 1360–1372 (2018).
Fleischmann, R. et al. Placebo-controlled trial of tofacitinib monotherapy in rheumatoid arthritis. N. Engl. J. Med. 367, 495–507 (2012).
Fleischmann, R. et al. Baricitinib, methotrexate, or combination in patients with rheumatoid arthritis and no or limited prior disease-modifying antirheumatic drug treatment. Arthritis Rheumatol. 69, 506–517 (2017).
Illei, G. G. et al. Tocilizumab in systemic lupus erythematosus: data on safety, preliminary efficacy, and impact on circulating plasma cells from an open-label phase I dosage-escalation study. Arthritis Rheum. 62, 542–552 (2010).
Shirota, Y. et al. Impact of anti-interleukin-6 receptor blockade on circulating T and B cell subsets in patients with systemic lupus erythematosus. Ann. Rheum. Dis. 72, 118–128 (2013).
Shima, Y. et al. The skin of patients with systemic sclerosis softened during the treatment with anti-IL-6 receptor antibody tocilizumab. Rheumatology 49, 2408–2412 (2010).
Su, T. I. et al. Rapamycin versus methotrexate in early diffuse systemic sclerosis: results from a randomized, single-blind pilot study. Arthritis Rheum. 60, 3821–3830 (2009).
Reitamo, S. et al. Efficacy of sirolimus (rapamycin) administered concomitantly with a subtherapeutic dose of cyclosporin in the treatment of severe psoriasis: a randomized controlled trial. Br. J. Dermatol. 145, 438–445 (2001).
Sun, F. et al. Effects of metformin on disease flares in patients with systemic lupus erythematosus: post hoc analyses from two randomised trials. Lupus Sci. Med. 7, e000429 (2020).
Wang, H., Li, T., Chen, S., Gu, Y. & Ye, S. Neutrophil extracellular trap mitochondrial DNA and its autoantibody in systemic lupus erythematosus and a proof-of-concept trial of metformin. Arthritis Rheumatol. 67, 3190–3200 (2015).
Khojah, H. M., Ahmed, S., Abdel-Rahman, M. S. & Elhakeim, E. H. Resveratrol as an effective adjuvant therapy in the management of rheumatoid arthritis: a clinical study. Clin. Rheumatol. 37, 2035–2042 (2018).
Marouf, B. H., Hussain, S. A., Ali, Z. S. & Ahmmad, R. S. Resveratrol supplementation reduces pain and inflammation in knee osteoarthritis patients treated with meloxicam: a randomized placebo-controlled study. J. Med. Food https://doi.org/10.1089/jmf.2017.4176 (2018).
Justice, J. N. et al. Senolytics in idiopathic pulmonary fibrosis: results from a first-in-human, open-label, pilot study. EBioMedicine 40, 554–563 (2019).
Hus, B. E. A. S. Tolerability, pharmacokinetics, and clinical outcomes following single-dose IA administration of UBX0101, a senolytic MDM2/p53 interaction inhibitor, in patients with knee OA [abstract]. Arthritis Rheumatol. https://doi.org/10.1002/art.41108 (2019).
Smolen, J. S. et al. Effect of interleukin-6 receptor inhibition with tocilizumab in patients with rheumatoid arthritis (OPTION study): a double-blind, placebo-controlled, randomised trial. Lancet 371, 987–997 (2008).
Richette, P. et al. Efficacy of tocilizumab in patients with hand osteoarthritis: double blind, randomised, placebo-controlled, multicentre trial. Ann. Rheum. Dis. 80, 349–355 (2020).
Khanna, D. et al. Safety and efficacy of subcutaneous tocilizumab in adults with systemic sclerosis (faSScinate): a phase 2, randomised, controlled trial. Lancet 387, 2630–2640 (2016).
Khanna, D. et al. Long-term safety and efficacy of tocilizumab in early systemic sclerosis-interstitial lung disease: open label extension of a phase 3 randomized controlled trial. Am. J. Respir. Crit. Care Med. 205, 674–684 (2021).
Opoka-Winiarska, V. et al. Long-term, interventional, open-label extension study evaluating the safety of tocilizumab treatment in patients with polyarticular-course juvenile idiopathic arthritis from Poland and Russia who completed the global, international CHERISH trial. Clin. Rheumatol. 37, 1807–1816 (2018).
Mease, P. J. et al. The efficacy and safety of clazakizumab, an anti-interleukin-6 monoclonal antibody, in a phase IIb study of adults with active psoriatic arthritis. Arthritis Rheumatol. 68, 2163–2173 (2016).
Wallace, D. J. et al. Efficacy and safety of an interleukin 6 monoclonal antibody for the treatment of systemic lupus erythematosus: a phase II dose-ranging randomised controlled trial. Ann. Rheum. Dis. 76, 534–542 (2017).
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Rheumatology thanks Kornelis van der Geest and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Alsaleh, G., Richter, F.C. & Simon, A.K. Age-related mechanisms in the context of rheumatic disease. Nat Rev Rheumatol 18, 694–710 (2022). https://doi.org/10.1038/s41584-022-00863-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41584-022-00863-8