Abstract
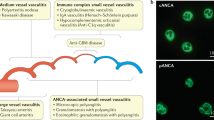
Anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) comprises granulomatosis with polyangiitis (GPA), microscopic polyangiitis (MPA) and eosinophilic granulomatosis with polyangiitis (EGPA), that share features of pauci-immune small-vessel vasculitis and the positivity of ANCA targeting proteinase-3 (PR3-ANCA) or myeloperoxidase (MPO-ANCA). AAV syndromes are rare, complex diseases and their aetio-pathogenesis is mainly driven by the interaction between environmental and genetic factors. In patients with GPA and MPA, the genetic associations are stronger with ANCA specificity (PR3- versus MPO-ANCA) than with the clinical diagnosis, which, in keeping with the known clinical and prognostic differences between PR3-ANCA-positive and MPO-ANCA-positive patients, supports an ANCA-based re-classification of these disorders. EGPA is also made up of genetically distinct subsets, which can be stratified on ANCA-status (MPO ANCA-positive versus ANCA-negative); these subsets differ in clinical phenotype and possibly in their response to treatment. Interestingly, MPO-ANCA-positive patients with either MPA or EGPA have overlapping genetic determinants, thus strengthening the concept that this EGPA subset is closely related to the other AAV syndromes. The genetics of AAV provides us with essential information to understand its varied phenotype. This Review discusses the main findings of genetic association studies in AAV, their pathogenic implications and their potential effect on classification, management and prognosis.
Key points
-
ANCA-associated vasculitides (AAV) are classified according to clinico-pathological features as granulomatosis with polyangiitis (GPA), microscopic polyangiitis (MPA) and eosinophilic granulomatosis with polyangiitis (EGPA), and have complex aetio-pathogenetic mechanisms.
-
In GPA and MPA, genetic associations are stronger with ANCA specificity (PR3- or MPO-ANCA) than with the clinical diagnosis.
-
Evidence of a distinct genetic background between PR3-ANCA-positive and MPO-ANCA-positive vasculitis is coherent with the demographic, clinical and prognostic data, and supports a re-classification of AAV according to ANCA specificity.
-
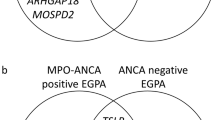
In EGPA, MPO-ANCA-positive and ANCA-negative subsets share associations with loci involved in eosinophilia and asthma, and differ for associations with HLA-DQ, IL-5 and GPA33.
-
The genetic differences between the two EGPA subsets support the ANCA-based dichotomy of this syndrome.
-
ANCA-positive disease, which often displays vasculitic features, is closely related to MPO-ANCA-positive vasculitis, whereas ANCA-negative disease, which shows a higher prevalence of eosinophilic manifestations, may be associated with mucosal barrier dysfunction.
-
Future studies focusing on genotype–phenotype, genotype–prognosis and pharmacogenomics are likely to improve our understanding of AAV and refine our approaches to patient management.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Jennette, J. C. et al. 2012 revised international Chapel Hill consensus conference nomenclature of Vasculitides. Arthritis Rheum. 65, 1–11 (2013).
Lanham, J. G., Elkon, K. B., Pusey, C. D. & Hughes, G. R. Systemic vasculitis with asthma and eosinophilia: a clinical approach to the Churg-Strauss syndrome. Medicine 63, 65–81 (1984).
Leavitt, R. Y. et al. The American College of Rheumatology 1990 criteria for the classification of Wegener’s granulomatosis. Arthritis Rheum. 33, 1101–1107 (1990).
Masi, A. T. et al. The American College of Rheumatology 1990 criteria for the classification of Churg-Strauss syndrome (allergic granulomatosis and angiitis). Arthritis Rheum. 33, 1094–1100 (1990).
Sorensen, S. F., Slot, O., Tvede, N. & Petersen, J. A prospective study of vasculitis patients collected in a five year period: evaluation of the Chapel Hill nomenclature. Ann. Rheum. Dis. 59, 478–482 (2000).
Watts, R. et al. Development and validation of a consensus methodology for the classification of the ANCA-associated vasculitides and polyarteritis nodosa for epidemiological studies. Ann. Rheum. Dis. 66, 222–227 (2007).
Robson, J. C. et al. 2022 American College of Rheumatology/European Alliance of Associations for rheumatology classification criteria for granulomatosis with polyangiitis. Arthritis Rheumatol. 74, 393–399 (2022).
Suppiah, R. et al. 2022 American College of Rheumatology/European Alliance of Associations for rheumatology classification criteria for microscopic polyangiitis. Arthritis Rheumatol. 74, 400–406 (2022).
Grayson, P. C. et al. 2022 American College of Rheumatology/European Alliance of Associations for rheumatology classification criteria for eosinophilic granulomatosis with polyangiitis. Arthritis Rheumatol. 74, 386–392 (2022).
Hagen, E. C. et al. Diagnostic value of standardized assays for anti-neutrophil cytoplasmic antibodies in idiopathic systemic vasculitis. EC/BCR Project for ANCA Assay Standardization. Kidney Int. 53, 743–753 (1998).
Falk, R. J. & Jennette, J. C. Anti-neutrophil cytoplasmic autoantibodies with specificity for myeloperoxidase in patients with systemic vasculitis and idiopathic necrotizing and crescentic glomerulonephritis. N. Engl. J. Med. 318, 1651–1657 (1988).
Goldschmeding, R. et al. Wegener’s granulomatosis autoantibodies identify a novel diisopropylfluorophosphate-binding protein in the lysosomes of normal human neutrophils. J. Clin. Invest. 84, 1577–1587 (1989).
Jennette, J. C., Hoidal, J. R. & Falk, R. J. Specificity of anti-neutrophil cytoplasmic autoantibodies for proteinase 3. Blood 75, 2263–2264 (1990).
Franssen, C., Gans, R., Kallenberg, C., Hageluken, C. & Hoorntje, S. Disease spectrum of patients with antineutrophil cytoplasmic autoantibodies of defined specificity: distinct differences between patients with anti-proteinase 3 and anti-myeloperoxidase autoantibodies. J. Intern. Med. 244, 209–216 (1998).
Lionaki, S. et al. Classification of antineutrophil cytoplasmic autoantibody vasculitides: the role of antineutrophil cytoplasmic autoantibody specificity for myeloperoxidase or proteinase 3 in disease recognition and prognosis. Arthritis Rheum. 64, 3452–3462 (2012).
Mahr, A. et al. Revisiting the classification of clinical phenotypes of anti-neutrophil cytoplasmic antibody-associated vasculitis: a cluster analysis. Ann. Rheum. Dis. 72, 1003–1010 (2013).
Miloslavsky, E. M. et al. Myeloperoxidase-antineutrophil cytoplasmic antibody (ANCA)-positive and ANCA-negative patients with granulomatosis with polyangiitis (Wegener’s): distinct patient subsets. Arthritis Rheumatol. 68, 2945–2952 (2016).
Schirmer, J. H. et al. Myeloperoxidase-antineutrophil cytoplasmic antibody (ANCA)-positive granulomatosis with polyangiitis (Wegener’s) is a clinically distinct subset of ANCA-associated vasculitis: a retrospective analysis of 315 patients from a German vasculitis referral center. Arthritis Rheumatol. 68, 2953–2963 (2016).
Guillevin, L. et al. Churg-Strauss syndrome. Clinical study and long-term follow-up of 96 patients. Medicine 78, 26–37 (1999).
Moosig, F. et al. A vasculitis centre based management strategy leads to improved outcome in eosinophilic granulomatosis and polyangiitis (Churg-Strauss, EGPA): monocentric experiences in 150 patients. Ann. Rheum. Dis. 72, 1011–1017 (2013).
Sable-Fourtassou, R. et al. Antineutrophil cytoplasmic antibodies and the Churg-Strauss syndrome. Ann. Intern. Med. 143, 632–638 (2005).
Sinico, R. A. et al. Prevalence and clinical significance of antineutrophil cytoplasmic antibodies in Churg-Strauss syndrome. Arthritis Rheum. 52, 2926–2935 (2005).
Comarmond, C. et al. Eosinophilic granulomatosis with polyangiitis (Churg-Strauss): clinical characteristics and long-term followup of the 383 patients enrolled in the French Vasculitis Study Group cohort. Arthritis Rheum. 65, 270–281 (2013).
Moiseev, S. et al. International consensus on ANCA testing in eosinophilic granulomatosis with polyangiitis. Am. J. Respir. Crit. Care Med. https://doi.org/10.1164/rccm.202005-1628SO (2020).
Jennette, J. C., Falk, R. J., Hu, P. & Xiao, H. Pathogenesis of antineutrophil cytoplasmic autoantibody-associated small-vessel vasculitis. Annu. Rev. Pathol. 8, 139–160 (2013).
Halbwachs-Mecarelli, L., Bessou, G., Lesavre, P., Lopez, S. & Witko-Sarsat, V. Bimodal distribution of proteinase 3 (PR3) surface expression reflects a constitutive heterogeneity in the polymorphonuclear neutrophil pool. FEBS Lett. 374, 29–33 (1995).
Falk, R. J., Terrell, R. S., Charles, L. A. & Jennette, J. C. Anti-neutrophil cytoplasmic autoantibodies induce neutrophils to degranulate and produce oxygen radicals in vitro. Proc. Natl Acad. Sci. USA. 87, 4115–4119 (1990).
Porges, A. J. et al. Anti-neutrophil cytoplasmic antibodies engage and activate human neutrophils via Fc gamma RIIa. J. Immunol. 153, 1271–1280 (1994).
Savage, C. O., Pottinger, B. E., Gaskin, G., Pusey, C. D. & Pearson, J. D. Autoantibodies developing to myeloperoxidase and proteinase 3 in systemic vasculitis stimulate neutrophil cytotoxicity toward cultured endothelial cells. Am. J. Pathol. 141, 335–342 (1992).
Xiao, H. et al. Antineutrophil cytoplasmic autoantibodies specific for myeloperoxidase cause glomerulonephritis and vasculitis in mice. J. Clin. Invest. 110, 955–963 (2002).
Xiao, H., Schreiber, A., Heeringa, P., Falk, R. J. & Jennette, J. C. Alternative complement pathway in the pathogenesis of disease mediated by anti-neutrophil cytoplasmic autoantibodies. Am. J. Pathol. 170, 52–64 (2007).
Schreiber, A. et al. C5a receptor mediates neutrophil activation and ANCA-induced glomerulonephritis. J. Am. Soc. Nephrol. 20, 289–298 (2009).
Jayne, D. R. W., Merkel, P. A., Schall, T. J., Bekker, P. & Group, A. S. Avacopan for the treatment of ANCA-associated vasculitis. N. Engl. J. Med. 384, 599–609 (2021).
Mellbye, O. J., Mollnes, T. E. & Steen, L. S. IgG subclass distribution and complement activation ability of autoantibodies to neutrophil cytoplasmic antigens (ANCA). Clin. Immunol. Immunopathol. 70, 32–39 (1994).
Csernok, E. et al. Cytokine profiles in Wegener’s granulomatosis: predominance of type 1 (Th1) in the granulomatous inflammation. Arthritis Rheum. 42, 742–750 (1999).
Schonermarck, U., Csernok, E., Trabandt, A., Hansen, H. & Gross, W. L. Circulating cytokines and soluble CD23, CD26 and CD30 in ANCA-associated vasculitides. Clin. Exp. Rheumatol. 18, 457–463 (2000).
Kiene, M. et al. Elevated interleukin-4 and interleukin-13 production by T cell lines from patients with Churg-Strauss syndrome. Arthritis Rheum. 44, 469–473 (2001).
Abdulahad, W. H., Stegeman, C. A., Limburg, P. C. & Kallenberg, C. G. Skewed distribution of Th17 lymphocytes in patients with Wegener’s granulomatosis in remission. Arthritis Rheum. 58, 2196–2205 (2008).
Nogueira, E. et al. Serum IL-17 and IL-23 levels and autoantigen-specific Th17 cells are elevated in patients with ANCA-associated vasculitis. Nephrol. Dial. Transplant. 25, 2209–2217 (2010).
Fagin, U. et al. Distinct proteinase 3-induced cytokine patterns in Wegener s granulomatosis, Churg-Strauss syndrome, and healthy controls. Clin. Exp. Rheumatol. 29 (1 Suppl 64), S57–S62 (2011).
Pinching, A. J. et al. Relapses in Wegener’s granulomatosis: the role of infection. Br. Med. J. 281, 836–838 (1980).
Raynauld, J. P., Bloch, D. A. & Fries, J. F. Seasonal variation in the onset of Wegener’s granulomatosis, polyarteritis nodosa and giant cell arteritis. J. Rheumatol. 20, 1524–1526 (1993).
Stegeman, C. A. et al. Association of chronic nasal carriage of Staphylococcus aureus and higher relapse rates in Wegener granulomatosis. Ann. Intern. Med. 120, 12–17 (1994).
Grau, R. G. Drug-induced vasculitis: new insights and a changing lineup of suspects. Curr. Rheumatol. Rep. 17, 71 (2015).
Watanabe, T. Vasculitis following influenza vaccination: a review of the literature. Curr. Rheumatol. Rev. 13, 188–196 (2017).
Prabhahar, A. et al. ANCA-associated vasculitis following ChAdOx1 nCoV19 vaccination: case-based review. Rheumatol. Int. 42, 749–758 (2022).
Yashiro, M. et al. Significantly high regional morbidity of MPO-ANCA-related angitis and/or nephritis with respiratory tract involvement after the 1995 great earthquake in Kobe (Japan). Am. J. Kidney Dis. 35, 889–895 (2000).
Hogan, S. L. et al. Association of silica exposure with anti-neutrophil cytoplasmic autoantibody small-vessel vasculitis: a population-based, case-control study. Clin. J. Am. Soc. Nephrol. 2, 290–299 (2007).
Lane, S. E., Watts, R. A., Bentham, G., Innes, N. J. & Scott, D. G. Are environmental factors important in primary systemic vasculitis? A case-control study. Arthritis Rheum. 48, 814–823 (2003).
Maritati, F. et al. Occupational exposures and smoking in eosinophilic granulomatosis with polyangiitis: a case-control study. Arthritis Rheumatol. 73, 1694–1702 (2021).
Fujimoto, S. et al. Comparison of the epidemiology of anti-neutrophil cytoplasmic antibody-associated vasculitis between Japan and the UK. Rheumatology 50, 1916–1920 (2011).
Watts, R. A. et al. Renal vasculitis in Japan and the UK-are there differences in epidemiology and clinical phenotype? Nephrol. Dial. Transplant. 23, 3928–3931 (2008).
Watts, R. A., Hatemi, G., Burns, J. C. & Mohammad, A. J. Global epidemiology of vasculitis. Nat. Rev. Rheumatol. 18, 22–34 (2022).
Weiner, M. et al. Proteinase-3 and myeloperoxidase serotype in relation to demographic factors and geographic distribution in anti-neutrophil cytoplasmic antibody-associated glomerulonephritis. Nephrol. Dial. Transplant. 34, 301–308 (2019).
Watts, R. A. et al. Epidemiology of vasculitis in Europe. Ann. Rheum. Dis. 60, 1156–1157 (2001).
Mohammad, A. J., Jacobsson, L. T., Mahr, A. D., Sturfelt, G. & Segelmark, M. Prevalence of Wegener’s granulomatosis, microscopic polyangiitis, polyarteritis nodosa and Churg-Strauss syndrome within a defined population in southern Sweden. Rheumatology 46, 1329–1337 (2007).
Watts, R. A. et al. Classification, epidemiology and clinical subgrouping of antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis. Nephrol. Dial. Transplant. 30, i14–i22 (2015).
Knight, A., Sandin, S. & Askling, J. Risks and relative risks of Wegener’s granulomatosis among close relatives of patients with the disease. Arthritis Rheum. 58, 302–307 (2008).
Manganelli, P., Giacosa, R., Fietta, P., Zanetti, A. & Neri, T. M. Familial vasculitides: Churg-Strauss syndrome and Wegener’s granulomatosis in 2 first-degree relatives. J. Rheumatol. 30, 618–621 (2003).
Staels, F. et al. Adult-onset ANCA-associated vasculitis in SAVI: extension of the phenotypic spectrum, case report and review of the literature. Front. Immunol. 11, 575219 (2020).
Lodi LM, M. V. et al. Type I interferon-related kidney disorders. Kidney Int. 101, 1142–1159 (2022).
Jagiello, P. et al. New genomic region for Wegener’s granulomatosis as revealed by an extended association screen with 202 apoptosis-related genes. Hum. Genet. 114, 468–477 (2004).
Vaglio, A. et al. HLA-DRB4 as a genetic risk factor for Churg-Strauss syndrome. Arthritis Rheum. 56, 3159–3166 (2007).
Mahr, A. D. et al. Alpha1-antitrypsin deficiency-related alleles Z and S and the risk of Wegener’s granulomatosis. Arthritis Rheum. 62, 3760–3767 (2010).
Gencik, M., Meller, S., Borgmann, S. & Fricke, H. Proteinase 3 gene polymorphisms and Wegener’s granulomatosis. Kidney Int. 58, 2473–2477 (2000).
Jagiello, P. et al. The PTPN22 620W allele is a risk factor for Wegener’s granulomatosis. Arthritis Rheum. 52, 4039–4043 (2005).
Willcocks, L. C. et al. Copy number of FCGR3B, which is associated with systemic lupus erythematosus, correlates with protein expression and immune complex uptake. J. Exp. Med. 205, 1573–1582 (2008).
Martorana, D. et al. Fcγ-receptor 3B (FCGR3B) copy number variations in patients with eosinophilic granulomatosis with polyangiitis. J. Allergy Clin. Immunol. 137, 1597–1599.e8 (2016).
Husmann, C. A. et al. Genetics of toll like receptor 9 in ANCA associated vasculitides. Ann. Rheum. Dis. 73, 890–896 (2014).
Lyons, P. A. et al. Genetically distinct subsets within ANCA-associated vasculitis. N. Engl. J. Med. 367, 214–223 (2012).
Xie, G. et al. Association of granulomatosis with polyangiitis (Wegener’s) with HLA-DPB1*04 and SEMA6A gene variants: evidence from genome-wide analysis. Arthritis Rheum. 65, 2457–2468 (2013).
Merkel, P. A. et al. Identification of functional and expression polymorphisms associated with risk for antineutrophil cytoplasmic autoantibody-associated vasculitis. Arthritis Rheumatol. 69, 1054–1066 (2017).
Lyons, P. A. et al. Genome-wide association study of eosinophilic granulomatosis with polyangiitis reveals genomic loci stratified by ANCA status. Nat. Commun. 10, 5120 (2019).
Ciavatta, D. J. et al. Epigenetic basis for aberrant upregulation of autoantigen genes in humans with ANCA vasculitis. J. Clin. Invest. 120, 3209–3219 (2010).
Yang, J. et al. Histone modification signature at myeloperoxidase and proteinase 3 in patients with anti-neutrophil cytoplasmic autoantibody-associated vasculitis. Clin. Epigenetics 8, 85 (2016).
Jones, B. E. et al. Gene-specific DNA methylation changes predict remission in patients with ANCA-associated vasculitis. J. Am. Soc. Nephrol. 28, 1175–1187 (2017).
Martorana, D. et al. PTPN22 R620W polymorphism in the ANCA-associated vasculitides. Rheumatology 51, 805–812 (2012).
Wieczorek, S. et al. Contrasting association of a non-synonymous leptin receptor gene polymorphism with Wegener’s granulomatosis and Churg-Strauss syndrome. Rheumatology 49, 907–914 (2010).
Fanciulli, M. et al. FCGR3B copy number variation is associated with susceptibility to systemic, but not organ-specific, autoimmunity. Nat. Genet. 39, 721–723 (2007).
Wieczorek, S. et al. Functionally relevant variations of the interleukin-10 gene associated with antineutrophil cytoplasmic antibody-negative Churg-Strauss syndrome, but not with Wegener’s granulomatosis. Arthritis Rheum. 58, 1839–1848 (2008).
Kamesh, L. et al. CT60 and +49 polymorphisms of CTLA 4 are associated with ANCA-positive small vessel vasculitis. Rheumatology 48, 1502–1505 (2009).
Dahlqvist, J. et al. Identification and functional characterization of a novel susceptibility locus for small vessel vasculitis with MPO-ANCA. Rheumatology https://doi.org/10.1093/rheumatology/keab912 (2021).
Heckmann, M. et al. The Wegener’s granulomatosis quantitative trait locus on chromosome 6p21.3 as characterised by tagSNP genotyping. Ann. Rheum. Dis. 67, 972–979 (2008).
Tsuchiya, N., Kobayashi, S., Hashimoto, H., Ozaki, S. & Tokunaga, K. Association of HLA-DRB1*0901-DQB1*0303 haplotype with microscopic polyangiitis in Japanese. Genes Immun. 7, 81–84 (2006).
Wieczorek, S., Hellmich, B., Gross, W. L. & Epplen, J. T. Associations of Churg-Strauss syndrome with the HLA-DRB1 locus, and relationship to the genetics of antineutrophil cytoplasmic antibody-associated vasculitides: comment on the article by Vaglio et al. Arthritis Rheum. 58, 329–330 (2008).
Kawasaki, A. et al. Protective role of HLA-DRB1*13:02 against microscopic polyangiitis and MPO-ANCA-positive vasculitides in a Japanese population: a case-control study. PLoS ONE 11, e0154393 (2016).
Tsuchiya, N. et al. Genetic background of Japanese patients with antineutrophil cytoplasmic antibody-associated vasculitis: association of HLA-DRB1*0901 with microscopic polyangiitis. J. Rheumatol. 30, 1534–1540 (2003).
Wieczorek, S. et al. A functionally relevant IRF5 haplotype is associated with reduced risk to Wegener’s granulomatosis. J. Mol. Med. 88, 413–421 (2010).
Kawasaki, A. et al. Association of ETS1 polymorphism with granulomatosis with polyangiitis and proteinase 3-anti-neutrophil cytoplasmic antibody positive vasculitis in a Japanese population. J. Hum. Genet. 63, 55–62 (2018).
Miyashita, R. et al. Association of killer cell immunoglobulin-like receptor genotypes with microscopic polyangiitis. Arthritis Rheum. 54, 992–997 (2006).
Wieczorek, S. et al. Novel association of the CD226 (DNAM-1) Gly307Ser polymorphism in Wegener’s granulomatosis and confirmation for multiple sclerosis in German patients. Genes Immun. 10, 591–595 (2009).
Mamegano, K. et al. Association of LILRA2 (ILT1, LIR7) splice site polymorphism with systemic lupus erythematosus and microscopic polyangiitis. Genes Immun. 9, 214–223 (2008).
Wang, H. Y. et al. Risk HLA class II alleles and amino acid residues in myeloperoxidase-ANCA-associated vasculitis. Kidney Int. 96, 1010–1019 (2019).
Elzouki, A. N., Segelmark, M., Wieslander, J. & Eriksson, S. Strong link between the alpha 1-antitrypsin PiZ allele and Wegener’s granulomatosis. J. Intern. Med. 236, 543–548 (1994).
Stanford, S. M. & Bottini, N. PTPN22: the archetypal non-HLA autoimmunity gene. Nat. Rev. Rheumatol. 10, 602–611 (2014).
Bottini, N. et al. A functional variant of lymphoid tyrosine phosphatase is associated with type I diabetes. Nat. Genet. 36, 337–338 (2004).
Begovich, A. B. et al. A missense single-nucleotide polymorphism in a gene encoding a protein tyrosine phosphatase (PTPN22) is associated with rheumatoid arthritis. Am. J. Hum. Genet. 75, 330–337 (2004).
Kyogoku, C. et al. Genetic association of the R620W polymorphism of protein tyrosine phosphatase PTPN22 with human SLE. Am. J. Hum. Genet. 75, 504–507 (2004).
Serrano, A. et al. Identification of the PTPN22 functional variant R620W as susceptibility genetic factor for giant cell arteritis. Ann. Rheum. Dis. 72, 1882–1886 (2013).
Tizaoui, K. et al. The role of PTPN22 in the pathogenesis of autoimmune diseases: a comprehensive review. Semin. Arthritis Rheum. 51, 513–522 (2021).
Giscombe, R., Wang, X., Huang, D. & Lefvert, A. K. Coding sequence 1 and promoter single nucleotide polymorphisms in the CTLA-4 gene in Wegener’s granulomatosis. J. Rheumatol. 29, 950–953 (2002).
Zhou, Y. et al. An analysis of CTLA-4 and proinflammatory cytokine genes in Wegener’s granulomatosis. Arthritis Rheum. 50, 2645–2650 (2004).
Slot, M. C. et al. Immunoregulatory gene polymorphisms are associated with ANCA-related vasculitis. Clin. Immunol. 128, 39–45 (2008).
Lee, Y. H., Choi, S. J., Ji, J. D. & Song, G. G. CTLA-4 and TNF-α promoter-308 A/G polymorphisms and ANCA-associated vasculitis susceptibility: a meta-analysis. Mol. Biol. Rep. 39, 319–326 (2012).
Takara, M., Kouki, T. & DeGroot, L. J. CTLA-4 AT-repeat polymorphism reduces the inhibitory function of CTLA-4 in Graves’ disease. Thyroid 13, 1083–1089 (2003).
Anjos, S., Nguyen, A., Ounissi-Benkalha, H., Tessier, M. C. & Polychronakos, C. A common autoimmunity predisposing signal peptide variant of the cytotoxic T-lymphocyte antigen 4 results in inefficient glycosylation of the susceptibility allele. J. Biol. Chem. 277, 46478–46486 (2002).
Igarashi, K., Kurosaki, T. & Roychoudhuri, R. BACH transcription factors in innate and adaptive immunity. Nat. Rev. Immunol. 17, 437–450 (2017).
Sawalha, A. H. & Dozmorov, M. G. Epigenomic functional characterization of genetic susceptibility variants in systemic vasculitis. J. Autoimmun. 67, 76–81 (2016).
Mahr, A. et al. Eosinophilic granulomatosis with polyangiitis (Churg-Strauss): evolutions in classification, etiopathogenesis, assessment and management. Curr. Opin. Rheumatol. 26, 16–23 (2014).
Williams, B. B. et al. Glycoprotein A33 deficiency: a new mouse model of impaired intestinal epithelial barrier function and inflammatory disease. Dis. Model. Mech. 8, 805–815 (2015).
GTEx Consortium. Human genomics. The genotype-tissue expression (GTEx) pilot analysis: multitissue gene regulation in humans. Science 348, 648–660 (2015).
Hui, C. C. et al. Thymic stromal lymphopoietin (TSLP) secretion from human nasal epithelium is a function of TSLP genotype. Mucosal Immunol. 8, 993–999 (2015).
Suppiah, R. et al. A model to predict cardiovascular events in patients with newly diagnosed Wegener’s granulomatosis and microscopic polyangiitis. Arthritis Care Res. 63, 588–596 (2011).
de Joode, A. A., Sanders, J. S. & Stegeman, C. A. Renal survival in proteinase 3 and myeloperoxidase ANCA-associated systemic vasculitis. Clin. J. Am. Soc. Nephrol. 8, 1709–1717 (2013).
Quintana, L. F. et al. ANCA serotype and histopathological classification for the prediction of renal outcome in ANCA-associated glomerulonephritis. Nephrol. Dial. Transpl. 29, 1764–1769 (2014).
Bischof, A. et al. Peripheral neuropathy in antineutrophil cytoplasmic antibody-associated vasculitides: Insights from the DCVAS study. Neurol. Neuroimmunol. Neuroinflamm. 6, e615 (2019).
Monti, S. et al. Association between age at disease onset of anti-neutrophil cytoplasmic antibody-associated vasculitis and clinical presentation and short-term outcomes. Rheumatology 60, 617–628 (2021).
Moiseev, S. et al. Association of venous thromboembolic events with skin, pulmonary and kidney involvement in ANCA-associated vasculitis: a multinational study. Rheumatology 60, 4654–4661 (2021).
Hauer, H. A. et al. Renal histology in ANCA-associated vasculitis: differences between diagnostic and serologic subgroups. Kidney Int. 61, 80–89 (2002).
Bettiol, A. et al. Risk of acute arterial and venous thromboembolic events in eosinophilic granulomatosis with polyangiitis (Churg-Strauss syndrome). Eur. Respir. J. 57, 2004158 (2021).
Knight, A., Sandin, S. & Askling, J. Increased risk of autoimmune disease in families with Wegener’s granulomatosis. J. Rheumatol. 37, 2553–2558 (2010).
Draibe, J. & Salama, A. D. Association of ANCA associated vasculitis and rheumatoid arthritis: a lesser recognized overlap syndrome. Springerplus 4, 50 (2015).
Itikyala, S., Pattanaik, D. & Raza, S. Systemic lupus erythematosus (SLE) and antineutrophil cytoplasmic antibody-associated vasculitis (AAV) overlap syndrome: case report and review of the literature. Case Rep. Rheumatol. 2019, 5013904 (2019).
Martin-Nares, E., Zuniga-Tamayo, D. & Hinojosa-Azaola, A. Prevalence of overlap of antineutrophil cytoplasmic antibody associated vasculitis with systemic autoimmune diseases: an unrecognized example of poliautoimmunity. Clin. Rheumatol. 38, 97–106 (2019).
Dendrou, C. A., Petersen, J., Rossjohn, J. & Fugger, L. HLA variation and disease. Nat. Rev. Immunol. 18, 325–339 (2018).
Rahmattulla, C. et al. Genetic variants in ANCA-associated vasculitis: a meta-analysis. Ann. Rheum. Dis. 75, 1687–1692 (2016).
Vafiadis, P. et al. Insulin expression in human thymus is modulated by INS VNTR alleles at the IDDM2 locus. Nat. Genet. 15, 289–292 (1997).
Stanescu, H. C. et al. Risk HLA-DQA1 and PLA2R1 alleles in idiopathic membranous nephropathy. N. Engl. J. Med. 364, 616–626 (2011).
Stegeman, C. A. Anti-neutrophil cytoplasmic antibody (ANCA) levels directed against proteinase-3 and myeloperoxidase are helpful in predicting disease relapse in ANCA-associated small-vessel vasculitis. Nephrol. Dial. Transpl. 17, 2077–2080 (2002).
Walsh, M. et al. Risk factors for relapse of antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheum. 64, 542–548 (2012).
Mohammad, A. J. et al. Pulmonary involvement in antineutrophil cytoplasmic antibodies (ANCA)-associated vasculitis: the influence of ANCA subtype. J. Rheumatol. 44, 1458–1467 (2017).
Tanna, A. et al. Long-term outcome of anti-neutrophil cytoplasm antibody-associated glomerulonephritis: evaluation of the international histological classification and other prognostic factors. Nephrol. Dial. Transpl. 30, 1185–1192 (2015).
Mohammad, A. J. & Segelmark, M. A population-based study showing better renal prognosis for proteinase 3 antineutrophil cytoplasmic antibody (ANCA)-associated nephritis versus myeloperoxidase ANCA-associated nephritis. J. Rheumatol. 41, 1366–1373 (2014).
Menez, S. et al. Predictors of renal outcomes in sclerotic class anti-neutrophil cytoplasmic antibody glomerulonephritis. Am. J. Nephrol. 48, 465–471 (2018).
Trivioli, G. et al. Slowly progressive anti-neutrophil cytoplasmic antibody-associated renal vasculitis: clinico-pathological characterization and outcome. Clin. Kidney J. 14, 332–340 (2021).
Flossmann, O. et al. Long-term patient survival in ANCA-associated vasculitis. Ann. Rheum. Dis. 70, 488–494 (2011).
Wallace, Z. S. et al. All-cause and cause-specific mortality in ANCA-associated vasculitis: overall and according to ANCA type. Rheumatology 59, 2308–2315 (2020).
Segelmark, M., Elzouki, A. N., Wieslander, J. & Eriksson, S. The PiZ gene of α1-antitrypsin as a determinant of outcome in PR3-ANCA-positive vasculitis. Kidney Int. 48, 844–850 (1995).
Chang, D. Y., Luo, H., Zhou, X. J., Chen, M. & Zhao, M. H. Association of HLA genes with clinical outcomes of ANCA-associated vasculitis. Clin. J. Am. Soc. Nephrol. 7, 1293–1299 (2012).
Chen, D. et al. Immunological interaction of HLA-DPB1 and proteinase 3 in ANCA vasculitis is associated with clinical disease activity. J. Am. Soc. Nephrol. https://doi.org/10.1681/ASN.2021081142 (2022).
Ohlsson, S., Bakoush, O., Tencer, J., Torffvit, O. & Segelmark, M. Monocyte chemoattractant protein 1 is a prognostic marker in ANCA-associated small vessel vasculitis. Mediators Inflamm. 2009, 584916 (2009).
Jönsson, N., Erlandsson, E., Gunnarsson, L., Pettersson, A. & Ohlsson, S. Monocyte chemoattractant protein-1 in antineutrophil cytoplasmic autoantibody-associated vasculitis: biomarker potential and association with polymorphisms in the MCP-1 and the CC chemokine receptor-2 gene. Mediators Inflamm. 2018, 6861257 (2018).
Comarmond, C. et al. Pulmonary fibrosis in antineutrophil cytoplasmic antibodies (ANCA)-associated vasculitis: a series of 49 patients and review of the literature. Medicine 93, 340–349 (2014).
Alba, M. A. et al. Interstitial lung disease in ANCA vasculitis. Autoimmun. Rev. 16, 722–729 (2017).
Zhao, W. et al. Clinical features and prognosis of microscopic polyangiitis with usual interstitial pneumonia compared with idiopathic pulmonary fibrosis. Clin. Respir. J. 13, 460–466 (2019).
Namba, N. et al. Association of MUC5B promoter polymorphism with interstitial lung disease in myeloperoxidase-antineutrophil cytoplasmic antibody-associated vasculitis. Ann. Rheum. Dis. 78, 1144–1146 (2019).
Seibold, M. A. et al. A common MUC5B promoter polymorphism and pulmonary fibrosis. N. Engl. J. Med. 364, 1503–1512 (2011).
Juge, P. A. et al. MUC5B promoter variant and rheumatoid arthritis with interstitial lung disease. N. Engl. J. Med. 379, 2209–2219 (2018).
Morgan, M. D. et al. Increased incidence of cardiovascular events in patients with antineutrophil cytoplasmic antibody-associated vasculitides: a matched-pair cohort study. Arthritis Rheum. 60, 3493–3500 (2009).
Kang, A. et al. High incidence of arterial and venous thrombosis in antineutrophil cytoplasmic antibody-associated vasculitis. J. Rheumatol. 46, 285–293 (2019).
Malik, R. et al. Common coding variant in SERPINA1 increases the risk for large artery stroke. Proc. Natl Acad. Sci. USA 114, 3613–3618 (2017).
Markus, H. S. et al. Evidence HDAC9 genetic variant associated with ischemic stroke increases risk via promoting carotid atherosclerosis. Stroke 44, 1220–1225 (2013).
Malhotra, R. et al. HDAC9 is implicated in atherosclerotic aortic calcification and affects vascular smooth muscle cell phenotype. Nat. Genet. 51, 1580–1587 (2019).
Jones, R. B. et al. Rituximab versus cyclophosphamide in ANCA-associated renal vasculitis: 2-year results of a randomised trial. Ann. Rheum. Dis. 74, 1178–1182 (2015).
Stone, J. H. et al. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N. Engl. J. Med. 363, 221–232 (2010).
Smith, R. M. et al. Rituximab as therapy to induce remission after relapse in ANCA-associated vasculitis. Ann. Rheum. Dis. 79, 1243–1249 (2020).
Walsh, M. et al. Plasma exchange and glucocorticoids in severe ANCA-associated vasculitis. N. Engl. J. Med. 382, 622–631 (2020).
Yates, M. et al. EULAR/ERA-EDTA recommendations for the management of ANCA-associated vasculitis. Ann. Rheum. Dis. 75, 1583–1594 (2016).
Hessels, A. C. et al. Clinical outcome in anti-neutrophil cytoplasmic antibody-associated vasculitis and gene variants of 11beta-hydroxysteroid dehydrogenase type 1 and the glucocorticoid receptor. Rheumatology 58, 447–454 (2019).
Quax, R. A. et al. Glucocorticoid sensitivity in health and disease. Nat. Rev. Endocrinol. 9, 670–686 (2013).
van Rossum, E. F. et al. Identification of the BclI polymorphism in the glucocorticoid receptor gene: association with sensitivity to glucocorticoids in vivo and body mass index. Clin. Endocrinol. 59, 585–592 (2003).
Alberici, F. et al. Association of a TNFSF13B (BAFF) regulatory region single nucleotide polymorphism with response to rituximab in antineutrophil cytoplasmic antibody-associated vasculitis. J. Allergy Clin. Immunol. 139, 1684–1687.e10 (2017).
Nossent, J. C., Lester, S., Zahra, D., Mackay, C. R. & Rischmueller, M. Polymorphism in the 5′ regulatory region of the B-lymphocyte activating factor gene is associated with the Ro/La autoantibody response and serum BAFF levels in primary Sjogren’s syndrome. Rheumatology 47, 1311–1316 (2008).
Fabris, M. et al. The TTTT B lymphocyte stimulator promoter haplotype is associated with good response to rituximab therapy in seropositive rheumatoid arthritis resistant to tumor necrosis factor blockers. Arthritis Rheum. 65, 88–97 (2013).
Taylor, R. P. & Lindorfer, M. A. Drug insight: the mechanism of action of rituximab in autoimmune disease-the immune complex decoy hypothesis. Nat. Clin. Pract. Rheumatol. 3, 86–95 (2007).
Lim, S. H. et al. Fc gamma receptor IIb on target B cells promotes rituximab internalization and reduces clinical efficacy. Blood 118, 2530–2540 (2011).
Ruyssen-Witrand, A. et al. Fcγ receptor type IIIA polymorphism influences treatment outcomes in patients with rheumatoid arthritis treated with rituximab. Ann. Rheum. Dis. 71, 875–877 (2012).
Cartin-Ceba, R. et al. The pharmacogenomic association of Fcγ receptors and cytochrome P450 enzymes with response to rituximab or cyclophosphamide treatment in antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheumatol. 69, 169–175 (2017).
Schirmer, J. H. et al. Cyclophosphamide treatment-induced leukopenia rates in ANCA-associated vasculitis are influenced by variant CYP450 2C9 genotypes. Pharmacogenomics 17, 367–374 (2016).
Cartin-Ceba, R., Keogh, K. A., Specks, U., Sethi, S. & Fervenza, F. C. Rituximab for the treatment of Churg-Strauss syndrome with renal involvement. Nephrol. Dial. Transpl. 26, 2865–2871 (2011).
Mohammad, A. J. et al. Rituximab for the treatment of eosinophilic granulomatosis with polyangiitis (Churg-Strauss). Ann. Rheum. Dis. 75, 396–401 (2016).
Teixeira, V., Mohammad, A. J., Jones, R. B., Smith, R. & Jayne, D. Efficacy and safety of rituximab in the treatment of eosinophilic granulomatosis with polyangiitis. RMD Open 5, e000905 (2019).
Wechsler, M. E. et al. Mepolizumab or placebo for eosinophilic granulomatosis with polyangiitis. N. Engl. J. Med. 376, 1921–1932 (2017).
Bettiol, A. et al. Mepolizumab for eosinophilic granulomatosis with polyangiitis: a European multicenter observational study. Arthritis Rheumatol. 74, 295–306 (2022).
Alberici, F. et al. FCGR3B polymorphism predicts relapse risk in eosinophilic granulomatosis with polyangiitis. Rheumatology 59, 3563–3566 (2020).
Acknowledgements
We gratefully thank Dr. Giovanni M. Rossi for providing the histological images included in Fig. 1.
Author information
Authors and Affiliations
Contributions
All authors researched data for the article. A.V., G.T., D.M., M.T. and A.K. contributed substantially to discussion of the content. A.V., G.T., A.M., D.M., A.K. and P.A.L. wrote the article. G.T., A.M., M.T., A.K. and P.A.L. reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Rheumatology thanks Ingeborg Bajema, Stephen McAdoo and the other, anonymous, reviewer for their contribution to the peer review of this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Abatacept for the Treatment of Relapsing, Non-Severe, Granulomatosis With Polyangiitis (Wegener’s): https://clinicaltrials.gov/ct2/show/NCT02108860
Rituximab and Belimumab Combination Therapy in PR3 Vasculitis (COMBIVAS): https://clinicaltrials.gov/ct2/show/NCT03967925
Supplementary information
Glossary
- DNA methylation
-
An epigenetic mechanism involved in the regulation of gene expression through the transfer of a methyl group to the 5′-carbon of a cytosine.
- Histone marks
-
Covalent post-translational modifications of histone proteins that affect chromatin structure and, consequently, gene expression.
- Linkage disequilibrium
-
Non-random association of alleles at different loci because of their physical proximity on a chromosome.
- Luciferase reporter assay
-
An assay used to determine the allele-specific effects of a genetic variant on the expression of a target gene by measuring luminescence emitted by a reporter gene.
- Pleiotropy-informed conditional false discovery rate
-
A method of exploiting the shared similarities between two diseases to detect additional genetic loci common to both.
- Mendelian randomization
-
An approach that uses genetic variation as an instrumental variable to explore the causal relationship between risk factors and clinical traits.
- BOLT-REML
-
A computationally efficient method for carrying out variance component analyses of large GWAS data sets. Such an approach allows an estimation of the variance of a trait that is explained by aggregated sets of SNPs rather than simply testing the significance of individual loci.
- Whole-genome/-exome sequencing
-
The analysis of either the entire genomic sequence of an individual or, in the case of exome sequencing, the protein-coding regions of the genome.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Trivioli, G., Marquez, A., Martorana, D. et al. Genetics of ANCA-associated vasculitis: role in pathogenesis, classification and management. Nat Rev Rheumatol 18, 559–574 (2022). https://doi.org/10.1038/s41584-022-00819-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41584-022-00819-y
This article is cited by
-
ANCA detection with solid phase chemiluminescence assay: diagnostic and severity association in vasculitis
Immunologic Research (2024)
-
Acute interstitial nephritis caused by ANCA-associated vasculitis: a case based review
Clinical Rheumatology (2024)
-
Association between the AKT1 single nucleotide polymorphism (rs2498786, rs2494752 and rs5811155) and microscopic polyangiitis risk in a Chinese population
Molecular Genetics and Genomics (2023)
-
Polyangiitis overlap syndrome: a rare clinical entity
Rheumatology International (2023)
-
Profile, Healthcare Resource Consumption and Related Costs in ANCA-Associated Vasculitis Patients: A Real-World Analysis in Italy
Advances in Therapy (2023)