Abstract
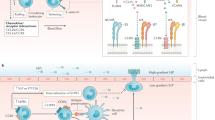
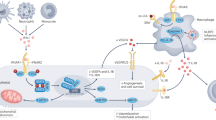
Sphingosine 1-phosphate (S1P), which acts via G protein-coupled S1P receptors (S1PRs), is a bioactive lipid essential for vascular integrity and lymphocyte trafficking. The S1P–S1PR signalling axis is a key component of the inflammatory response in autoimmune rheumatic diseases. Several drugs that target S1PRs have been approved for the treatment of multiple sclerosis and inflammatory bowel disease and are under clinical testing for patients with systemic lupus erythematosus (SLE). Preclinical studies support the hypothesis that targeting the S1P–S1PR axis would be beneficial to patients with SLE, rheumatoid arthritis (RA) and systemic sclerosis (SSc) by reducing pathological inflammation. Whereas most preclinical research and development efforts are focused on reducing lymphocyte trafficking, protective effects of circulating S1P on endothelial S1PRs, which maintain the vascular barrier and enable blood circulation while dampening leukocyte extravasation, have been largely overlooked. In this Review, we take a holistic view of S1P–S1PR signalling in lymphocyte and vascular pathobiology. We focus on the potential of S1PR modulators for the treatment of SLE, RA and SSc and summarize the rationale, pathobiology and evidence from preclinical models and clinical studies. Improved understanding of S1P pathobiology in autoimmune rheumatic diseases and S1PR therapeutic modulation is anticipated to lead to efficacious and safer management of these diseases.
Key points
-
Autoimmune rheumatic diseases are complex and heterogeneous diseases that have fundamental pathophysiological pathways in common.
-
These dysregulated pathways include lymphocyte autoreactivity, myeloid and endothelial cell activation, and extravasation of inflammatory mediators into tissues.
-
Chronic inflammatory damage in autoimmune rheumatic disease leads to end organ damage.
-
Sphingosine 1-phosphate receptor 1 (S1PR1) is expressed on leukocytes and endothelial cells and is an important mediator of lymphocyte trafficking, regulatory T/T helper 17 cell homeostasis and vascular permeability.
-
Use of S1PR1 modulators in preclinical studies of systemic lupus erythematosus, rheumatoid arthritis and systemic sclerosis has shown promise in attenuating inflammatory injury and end organ damage.
-
Modulation of S1PR1 signalling in leukocytes and/or endothelial cells warrants further evaluation in clinical studies in autoimmune rheumatic diseases.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Lee, M. J. et al. Vascular endothelial cell adherens junction assembly and morphogenesis induced by sphingosine-1-phosphate. Cell 99, 301–312 (1999).
Galvani, S. et al. HDL-bound sphingosine 1-phosphate acts as a biased agonist for the endothelial cell receptor S1P1 to limit vascular inflammation. Sci. Signal. 8, ra79 (2015).
Ruiz, M. et al. High-density lipoprotein-associated apolipoprotein M limits endothelial inflammation by delivering sphingosine-1-phosphate to the sphingosine-1-phosphate receptor 1. Arterioscler. Thromb. Vasc. Biol. 37, 118–129 (2017).
Jiang, H. et al. Sphingosine 1-phosphate receptor 1 (S1PR1) agonist CYM5442 inhibits expression of intracellular adhesion molecule 1 (ICAM1) in endothelial cells infected with influenza A viruses. PLoS One 12, e0175188 (2017).
Teijaro, J. R. et al. Endothelial cells are central orchestrators of cytokine amplification during influenza virus infection. Cell 146, 980–991 (2011).
Schwab, S. R. et al. Lymphocyte sequestration through S1P lyase inhibition and disruption of S1P gradients. Science 309, 1735–1739 (2005).
Ramos-Perez, W. D. et al. A map of the distribution of sphingosine 1-phosphate in the spleen. Nat. Immunol. 16, 1245–52. (2015).
Pappu, R. et al. Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine-1-phosphate. Science 316, 295–298 (2007).
Cyster, J. G. & Schwab, S. R. Sphingosine-1-phosphate and lymphocyte egress from lymphoid organs. Annu. Rev. Immunol. 30, 69–94 (2012).
Cartier, A. & Hla, T. Sphingosine 1-phosphate: lipid signaling in pathology and therapy. Science 366, eaar5551 (2019).
Proia, R. L. & Hla, T. Emerging biology of sphingosine-1-phosphate: its role in pathogenesis and therapy. J. Clin. Invest. 125, 1379–1387 (2015).
Sanchez, T. et al. PTEN as an effector in the signaling of antimigratory G protein-coupled receptor. Proc. Natl Acad. Sci. USA 102, 4312–4317 (2005).
Sanchez, T. et al. Induction of vascular permeability by the sphingosine-1-phosphate receptor-2 (S1P2R) and its downstream effectors ROCK and PTEN. Arterioscler. Thromb. Vasc. Biol. 27, 1312–1318 (2007).
Cruz-Orengo, L. et al. Enhanced sphingosine-1-phosphate receptor 2 expression underlies female CNS autoimmunity susceptibility. J. Clin. Invest. 124, 2571–84. (2014).
Michaud, J., Im, D. S. & Hla, T. Inhibitory role of sphingosine 1-phosphate receptor 2 in macrophage recruitment during inflammation. J. Immunol. 184, 1475–1483 (2010).
Laidlaw, B. J. et al. Sphingosine-1-phosphate receptor 2 restrains egress of γδ T cells from the skin. J. Exp. Med. 216, 1487–1496 (2019).
Muppidi, J. R. et al. Loss of signalling via Gα13 in germinal centre B-cell-derived lymphoma. Nature 516, 254–258 (2014).
Sun, X. et al. Sphingosine-1-phosphate receptor-3 is a novel biomarker in acute lung injury. Am. J. Respir. Cell Mol. Biol. 47, 628–636 (2012).
Cantalupo, A. et al. S1PR1 (sphingosine-1-phosphate receptor 1) signaling regulates blood flow and pressure. Hypertension 70, 426–434 (2017).
Murakami, K. et al. Knock out of S1P3 receptor signaling attenuates inflammation and fibrosis in bleomycin-induced lung injury mice model. PLoS One 9, e106792 (2014).
Takuwa, N. et al. S1P3-mediated cardiac fibrosis in sphingosine kinase 1 transgenic mice involves reactive oxygen species. Cardiovasc. Res. 85, 484–93. (2010).
Group, C. C. H. W. Meta-analysis of rare and common exome chip variants identifies S1PR4 and other loci influencing blood cell traits. Nat. Genet. 48, 867–876 (2016).
Drouillard, A. et al. S1PR5 is essential for human natural killer cell migration toward sphingosine-1 phosphate. J. Allergy Clin. Immunol. 141, 2265–2268.e1 (2018).
Kharel, Y. et al. Sphingosine kinase 2 is required for modulation of lymphocyte traffic by FTY720. J. Biol. Chem. 280, 36865–36872 (2005).
Chun, J. & Hartung, H. Mechanism of action of oral fingolimod (FTY720) in multiple sclerosis. Clin. Neuropharmacol. 33, 91–101 (2010).
Fryer, R. M. et al. The clinically-tested S1P receptor agonists, FTY720 and BAF312, demonstrate subtype-specific bradycardia (S1P1) and hypertension (S1P3) in rat. PLoS One 7, e52985 (2012).
Camm, J. et al. Cardiac and vascular effects of fingolimod: mechanistic basis and clinical implications. Am. Heart J. 168, 632–644 (2014).
Gonzalez-Cabrera, P. J. et al. S1P signaling: new therapies and opportunities. F1000Prime Rep. 6, 109 (2014).
Oo, M. L. et al. Engagement of S1P1-degradative mechanisms leads to vascular leak in mice. J. Clin. Invest. 121, 2290–2300 (2011).
Jain, N. & Bhatti, M. T. Fingolimod-associated macular edema: incidence, detection, and management. Neurology 78, 672–680 (2012).
Chae, S. S., Proia, R. L. & Hla, T. Constitutive expression of the S1P1 receptor in adult tissues. Prostaglandins Other Lipid Mediat. 73, 141–150 (2004).
Yanagida, K. et al. Sphingosine 1-phosphate receptor signaling establishes AP-1 gradients to allow for retinal endothelial cell specialization. Dev. Cell 52, 779–793.e7 (2020).
Ancellin, N. et al. Extracellular export of sphingosine kinase-1 enzyme. Sphingosine 1-phosphate generation and the induction of angiogenic vascular maturation. J. Biol. Chem. 277, 6667–6675 (2002).
Igarashi, N. et al. Sphingosine kinase 2 is a nuclear protein and inhibits DNA synthesis. J. Biol. Chem. 278, 46832–46839 (2003).
Allende, M. L. et al. Mice deficient in sphingosine kinase 1 are rendered lymphopenic by FTY720. J. Biol. Chem. 279, 52487–52492 (2004).
Zemann, B. et al. Sphingosine kinase type 2 is essential for lymphopenia induced by the immunomodulatory drug FTY720. Blood 107, 1454–1458 (2006).
Kharel, Y. et al. Sphingosine kinase type 2 inhibition elevates circulating sphingosine 1-phosphate. Biochem. J. 447, 149–157 (2012).
Kharel, Y. et al. Sphingosine kinase 2 inhibition and blood sphingosine 1-phosphate levels. J. Pharmacol. Exp. Ther. 355, 23–31 (2015).
Mizugishi, K. et al. Essential role for sphingosine kinases in neural and vascular development. Mol. Cell Biol. 25, 11113–11121 (2005).
Camerer, E. et al. Sphingosine-1-phosphate in the plasma compartment regulates basal and inflammation-induced vascular leak in mice. J. Clin. Invest. 119, 1871–1879 (2009).
Smyth, S. S. et al. Roles for lysophosphatidic acid signaling in vascular development and disease. Biochim. Biophys. Acta 1865, 158734 (2020).
Engelbrecht, E., MacRae, C. A. & Hla, T. Lysolipids in vascular development, biology, and disease. Arterioscler. Thromb. Vasc. Biol. 41, 564–584 (2021).
Panchatcharam, M. et al. Mice with targeted inactivation of ppap2b in endothelial and hematopoietic cells display enhanced vascular inflammation and permeability. Arterioscler. Thromb. Vasc. Biol. 34, 837–845 (2014).
Chatterjee, I. et al. Endothelial lipid phosphate phosphatase-3 deficiency that disrupts the endothelial barrier function is a modifier of cardiovascular development. Cardiovasc. Res. 111, 105–118 (2016).
Breart, B. et al. Lipid phosphate phosphatase 3 enables efficient thymic egress. J. Exp. Med. 208, 1267–1278 (2011).
Pham, T. H. et al. Lymphatic endothelial cell sphingosine kinase activity is required for lymphocyte egress and lymphatic patterning. J. Exp. Med. 207, 17–27 (2010).
Venkataraman, K. et al. Vascular endothelium as a contributor of plasma sphingosine 1-phosphate. Circ. Res. 102, 669–676 (2008).
Xiong, Y. et al. Erythrocyte-derived sphingosine 1-phosphate is essential for vascular development. J. Clin. Invest. 124, 4823–4828 (2014).
Urtz, N. et al. Sphingosine 1-cphosphate produced by sphingosine kinase 2 intrinsically controls platelet aggregation in vitro and in vivo. Circ. Res. 117, 376–387 (2015).
Chandrakanthan, M. et al. Deletion of Mfsd2b impairs thrombotic functions of platelets. Nat. Commun. 12, 2286 (2021).
Jonnalagadda, D. et al. Granule-mediated release of sphingosine-1-phosphate by activated platelets. Biochim. Biophys. Acta 1841, 1581–1589 (2014).
Gazit, S. L. et al. Platelet and erythrocyte sources of S1P are redundant for vascular development and homeostasis, but both rendered essential after plasma S1P depletion in anaphylactic shock. Circ. Res. 119, e110–126 (2016).
Schaphorst, K. L. et al. Role of sphingosine-1 phosphate in the enhancement of endothelial barrier integrity by platelet-released products. Am. J. Physiol. Lung Cell Mol. Physiol. 285, L258–L267 (2003).
Herzog, B. H. et al. Podoplanin maintains high endothelial venule integrity by interacting with platelet CLEC-2. Nature 502, 105–109 (2013).
Kawahara, A. et al. The sphingolipid transporter spns2 functions in migration of zebrafish myocardial precursors. Science 323, 524–527 (2009).
Vu, T. M. et al. Mfsd2b is essential for the sphingosine-1-phosphate export in erythrocytes and platelets. Nature 550, 524–528 (2017).
Fukuhara, S. et al. The sphingosine-1-phosphate transporter Spns2 expressed on endothelial cells regulates lymphocyte trafficking in mice. J. Clin. Invest. 122, 1416–1426 (2012).
Hisano, Y. et al. Mouse SPNS2 functions as a sphingosine-1-phosphate transporter in vascular endothelial cells. PLoS One 7, e38941 (2012).
Ding, B. S. et al. Aging suppresses sphingosine-1-phosphate chaperone ApoM in circulation resulting in maladaptive organ repair. Dev. Cell 53, 677–690.e4 (2020).
Winkler, M. S. et al. Decreased serum concentrations of sphingosine-1-phosphate in sepsis. Crit. Care 19, 372 (2015).
Winkler, M. S. et al. Sphingosine-1-phosphate: a potential biomarker and therapeutic target for endothelial dysfunction and sepsis? Shock 47, 666–672 (2017).
Christoffersen, C. et al. Endothelium-protective sphingosine-1-phosphate provided by HDL-associated apolipoprotein M. Proc. Natl Acad. Sci. USA 108, 9613–9618 (2011).
Christoffersen, C. et al. The apolipoprotein M/S1P axis controls triglyceride metabolism and brown fat activity. Cell Rep. 22, 175–188 (2018).
Christoffersen, C. et al. Effect of apolipoprotein M on high density lipoprotein metabolism and atherosclerosis in low density lipoprotein receptor knock-out mice. J. Biol. Chem. 283, 1839–1847 (2008).
Elsoe, S. et al. Apolipoprotein M binds oxidized phospholipids and increases the antioxidant effect of HDL. Atherosclerosis 221, 91–97 (2012).
Dahlback, B. & Nielsen, L. B. Apolipoprotein M — a novel player in high-density lipoprotein metabolism and atherosclerosis. Curr. Opin. Lipidol. 17, 291–295 (2006).
Blaho, V. A. et al. HDL-bound sphingosine-1-phosphate restrains lymphopoiesis and neuroinflammation. Nature 523, 342–346 (2015).
Wilkerson, B. A. et al. Sphingosine 1-phosphate (S1P) carrier-dependent regulation of endothelial barrier: high density lipoprotein (HDL)-S1P prolongs endothelial barrier enhancement as compared with albumin-S1P via effects on levels, trafficking, and signaling of S1P1. J. Biol. Chem. 287, 44645–44653 (2012).
Swendeman, S. L. et al. An engineered S1P chaperone attenuates hypertension and ischemic injury. Sci. Signal. 10, eaal2722 (2017).
Christensen, P. M. et al. Impaired endothelial barrier function in apolipoprotein M-deficient mice is dependent on sphingosine-1-phosphate receptor 1. FASEB J. 30, 2351–2359 (2016).
Matloubian, M. et al. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature 427, 355–360 (2004).
Allende, M. L. et al. Expression of the sphingosine 1-phosphate receptor, S1P1, on T-cells controls thymic emigration. J. Biol. Chem. 279, 15396–15401 (2004).
Allende, M. L. et al. S1P1 receptor directs the release of immature B cells from bone marrow into blood. J. Exp. Med. 207, 1113–1124 (2010).
Mendoza, A. et al. The transporter Spns2 is required for secretion of lymph but not plasma sphingosine-1-phosphate. Cell Rep. 2, 1104–1110 (2012).
Pham, T. H. et al. S1P1 receptor signaling overrides retention mediated by Gαi-coupled receptors to promote T cell egress. Immunity 28, 122–133 (2008).
Birker-Robaczewska, M. et al. S1P1 modulator-induced Gαi signaling and β-arrestin recruitment are both necessary to induce rapid and efficient reduction of blood lymphocyte count in vivo. Mol. Pharmacol. 93, 109–118 (2018).
Sykes, D. A. et al. Investigating the molecular mechanisms through which FTY720-P causes persistent S1P1 receptor internalization. Br. J. Pharmacol. 171, 4797–4807 (2014).
Lim, K. G. et al. FTY720 analogues as sphingosine kinase 1 inhibitors: enzyme inhibition kinetics, allosterism, proteasomal degradation, and actin rearrangement in MCF-7 breast cancer cells. J. Biol. Chem. 286, 18633–18640 (2011).
Mehling, M. et al. FTY720 therapy exerts differential effects on T cell subsets in multiple sclerosis. Neurology 71, 1261–1267 (2008).
Harris, S. et al. Effect of the sphingosine-1-phosphate receptor modulator ozanimod on leukocyte subtypes in relapsing MS. Neurol. Neuroimmunol. Neuroinflamm. 7, e839 (2020).
Luna, G. et al. Infection risks among patients with multiple sclerosis treated with fingolimod, natalizumab, rituximab, and injectable therapies. JAMA Neurol. 77, 184–191 (2020).
Zhao, Z. et al. Incidence and risk of infection associated with fingolimod in patients with multiple sclerosis: a systematic review and meta-analysis of 8,448 patients from 12 randomized controlled trials. Front. Immunol. 12, 611711 (2021).
Shiow, L. R. et al. CD69 acts downstream of interferon-alpha/beta to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature 440, 540–544 (2006).
Baeyens, A. et al. Monocyte-derived S1P in the lymph node regulates immune responses. Nature 592, 290–295 (2021).
Mike, E. V. et al. Neuropsychiatric systemic lupus erythematosus is dependent on sphingosine-1-phosphate signaling. Front. Immunol. 9, 2189 (2018).
Wenderfer, S. E., Stepkowski, S. M. & Braun, M. C. Increased survival and reduced renal injury in MRL/lpr mice treated with a novel sphingosine-1-phosphate receptor agonist. Kidney Int. 74, 1319–1326 (2008).
Sugahara, K. et al. Amiselimod (MT-1303), a novel sphingosine 1-phosphate receptor-1 modulator, potently inhibits the progression of lupus nephritis in two murine SLE models. J. Immunol. Res. 2019, 5821589 (2019).
Suzuki, S. et al. A new immunosuppressant, FTY720, induces bcl-2-associated apoptotic cell death in human lymphocytes. Immunology 89, 518–523 (1996).
Nagahara, Y. et al. Evidence that FTY720 induces T cell apoptosis in vivo. Immunopharmacology 48, 75–85 (2000).
Suzuki, S. et al. The in vivo induction of lymphocyte apoptosis in MRL-lpr/lpr mice treated with FTY720. Clin. Exp. Immunol. 107, 103–111 (1997).
Morris, M. A. et al. Transient T cell accumulation in lymph nodes and sustained lymphopenia in mice treated with FTY720. Eur. J. Immunol. 35, 3570–3580 (2005).
Han, Y. et al. FTY720 abrogates collagen-induced arthritis by hindering dendritic cell migration to local lymph nodes. J. Immunol. 195, 4126–4135 (2015).
Lan, Y. Y. et al. The sphingosine-1-phosphate receptor agonist FTY720 modulates dendritic cell trafficking in vivo. Am. J. Transpl. 5, 2649–2659. (2005).
Han, S. et al. FTY720 suppresses humoral immunity by inhibiting germinal center reaction. Blood 104, 4129–4133 (2004).
Moser, T. et al. The role of TH17 cells in multiple sclerosis: therapeutic implications. Autoimmun. Rev. 19, 102647 (2020).
Eken, A. et al. S1P1 deletion differentially affects TH17 and regulatory T cells. Sci. Rep. 7, 12905 (2017).
Liao, J. J., Huang, M. C. & Goetzl, E. J. Cutting edge: alternative signaling of Th17 cell development by sphingosine 1-phosphate. J. Immunol. 178, 5425–5428 (2007).
Garris, C. S. et al. Defective sphingosine 1-phosphate receptor 1 (S1P1) phosphorylation exacerbates TH17-mediated autoimmune neuroinflammation. Nat. Immunol. 14, 1166–1172 (2013).
Khattri, R. et al. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat. Immunol. 4, 337–342 (2003).
Fontenot, J. D., Gavin, M. A. & Rudensky, A. Y. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 4, 330–336 (2003).
Ochs, H. D., Gambineri, E. & Torgerson, T. R. IPEX, FOXP3 and regulatory T-cells: a model for autoimmunity. Immunol. Res. 38, 112–121 (2007).
Ohl, K. & Tenbrock, K. Regulatory T cells in systemic lupus erythematosus. Eur. J. Immunol. 45, 344–355 (2015).
Scalapino, K. J. et al. Suppression of disease in New Zealand Black/New Zealand White lupus-prone mice by adoptive transfer of ex vivo expanded regulatory T cells. J. Immunol. 177, 1451–1459 (2006).
Liu, G. et al. The receptor S1P1 overrides regulatory T cell-mediated immune suppression through Akt-mTOR. Nat. Immunol. 10, 769–777 (2009).
Priceman, S. J. et al. S1PR1 is crucial for accumulation of regulatory T cells in tumors via STAT3. Cell Rep. 6, 992–999 (2014).
Sawicka, E. et al. The sphingosine 1-phosphate receptor agonist FTY720 differentially affects the sequestration of CD4+/CD25+ T-regulatory cells and enhances their functional activity. J. Immunol. 175, 7973–7980 (2005).
Daniel, C. et al. FTY720 ameliorates Th1-mediated colitis in mice by directly affecting the functional activity of CD4+CD25+ regulatory T cells. J. Immunol. 178, 2458–2468 (2007).
Miller, D. C. et al. The CII-specific autoimmune T-cell response develops in the presence of FTY720 but is regulated by enhanced Treg cells that inhibit the development of autoimmune arthritis. Arthritis Res. Ther. 18, 8 (2016).
Haas, J. et al. Fingolimod does not impair T-cell release from the thymus and beneficially affects Treg function in patients with multiple sclerosis. Mult. Scler. 21, 1521–1532 (2015).
Hjorth, M., Dandu, N. & Mellergard, J. Treatment effects of fingolimod in multiple sclerosis: selective changes in peripheral blood lymphocyte subsets. PLoS One 15, e0228380 (2020).
Muls, N. et al. Fingolimod increases CD39-expressing regulatory T cells in multiple sclerosis patients. PLoS One 9, e113025 (2014).
Dominguez-Villar, M. et al. Fingolimod modulates T cell phenotype and regulatory T cell plasticity in vivo. J. Autoimmun. 96, 40–49 (2019).
Serpero, L. D. et al. Fingolimod modulates peripheral effector and regulatory T cells in MS patients. J. Neuroimmune Pharmacol. 8, 1106–1113 (2013).
Ghadiri, M. et al. Pre-treatment T-cell subsets associate with fingolimod treatment responsiveness in multiple sclerosis. Sci. Rep. 10, 356 (2020).
Liu, Y. et al. Edg-1, the G protein-coupled receptor for sphingosine-1-phosphate, is essential for vascular maturation. J. Clin. Invest. 106, 951–961 (2000).
Ben Shoham, A. et al. S1P1 inhibits sprouting angiogenesis during vascular development. Development 139, 3859–3869 (2012).
Jung, B. et al. Flow-regulated endothelial S1P receptor-1 signaling sustains vascular development. Dev. Cell 23, 600–610 (2012).
Gaengel, K. et al. The sphingosine-1-phosphate receptor S1PR1 restricts sprouting angiogenesis by regulating the interplay between VE-cadherin and VEGFR2. Dev. Cell 23, 587–599 (2012).
Cartier, A. et al. Endothelial sphingosine 1-phosphate receptors promote vascular normalization and antitumor therapy. Proc. Natl Acad. Sci. USA 117, 3157–3166 (2020).
Natarajan, V. et al. Sphingosine-1-phosphate, FTY720, and sphingosine-1-phosphate receptors in the pathobiology of acute lung injury. Am. J. Respir. Cell Mol. Biol. 49, 6–17 (2013).
Burg, N. et al. Sphingosine 1-phosphate receptor 1 signaling maintains endothelial cell barrier function and protects against immune complex-induced vascular injury. Arthritis Rheumatol. 70, 1879–1889 (2018).
Yanagida, K. et al. Size-selective opening of the blood-brain barrier by targeting endothelial sphingosine 1-phosphate receptor 1. Proc. Natl Acad. Sci. USA 114, 4531–4536 (2017).
Stone, M. L. et al. Sphingosine-1-phosphate receptor 1 agonism attenuates lung ischemia-reperfusion injury. Am. J. Physiol. Lung Cell Mol. Physiol. 308, L1245–L1252 (2015).
Shea, B. S. et al. Prolonged exposure to sphingosine 1-phosphate receptor-1 agonists exacerbates vascular leak, fibrosis, and mortality after lung injury. Am. J. Respir. Cell Mol. Biol. 43, 662–673 (2010).
Igarashi, J., Bernier, S. G. & Michel, T. Sphingosine 1-phosphate and activation of endothelial nitric-oxide synthase. differential regulation of Akt and MAP kinase pathways by EDG and bradykinin receptors in vascular endothelial cells. J. Biol. Chem. 276, 12420–12426 (2001).
Guan, Z. et al. Sphingosine-1-phosphate evokes unique segment-specific vasoconstriction of the renal microvasculature. J. Am. Soc. Nephrol. 25, 1774–1785 (2014).
Guan, Z. et al. Mechanisms of sphingosine-1-phosphate-mediated vasoconstriction of rat afferent arterioles. Acta Physiol. https://doi.org/10.1111/apha.12913 (2018).
Wolfrum, C., Poy, M. N. & Stoffel, M. Apolipoprotein M is required for prebeta-HDL formation and cholesterol efflux to HDL and protects against atherosclerosis. Nat. Med. 11, 418–422 (2005).
Silver, R. M., Metcalf, J. F. & LeRoy, E. C. Interstitial lung disease in scleroderma. Immune complexes in sera and bronchoalveolar lavage fluid. Arthritis Rheum. 29, 525–531 (1986).
Murdaca, G. et al. Endothelial dysfunction in rheumatic autoimmune diseases. Atherosclerosis 224, 309–317 (2012).
McGinley, M. & Cohen, J. A. Sphingosine 1-phosphate receptor modulators in multiple sclerosis and other conditions. Lancet 398, 1184–1194 (2021).
Perez-Jeldres, T., Alvarez-Lobos, M. & Rivera-Nieves, J. Targeting sphingosine-1-phosphate signaling in immune-mediated diseases: beyond multiple sclerosis. Drugs 81, 985–1002 (2021).
Frangou, E., Georgakis, S. & Bertsias, G. Update on the cellular and molecular aspects of lupus nephritis. Clin. Immunol. 216, 108445 (2020).
Katsuyama, T., Tsokos, G. C. & Moulton, V. R. Aberrant T cell signaling and subsets in systemic lupus erythematosus. Front. Immunol. 9, 1088 (2018).
Hamilton, J. A., Hsu, H. C. & Mountz, J. D. Autoreactive B cells in SLE, villains or innocent bystanders? Immunol. Rev. 292, 120–138 (2019).
Mancardi, D. et al. Endothelial dysfunction and cardiovascular risk in lupus nephritis: New roles for old players? Eur. J. Clin. Invest. 51, e13441 (2021).
Anders, H. J. et al. Lupus nephritis. Nat. Rev. Dis. Prim. 6, 7 (2020).
Jorge, A. M. et al. Unchanging premature mortality trends in systemic lupus erythematosus: a general population-based study (1999–2014). Rheumatology 57, 337–344 (2018).
Moghaddam, B. et al. All-cause and cause-specific mortality in systemic lupus erythematosus: a population-based study. Rheumatology 61, 367–376 (2021).
Ocampo-Piraquive, V. et al. Mortality in systemic lupus erythematosus: causes, predictors and interventions. Expert. Rev. Clin. Immunol. 14, 1043–1053 (2018).
Ballocca, F. et al. Predictors of cardiovascular events in patients with systemic lupus erythematosus (SLE): a systematic review and meta-analysis. Eur. J. Prev. Cardiol. 22, 1435–1441 (2015).
Watson, L. et al. Increased serum concentration of sphingosine-1-phosphate in juvenile-onset systemic lupus erythematosus. J. Clin. Immunol. 32, 1019–1025 (2012).
Patyna, S. et al. Blood ceramides as novel markers for renal impairment in systemic lupus erythematosus. Prostaglandins Other Lipid Mediat. 144, 106348 (2019).
Checa, A. et al. Dysregulations in circulating sphingolipids associate with disease activity indices in female patients with systemic lupus erythematosus: a cross-sectional study. Lupus 26, 1023–1033 (2017).
Du, W. et al. Low apolipoprotein M serum levels correlate with systemic lupus erythematosus disease activity and apolipoprotein M gene polymorphisms with lupus. Lipids Health Dis. 16, 88 (2017).
Tyden, H. et al. Low plasma concentrations of apolipoprotein M are associated with disease activity and endothelial dysfunction in systemic lupus erythematosus. Arthritis Res. Ther. 21, 110 (2019).
Axtell, A. L., Gomari, F. A. & Cooke, J. Assessing endothelial vasodilator function with the Endo-PAT 2000. J. Vis. Exp. https://doi.org/10.3791/2167 (2010).
Feingold, K. R. et al. Infection and inflammation decrease apolipoprotein M expression. Atherosclerosis 199, 19–26 (2008).
Crow, M. K. Interferon-alpha: a therapeutic target in systemic lupus erythematosus. Rheum. Dis. Clin. North. Am. 36, 173–186 (2010).
Crow, M. K. & Wohlgemuth, J. Microarray analysis of gene expression in lupus. Arthritis Res. Ther. 5, 279–287 (2003).
Niewold, T. B. et al. High serum IFN-α activity is a heritable risk factor for systemic lupus erythematosus. Genes. Immun. 8, 492–502 (2007).
Ronnblom, L., Alm, G. V. & Eloranta, M. L. The type I interferon system in the development of lupus. Semin. Immunol. 23, 113–121 (2011).
Zhao, J. et al. Combination of sphingosine-1-phosphate receptor 1 (S1PR1) agonist and antiviral drug: a potential therapy against pathogenic influenza virus. Sci. Rep. 9, 5272 (2019).
Garcia-Romo, G. S. et al. Netting neutrophils are major inducers of type I IFN production in pediatric systemic lupus erythematosus. Sci. Transl. Med. 3, 73ra20 (2011).
Blanco, L. P. et al. RNA externalized by neutrophil extracellular traps promotes inflammatory pathways in endothelial cells. Arthritis Rheumatol. 73, 2282–2292 (2021).
Taylor Meadows, K. R. et al. Ozanimod (RPC1063), a selective S1PR1 and S1PR5 modulator, reduces chronic inflammation and alleviates kidney pathology in murine systemic lupus erythematosus. PLoS One 13, e0193236 (2018).
Strasser, D. S. et al. Preclinical to clinical translation of cenerimod, a novel S1P1 receptor modulator, in systemic lupus erythematosus. RMD Open 6, e001261 (2020).
Liu, J. et al. Genomic view of systemic autoimmunity in MRLlpr mice. Genes Immun. 7, 156–168 (2006).
Singer, G. G. et al. Apoptosis, Fas and systemic autoimmunity: the MRL-lpr/lpr model. Curr. Opin. Immunol. 6, 913–920 (1994).
Okazaki, H. et al. Effects of FTY720 in MRL-lpr/lpr mice: therapeutic potential in systemic lupus erythematosus. J. Rheumatol. 29, 707–716 (2002).
Shi, D. et al. FTY720 attenuates behavioral deficits in a murine model of systemic lupus erythematosus. Brain Behav. Immun. 70, 293–304 (2018).
Subramanian, S. et al. A Tlr7 translocation accelerates systemic autoimmunity in murine lupus. Proc. Natl Acad. Sci. USA 103, 9970–9975 (2006).
Ando, S. et al. FTY720 exerts a survival advantage through the prevention of end-stage glomerular inflammation in lupus-prone BXSB mice. Biochem. Biophys. Res. Commun. 394, 804–810 (2010).
Comi, G. et al. Benefit-risk profile of sphingosine-1-phosphate receptor modulators in relapsing and secondary progressive multiple sclerosis. Drugs 77, 1755–1768 (2017).
Piali, L. et al. Cenerimod, a novel selective S1P1 receptor modulator with unique signaling properties. Pharmacol. Res. Perspect. 5, e00370 (2017).
Sugahara, K. et al. Amiselimod, a novel sphingosine 1-phosphate receptor-1 modulator, has potent therapeutic efficacy for autoimmune diseases, with low bradycardia risk. Br. J. Pharmacol. 174, 15–27 (2017).
Tanaka, Y. et al. Amiselimod, a sphingosine 1-phosphate receptor-1 modulator, for systemic lupus erythematosus: a multicenter, open-label exploratory study. Lupus 29, 1902–1913 (2020).
Smolen, J. S., Aletaha, D. & McInnes, I. B. Rheumatoid arthritis. Lancet 388, 2023–2038 (2016).
Buch, M. H., Eyre, S. & McGonagle, D. Persistent inflammatory and non-inflammatory mechanisms in refractory rheumatoid arthritis. Nat. Rev. Rheumatol. 17, 17–33 (2021).
Solomon, D. H. et al. Explaining the cardiovascular risk associated with rheumatoid arthritis: traditional risk factors versus markers of rheumatoid arthritis severity. Ann. Rheum. Dis. 69, 1920–1925 (2010).
Amigues, I. et al. Myocardial inflammation, measured using 18-fluorodeoxyglucose positron emission tomography with computed tomography, is associated with disease activity in rheumatoid arthritis. Arthritis Rheumatol. 71, 496–506 (2019).
Jagpal, A. & Navarro-Millan, I. Cardiovascular co-morbidity in patients with rheumatoid arthritis: a narrative review of risk factors, cardiovascular risk assessment and treatment. BMC Rheumatol. 2, 10 (2018).
Solomon, D. H. et al. Cardiovascular morbidity and mortality in women diagnosed with rheumatoid arthritis. Circulation 107, 1303–1307 (2003).
Sluiter, T. J. et al. Endothelial barrier function and leukocyte transmigration in atherosclerosis. Biomedicines 9, 328 (2021).
Nofer, J. R. High-density lipoprotein, sphingosine 1-phosphate, and atherosclerosis. J. Clin. Lipidol. 2, 4–11 (2008).
Kitano, M. et al. Sphingosine 1-phosphate/sphingosine 1-phosphate receptor 1 signaling in rheumatoid synovium: regulation of synovial proliferation and inflammatory gene expression. Arthritis Rheum. 54, 742–753 (2006).
Endresen, G. K. Evidence for activation of platelets in the synovial fluid from patients with rheumatoid arthritis. Rheumatol. Int. 9, 19–24 (1989).
Boilard, E. et al. Platelets amplify inflammation in arthritis via collagen-dependent microparticle production. Science 327, 580–583 (2010).
Zhao, C. et al. Chemical hypoxia brings to light altered autocrine sphingosine-1-phosphate signalling in rheumatoid arthritis synovial fibroblasts. Mediators Inflamm. 2015, 436525 (2015).
Lai, W. Q., Melendez, A. J. & Leung, B. Role of sphingosine kinase and sphingosine-1-phosphate in inflammatory arthritis. World J. Biol. Chem. 1, 321–326 (2010).
Zhao, C. et al. Specific and overlapping sphingosine-1-phosphate receptor functions in human synoviocytes: impact of TNF-alpha. J. Lipid Res. 49, 2323–2337 (2008).
Ishii, M. et al. Sphingosine-1-phosphate mobilizes osteoclast precursors and regulates bone homeostasis. Nature 458, 524–528 (2009).
Jaigirdar, S. A. et al. Sphingosine-1-phosphate promotes the persistence of activated CD4 T cells in inflamed sites. Front. Immunol. 8, 1627 (2017).
Ledgerwood, L. G. et al. The sphingosine 1-phosphate receptor 1 causes tissue retention by inhibiting the entry of peripheral tissue T lymphocytes into afferent lymphatics. Nat. Immunol. 9, 42–53 (2008).
Lai, W. Q. et al. Anti-inflammatory effects of sphingosine kinase modulation in inflammatory arthritis. J. Immunol. 181, 8010–8017 (2008).
Lai, W. Q. et al. Distinct roles of sphingosine kinase 1 and 2 in murine collagen-induced arthritis. J. Immunol. 183, 2097–2103 (2009).
Baker, D. A. et al. Genetic sphingosine kinase 1 deficiency significantly decreases synovial inflammation and joint erosions in murine TNF-alpha-induced arthritis. J. Immunol. 185, 2570–2579 (2010).
Pi, X. et al. Sphingosine kinase 1-mediated inhibition of Fas death signaling in rheumatoid arthritis B lymphoblastoid cells. Arthritis Rheum. 54, 754–764 (2006).
Michaud, J. et al. Normal acute and chronic inflammatory responses in sphingosine kinase 1 knockout mice. FEBS Lett. 580, 4607–4612 (2006).
Baker, D. A. et al. Impact of sphingosine kinase 2 deficiency on the development of TNF-alpha-induced inflammatory arthritis. Rheumatol. Int. 33, 2677–2681 (2013).
Yoshimoto, T. et al. Positive modulation of IL-12 signaling by sphingosine kinase 2 associating with the IL-12 receptor beta 1 cytoplasmic region. J. Immunol. 171, 1352–1359 (2003).
Pyne, S., Adams, D. R. & Pyne, N. J. Sphingosine kinases as druggable targets. Handb. Exp. Pharmacol. 259, 49–76 (2020).
Fitzpatrick, L. R. et al. Attenuation of arthritis in rodents by a novel orally-available inhibitor of sphingosine kinase. Inflammopharmacology 19, 75–87 (2011).
Hu, H. J. et al. Common variants at the promoter region of the APOM confer a risk of rheumatoid arthritis. Exp. Mol. Med. 43, 613–621 (2011).
Park, Y. J. et al. The APOM polymorphism as a novel risk factor for dyslipidaemia in rheumatoid arthritis: a possible shared link between disease susceptibility and dyslipidaemia. Clin. Exp. Rheumatol. 31, 180–188 (2013).
Huang, Y. et al. Apolipoprotein m (APOM) levels and APOM rs805297 G/T polymorphism are associated with increased risk of rheumatoid arthritis. Joint Bone Spine 81, 32–36 (2014).
van Halm, V. P. et al. Lipids and inflammation: serial measurements of the lipid profile of blood donors who later developed rheumatoid arthritis. Ann. Rheum. Dis. 66, 184–188 (2007).
Matsuura, M. et al. Effect of FTY720, a novel immunosuppressant, on adjuvant-induced arthritis in rats. Inflamm. Res. 49, 404–410 (2000).
Matsuura, M., Imayoshi, T. & Okumoto, T. Effect of FTY720, a novel immunosuppressant, on adjuvant- and collagen-induced arthritis in rats. Int. J. Immunopharmacol. 22, 323–331 (2000).
Zhu, C. et al. FTY720 inhibits the development of collagen-induced arthritis in mice by suppressing the recruitment of CD4+ T lymphocytes. Drug Des. Devel. Ther. 15, 1981–1992 (2021).
Wang, F. et al. Reduction of CD4 positive T cells and improvement of pathological changes of collagen-induced arthritis by FTY720. Eur. J. Pharmacol. 573, 230–240 (2007).
Jin, J. et al. Sphingosine-1-phosphate receptor subtype 1 (S1P1) modulator IMMH001 regulates adjuvant- and collagen-induced arthritis. Front. Pharmacol. 10, 1085 (2019).
Sakaguchi, N. et al. Altered thymic T-cell selection due to a mutation of the ZAP-70 gene causes autoimmune arthritis in mice. Nature 426, 454–460 (2003).
Tsunemi, S. et al. Effects of the novel immunosuppressant FTY720 in a murine rheumatoid arthritis model. Clin. Immunol. 136, 197–204 (2010).
Karuppuchamy, T. et al. Sphingosine-1-phosphate receptor-1 (S1P1) is expressed by lymphocytes, dendritic cells, and endothelium and modulated during inflammatory bowel disease. Mucosal Immunol. 10, 162–171 (2017).
Fujii, Y. et al. Amelioration of collagen-induced arthritis by a novel S1P1 antagonist with immunomodulatory activities. J. Immunol. 188, 206–215 (2012).
Bigaud, M. et al. Pathophysiological consequences of a break in S1P1-dependent homeostasis of vascular permeability revealed by S1P1 competitive antagonism. PLoS One 11, e0168252 (2016).
Bongartz, T. et al. Incidence and mortality of interstitial lung disease in rheumatoid arthritis: a population-based study. Arthritis Rheum. 62, 1583–1591 (2010).
Kadura, S. & Raghu, G. Rheumatoid arthritis-interstitial lung disease: manifestations and current concepts in pathogenesis and management. Eur. Respir. Rev. 30, 210011 (2021).
Huang, S. et al. Rheumatoid arthritis-associated interstitial lung disease: current update on prevalence, risk factors, and pharmacologic treatment. Curr. Treatm. Opt. Rheumatol. 6, 337–353 (2020).
Bagdanoff, J. T. et al. Inhibition of sphingosine 1-phosphate lyase for the treatment of rheumatoid arthritis: discovery of (E)-1-(4-((1R,2S,3R)-1,2,3,4-tetrahydroxybutyl)-1H-imidazol-2-yl)ethanone oxime (LX2931) and (1R,2S,3R)-1-(2-(isoxazol-3-yl)-1H-imidazol-4-yl)butane-1,2,3,4-tetraol (LX2932). J. Med. Chem. 53, 8650–8662 (2010).
Fleischmann, R. Novel small-molecular therapeutics for rheumatoid arthritis. Curr. Opin. Rheumatol. 24, 335–341 (2012).
Allende, M. L. et al. Sphingosine-1-phosphate lyase deficiency produces a pro-inflammatory response while impairing neutrophil trafficking. J. Biol. Chem. 286, 7348–7358 (2011).
Ruaro, B. et al. The treatment of lung involvement in systemic sclerosis. Pharmaceuticals 14, 154 (2021).
Denton, C. P., Wells, A. U. & Coghlan, J. G. Major lung complications of systemic sclerosis. Nat. Rev. Rheumatol. 14, 511–527 (2018).
Volkmann, E. R. & Fischer, A. Update on morbidity and mortality in systemic sclerosis-related interstitial lung disease. J. Scleroderma Relat. Disord. 6, 11–20 (2021).
Tashkin, D. P. et al. Mycophenolate mofetil versus oral cyclophosphamide in scleroderma-related interstitial lung disease (SLS II): a randomised controlled, double-blind, parallel group trial. Lancet Respir. Med. 4, 708–719 (2016).
Chung, L. et al. Survival and predictors of mortality in systemic sclerosis-associated pulmonary arterial hypertension: outcomes from the pulmonary hypertension assessment and recognition of outcomes in scleroderma registry. Arthritis Care Res. 66, 489–495 (2014).
Rubio-Rivas, M. et al. Mortality and survival in systemic sclerosis: systematic review and meta-analysis. Semin. Arthritis Rheum. 44, 208–219 (2014).
Teijaro, J. R. et al. S1PR1-mediated IFNAR1 degradation modulates plasmacytoid dendritic cell interferon-alpha autoamplification. Proc. Natl Acad. Sci. USA 113, 1351–1356 (2016).
Assassi, S. et al. Systemic sclerosis and lupus: points in an interferon-mediated continuum. Arthritis Rheum. 62, 589–598 (2010).
Brkic, Z. et al. The interferon type I signature is present in systemic sclerosis before overt fibrosis and might contribute to its pathogenesis through high BAFF gene expression and high collagen synthesis. Ann. Rheum. Dis. 75, 1567–1573 (2016).
Goldberg, A. et al. Dose-escalation of human anti-interferon-alpha receptor monoclonal antibody MEDI-546 in subjects with systemic sclerosis: a phase 1, multicenter, open label study. Arthritis Res. Ther. 16, R57 (2014).
Ciechomska, M. & Skalska, U. Targeting interferons as a strategy for systemic sclerosis treatment. Immunol. Lett. 195, 45–54 (2018).
Chrobak, I. et al. Interferon-gamma promotes vascular remodeling in human microvascular endothelial cells by upregulating endothelin (ET)-1 and transforming growth factor (TGF) β2. J. Cell Physiol. 228, 1774–1783 (2013).
Ah Kioon, M. D. et al. Plasmacytoid dendritic cells promote systemic sclerosis with a key role for TLR8. Sci. Transl. Med. 10, eaam8458 (2018).
Radstake, T. R. et al. The pronounced Th17 profile in systemic sclerosis (SSc) together with intracellular expression of TGFβ and IFNγ distinguishes SSc phenotypes. PLoS One 4, e5903 (2009).
Gabsi, A. et al. TH17 cells expressing CD146 are significantly increased in patients with systemic sclerosis. Sci. Rep. 9, 17721 (2019).
Goncalves, R. S. G. et al. IL-17 and related cytokines involved in systemic sclerosis: perspectives. Autoimmunity 51, 1–9 (2018).
Park, M. J. et al. IL-1-IL-17 signaling axis contributes to fibrosis and inflammation in two different murine models of systemic sclerosis. Front. Immunol. 9, 1611 (2018).
Moon, J. et al. Metformin ameliorates scleroderma via inhibiting Th17 cells and reducing mTOR-STAT3 signaling in skin fibroblasts. J. Transl. Med. 19, 192 (2021).
Frantz, C. et al. Regulatory T cells in systemic sclerosis. Front. Immunol. 9, 2356 (2018).
Igarashi, J. & Michel, T. Sphingosine-1-phosphate and modulation of vascular tone. Cardiovasc. Res. 82, 212–220 (2009).
Frech, T. M. et al. Vascular leak is a central feature in the pathogenesis of systemic sclerosis. J. Rheumatol. 39, 1385–1391 (2012).
Bruni, C. et al. Vascular leaking, a pivotal and early pathogenetic event in systemic sclerosis: should the door be closed? Front. Immunol. 9, 2045 (2018).
Fleming, J. N. & Schwartz, S. M. The pathology of scleroderma vascular disease. Rheum. Dis. Clin. North Am. 34, 41–55 (2008).
Asano, Y. et al. Endothelial Fli1 deficiency impairs vascular homeostasis: a role in scleroderma vasculopathy. Am. J. Pathol. 176, 1983–1998 (2010).
Shea, B. S. et al. Uncoupling of the profibrotic and hemostatic effects of thrombin in lung fibrosis. JCI Insight 2, e86608 (2017).
Knipe, R. S. et al. Endothelial-specific loss of sphingosine-1-phosphate receptor 1 increases vascular permeability and exacerbates bleomycin-induced pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 66, 38–52 (2021).
Kano, M. et al. Attenuation of murine sclerodermatous models by the selective S1P1 receptor modulator cenerimod. Sci. Rep. 9, 658 (2019).
Schmidt, K. G. et al. Sphingosine-1-phosphate receptor 5 modulates early-stage processes during fibrogenesis in a mouse model of systemic sclerosis: a pilot study. Front. Immunol. 8, 1242 (2017).
Salmon, J. E. & Roman, M. J. Subclinical atherosclerosis in rheumatoid arthritis and systemic lupus erythematosus. Am. J. Med. 121, S3–S8 (2008).
Roman, M. J. et al. Prevalence and correlates of accelerated atherosclerosis in systemic lupus erythematosus. N. Engl. J. Med. 349, 2399–2406 (2003).
Villanueva, E. et al. Netting neutrophils induce endothelial damage, infiltrate tissues, and expose immunostimulatory molecules in systemic lupus erythematosus. J. Immunol. 187, 538–552 (2011).
Author information
Authors and Affiliations
Contributions
N.B. researched data for the article. All authors contributed substantially to discussion of the content. N.B. wrote the article. All authors reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
T.H. declares the following potential competing interests in 2021–2022: consultancy — Arena Pharmaceuticals, Bristol Myers-Squibb Inc., Janssen Inc; inventor — patents on ApoM-Fc, S1PR2 modulators; speakers fees — Pfizer Inc. The other authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Rheumatology thanks S. Bourgoin, H. Radeke and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Burg, N., Salmon, J.E. & Hla, T. Sphingosine 1-phosphate receptor-targeted therapeutics in rheumatic diseases. Nat Rev Rheumatol 18, 335–351 (2022). https://doi.org/10.1038/s41584-022-00784-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41584-022-00784-6
This article is cited by
-
Gut liver brain axis in diseases: the implications for therapeutic interventions
Signal Transduction and Targeted Therapy (2023)
-
Targeted Small Molecules for Systemic Lupus Erythematosus: Drugs in the Pipeline
Drugs (2023)
-
Silence of S1PR4 Represses the Activation of Fibroblast-like Synoviocytes by Regulating IL-17/STAT3 Signaling Pathway
Inflammation (2023)