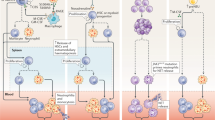
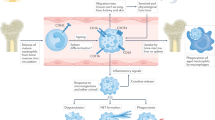
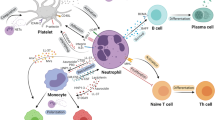
Abstract
Vascular pathologies underpin and intertwine autoimmune rheumatic diseases and cardiovascular conditions, and atherosclerosis is increasingly recognized as the leading cause of morbidity in conditions such as systemic lupus erythematosus (SLE), rheumatoid arthritis and antineutrophil cytoplasmic antibody-associated vasculitis. Neutrophils, important cells in the innate immune system, exert their functional effects in tissues via a variety of mechanisms, including the generation of neutrophil extracellular traps and the production of reactive oxygen species. Neutrophils have been implicated in the pathogenesis of several rheumatic diseases, and can also intimately interact with the vascular system, either through modulating endothelial barriers at the blood–vessel interface, or through associations with platelets. Emerging data suggest that neutrophils also have an important role maintaining homeostasis in individual organs and can protect the vascular system. Furthermore, studies using high-dimensional omics technologies have advanced our understanding of neutrophil diversity, and immature neutrophils are receiving new attention in rheumatic diseases including SLE and systemic vasculitis. Developments in genomic, imaging and organoid technologies are beginning to enable more in-depth investigations into the pathophysiology of vascular inflammation in rheumatic diseases, making now a good time to re-examine the full scope of roles of neutrophils in these processes.
Key points
-
Neutrophils are heterogeneous and have diversified phenotypes and functions, such as reactive oxygen species-producing immature neutrophils and neutrophil extracellular trap-producing mature neutrophils.
-
Neutrophils participate in the progression of disease from onset to chronic inflammation affecting multiple organs and tissues in rheumatic diseases such as rheumatoid arthritis, systemic lupus erythematosus and vasculitis.
-
Vascular inflammation in rheumatic diseases is associated with cardiovascular complications, such as atherosclerosis, which are a leading cause of morbidity and mortality.
-
Neutrophils, both mature and immature, have an essential role in initial endothelial activation and dysfunction associated with vascular inflammation and atherosclerosis.
-
Immune complexes in rheumatic diseases with well-defined autoantibodies excessively activate neutrophils to induce endothelial damage.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Summers, C. et al. Neutrophil kinetics in health and disease. Trends Immunol. 31, 318–324 (2010).
Silvestre-Roig, C., Fridlender, Z. G., Glogauer, M. & Scapini, P. Neutrophil diversity in health and disease. Trends Immunol. 40, 565–583 (2019).
Luqmani, R. Maintenance of clinical remission in ANCA-associated vasculitis. Nat. Rev. Rheumatol. 9, 127–132 (2013).
Nakazawa, D., Masuda, S., Tomaru, U. & Ishizu, A. Pathogenesis and therapeutic interventions for ANCA-associated vasculitis. Nat. Rev. Rheumatol. 15, 91–101 (2019).
Koster, M. J., Warrington, K. J. & Matteson, E. L. Morbidity and mortality of large-vessel vasculitides. Curr. Rheumatol. Rep. 22, 86 (2020).
Liu, Y. & Kaplan, M. J. Cardiovascular disease in systemic lupus erythematosus: an update. Curr. Opin. Rheumatol. 30, 441–448 (2018).
Skeoch, S. & Bruce, I. N. Atherosclerosis in rheumatoid arthritis: is it all about inflammation? Nat. Rev. Rheumatol. 11, 390–400 (2015).
Soehnlein, O., Steffens, S., Hidalgo, A. & Weber, C. Neutrophils as protagonists and targets in chronic inflammation. Nat. Rev. Immunol. 17, 248–261 (2017).
Nicolas-Avila, J. A., Adrover, J. M. & Hidalgo, A. Neutrophils in homeostasis, immunity, and cancer. Immunity 46, 15–28 (2017).
Casanova-Acebes, M. et al. Neutrophils instruct homeostatic and pathological states in naive tissues. J. Exp. Med. 215, 2778–2795 (2018).
Ballesteros, I. et al. Co-option of neutrophil fates by tissue environments. Cell 183, 1282–1297.e18 (2020).
Khoyratty, T. E. et al. Distinct transcription factor networks control neutrophil-driven inflammation. Nat. Immunol. 22, 1093–1106 (2021).
Cossio, I., Lucas, D. & Hidalgo, A. Neutrophils as regulators of the hematopoietic niche. Blood 133, 2140–2148 (2019).
Manz, M. G. & Boettcher, S. Emergency granulopoiesis. Nat. Rev. Immunol. 14, 302–314 (2014).
Scapini, P., Marini, O., Tecchio, C. & Cassatella, M. A. Human neutrophils in the saga of cellular heterogeneity: insights and open questions. Immunol. Rev. 273, 48–60 (2016).
Grieshaber-Bouyer, R. & Nigrovic, P. A. Neutrophil heterogeneity as therapeutic opportunity in immune-mediated disease. Front. Immunol. 10, 346 (2019).
Deniset, J. F. & Kubes, P. Neutrophil heterogeneity: bona fide subsets or polarization states? J. Leukoc. Biol. 103, 829–838 (2018).
Hacbarth, E. & Kajdacsy-Balla, A. Low density neutrophils in patients with systemic lupus erythematosus, rheumatoid arthritis, and acute rheumatic fever. Arthritis Rheum. 29, 1334–1342 (1986).
Denny, M. F. et al. A distinct subset of proinflammatory neutrophils isolated from patients with systemic lupus erythematosus induces vascular damage and synthesizes type I IFNs. J. Immunol. 184, 3284–3297 (2010).
Midgley, A. & Beresford, M. W. Increased expression of low density granulocytes in juvenile-onset systemic lupus erythematosus patients correlates with disease activity. Lupus 25, 407–411 (2016).
Wright, H. L., Makki, F. A., Moots, R. J. & Edwards, S. W. Low-density granulocytes: functionally distinct, immature neutrophils in rheumatoid arthritis with altered properties and defective TNF signalling. J. Leukoc. Biol. 101, 599–611 (2017).
Grayson, P. C. et al. Neutrophil-related gene expression and low-density granulocytes associated with disease activity and response to treatment in antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheumatol. 67, 1922–1932 (2015).
Wang, L. et al. ROS-producing immature neutrophils in giant cell arteritis are linked to vascular pathologies. JCI Insight 5, e139163 (2020).
Jones, B. E. et al. ANCA autoantigen gene expression highlights neutrophil heterogeneity where expression in normal-density neutrophils correlates with ANCA-induced activation. Kidney Int. 98, 744–757 (2020).
Mistry, P. et al. Transcriptomic, epigenetic, and functional analyses implicate neutrophil diversity in the pathogenesis of systemic lupus erythematosus. Proc. Natl Acad. Sci. USA 116, 25222–25228 (2019).
Winterbourn, C. C., Kettle, A. J. & Hampton, M. B. Reactive oxygen species and neutrophil function. Annu. Rev. Biochem. 85, 765–792 (2016).
Glennon-Alty, L., Hackett, A. P., Chapman, E. A. & Wright, H. L. Neutrophils and redox stress in the pathogenesis of autoimmune disease. Free Radic. Biol. Med. 125, 25–35 (2018).
Davies, M. J. Myeloperoxidase: mechanisms, reactions and inhibition as a therapeutic strategy in inflammatory diseases. Pharmacol. Ther. 218, 107685 (2021).
Forman, H. J. & Zhang, H. Targeting oxidative stress in disease: promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 20, 689–709 (2021).
Cunninghame Graham, D. S. et al. Association of NCF2, IKZF1, IRF8, IFIH1, and TYK2 with systemic lupus erythematosus. PLoS Genet. 7, e1002341 (2011).
Gateva, V. et al. A large-scale replication study identifies TNIP1, PRDM1, JAZF1, UHRF1BP1 and IL10 as risk loci for systemic lupus erythematosus. Nat. Genet. 41, 1228–1233 (2009).
Yokoyama, N. et al. Association of NCF1 polymorphism with systemic lupus erythematosus and systemic sclerosis but not with ANCA-associated vasculitis in a Japanese population. Sci. Rep. 9, 16366 (2019).
Zhao, J. et al. A missense variant in NCF1 is associated with susceptibility to multiple autoimmune diseases. Nat. Genet. 49, 433–437 (2017).
Olsson, L. M. et al. A single nucleotide polymorphism in the NCF1 gene leading to reduced oxidative burst is associated with systemic lupus erythematosus. Ann. Rheum. Dis. 76, 1607–1613 (2017).
Bengtsson, A. A. et al. Low production of reactive oxygen species in granulocytes is associated with organ damage in systemic lupus erythematosus. Arthritis Res. Ther. 16, R120 (2014).
De Ravin, S. S. et al. Chronic granulomatous disease as a risk factor for autoimmune disease. J. Allergy Clin. Immunol. 122, 1097–1103 (2008).
Kelkka, T. et al. Reactive oxygen species deficiency induces autoimmunity with type 1 interferon signature. Antioxid. Redox Signal. 21, 2231–2245 (2014).
Lightfoot, Y. L. & Kaplan, M. J. Disentangling the role of neutrophil extracellular traps in rheumatic diseases. Curr. Opin. Rheumatol. 29, 65–70 (2017).
Tan, C., Aziz, M. & Wang, P. The vitals of NETs. J. Leukoc. Biol. 110, 797–808 (2020).
Gupta, S. & Kaplan, M. J. The role of neutrophils and NETosis in autoimmune and renal diseases. Nat. Rev. Nephrol. 12, 402–413 (2016).
Lande, R. et al. Neutrophils activate plasmacytoid dendritic cells by releasing self-DNA–peptide complexes in systemic lupus erythematosus. Sci. Transl. Med. 3, 73ra19 (2011).
Lood, C. et al. Neutrophil extracellular traps enriched in oxidized mitochondrial DNA are interferogenic and contribute to lupus-like disease. Nat. Med. 22, 146–153 (2016).
Khandpur, R. et al. NETs are a source of citrullinated autoantigens and stimulate inflammatory responses in rheumatoid arthritis. Sci. Transl. Med. 5, 178ra140 (2013).
Kessenbrock, K. et al. Netting neutrophils in autoimmune small-vessel vasculitis. Nat. Med. 15, 623–625 (2009).
Nakazawa, D. et al. Enhanced formation and disordered regulation of NETs in myeloperoxidase-ANCA-associated microscopic polyangiitis. J. Am. Soc. Nephrol. 25, 990–997 (2014).
Warnatsch, A., Ioannou, M., Wang, Q. & Papayannopoulos, V. Inflammation. Neutrophil extracellular traps license macrophages for cytokine production in atherosclerosis. Science 349, 316–320 (2015).
Othman, A., Sekheri, M. & Filep, J. G. Roles of neutrophil granule proteins in orchestrating inflammation and immunity. FEBS J. https://doi.org/10.1111/febs.15803 (2021).
Yin, C. & Heit, B. Armed for destruction: formation, function and trafficking of neutrophil granules. Cell Tissue Res. 371, 455–471 (2018).
Mayadas, T. N., Cullere, X. & Lowell, C. A. The multifaceted functions of neutrophils. Annu. Rev. Pathol. 9, 181–218 (2014).
von der Mohlen, M. A., van der Poll, T., Jansen, J., Levi, M. & van Deventer, S. J. Release of bactericidal/permeability-increasing protein in experimental endotoxemia and clinical sepsis. Role of tumor necrosis factor. J. Immunol. 156, 4969–4973 (1996).
Kegerreis, B. J. et al. Genomic identification of low-density granulocytes and analysis of their role in the pathogenesis of systemic lupus erythematosus. J. Immunol. 202, 3309–3317 (2019).
Ohlsson, S. M. et al. Neutrophils from vasculitis patients exhibit an increased propensity for activation by anti-neutrophil cytoplasmic antibodies. Clin. Exp. Immunol. 176, 363–372 (2014).
Walls, C. A. et al. A novel 4-dimensional live-cell imaging system to study leukocyte–endothelial dynamics in ANCA-associated vasculitis. Autoimmunity 53, 148–155 (2020).
Brachemi, S. et al. Increased membrane expression of proteinase 3 during neutrophil adhesion in the presence of anti proteinase 3 antibodies. J. Am. Soc. Nephrol. 18, 2330–2339 (2007).
von Vietinghoff, S. et al. NB1 mediates surface expression of the ANCA antigen proteinase 3 on human neutrophils. Blood 109, 4487–4493 (2007).
Rarok, A. A., Stegeman, C. A., Limburg, P. C. & Kallenberg, C. G. Neutrophil membrane expression of proteinase 3 (PR3) is related to relapse in PR3-ANCA-associated vasculitis. J. Am. Soc. Nephrol. 13, 2232–2238 (2002).
Schreiber, A., Busjahn, A., Luft, F. C. & Kettritz, R. Membrane expression of proteinase 3 is genetically determined. J. Am. Soc. Nephrol. 14, 68–75 (2003).
Versteeg, H. H., Heemskerk, J. W., Levi, M. & Reitsma, P. H. New fundamentals in hemostasis. Physiol. Rev. 93, 327–358 (2013).
Dib, P. R. B. et al. Innate immune receptors in platelets and platelet–leukocyte interactions. J. Leukoc. Biol. 108, 1157–1182 (2020).
Clark, S. R. et al. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat. Med. 13, 463–469 (2007).
Sreeramkumar, V. et al. Neutrophils scan for activated platelets to initiate inflammation. Science 346, 1234–1238 (2014).
Schrottmaier, W. C., Mussbacher, M., Salzmann, M. & Assinger, A. Platelet-leukocyte interplay during vascular disease. Atherosclerosis 307, 109–120 (2020).
Kolaczkowska, E. & Kubes, P. Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 13, 159–175 (2013).
Phillipson, M. & Kubes, P. The neutrophil in vascular inflammation. Nat. Med. 17, 1381–1390 (2011).
Sadik, C. D., Kim, N. D. & Luster, A. D. Neutrophils cascading their way to inflammation. Trends Immunol. 32, 452–460 (2011).
Williams, M. R., Azcutia, V., Newton, G., Alcaide, P. & Luscinskas, F. W. Emerging mechanisms of neutrophil recruitment across endothelium. Trends Immunol. 32, 461–469 (2011).
Rock, K. L., Latz, E., Ontiveros, F. & Kono, H. The sterile inflammatory response. Annu. Rev. Immunol. 28, 321–342 (2010).
Sadik, C. D., Miyabe, Y., Sezin, T. & Luster, A. D. The critical role of C5a as an initiator of neutrophil-mediated autoimmune inflammation of the joint and skin. Semin. Immunol. 37, 21–29 (2018).
Jennette, J. C. & Falk, R. J. Pathogenesis of antineutrophil cytoplasmic autoantibody-mediated disease. Nat. Rev. Rheumatol. 10, 463–473 (2014).
Schreiber, A. et al. Necroptosis controls NET generation and mediates complement activation, endothelial damage, and autoimmune vasculitis. Proc. Natl Acad. Sci. USA 114, E9618–E9625 (2017).
Kraaij, T. et al. Excessive neutrophil extracellular trap formation in ANCA-associated vasculitis is independent of ANCA. Kidney Int. 94, 139–149 (2018).
Popat, R. J. & Robson, M. G. Neutrophils are not consistently activated by antineutrophil cytoplasmic antibodies in vitro. Ann. Rheum. Dis. 78, 709–711 (2019).
Hattanda, F. et al. The presence of anti-neutrophil extracellular trap antibody in patients with microscopic polyangiitis. Rheumatology 58, 1293–1298 (2019).
Shida, H. et al. Anti-neutrophil extracellular trap antibody in a patient with relapse of anti-neutrophil cytoplasmic antibody-associated vasculitis: a case report. BMC Nephrol. 19, 145 (2018).
Gill, E. E. et al. Different disease endotypes in phenotypically similar vasculitides affecting small-to-medium sized blood vessels. Front. Immunol. 12, 638571 (2021).
Wallace, Z. S. et al. All-cause and cause-specific mortality in ANCA-associated vasculitis: overall and according to ANCA type. Rheumatology 59, 2308–2315 (2020).
Houben, E. et al. Cardiovascular events in anti-neutrophil cytoplasmic antibody-associated vasculitis: a meta-analysis of observational studies. Rheumatology 57, 555–562 (2018).
de Leeuw, K. et al. Accelerated atherosclerosis in patients with Wegener’s granulomatosis. Ann. Rheum. Dis. 64, 753–759 (2005).
Ito, T., Kakuuchi, M. & Maruyama, I. Endotheliopathy in septic conditions: mechanistic insight into intravascular coagulation. Crit. Care 25, 95 (2021).
Eichhorn, J. et al. Anti-endothelial cell antibodies in Takayasu arteritis. Circulation 94, 2396–2401 (1996).
Nityanand, S., Mishra, K., Shrivastava, S., Holm, G. & Lefvert, A. K. Autoantibodies against cardiolipin and endothelial cells in Takayasu’s arteritis: prevalence and isotype distribution. Br. J. Rheumatol. 36, 923–924 (1997).
Mutoh, T. et al. Identification of two major autoantigens negatively regulating endothelial activation in Takayasu arteritis. Nat. Commun. 11, 1253 (2020).
Cats, H. A., Tervaert, J. W., van Wijk, R., Limburg, P. C. & Kallenberg, C. G. Anti-neutrophil cytoplasmic antibodies in giant cell arteritis and polymyalgia rheumatica. Adv. Exp. Med. Biol. 336, 363–366 (1993).
Baerlecken, N. T. et al. Association of ferritin autoantibodies with giant cell arteritis/polymyalgia rheumatica. Ann. Rheum. Dis. 71, 943–947 (2012).
Espinosa, G. et al. Antiphospholipid antibodies and thrombophilic factors in giant cell arteritis. Semin. Arthritis Rheum. 31, 12–20 (2001).
Duhaut, P. et al. Anticardiolipin antibodies and giant cell arteritis: a prospective, multicenter case-control study. Groupe de Recherche sur l’Artérite à Cellules Géantes. Arthritis Rheum. 41, 701–709 (1998).
Nadkarni, S. et al. Investigational analysis reveals a potential role for neutrophils in giant-cell arteritis disease progression. Circ. Res. 114, 242–248 (2014).
van Sleen, Y. et al. Leukocyte dynamics reveal a persistent myeloid dominance in giant cell arteritis and polymyalgia rheumatica. Front. Immunol. 10, 1981 (2019).
Oh, L. J. et al. Full blood count as an ancillary test to support the diagnosis of giant cell arteritis. Intern. Med. J. 48, 408–413 (2018).
Palamidas, D. A. et al. Neutrophil extracellular traps in giant cell arteritis biopsies: presentation, localization and co-expression with inflammatory cytokines. Rheumatology https://doi.org/10.1093/rheumatology/keab505 (2021).
Matsumoto, K. et al. Interleukin-1 pathway in active large vessel vasculitis patients with a poor prognosis: a longitudinal transcriptome analysis. Clin. Transl. Immunology 10, 1307 (2021).
Robson, J. C. et al. Which patients with giant cell arteritis will develop cardiovascular or cerebrovascular disease? A clinical practice research datalink study. J. Rheumatol. 43, 1085–1092 (2016).
Monti, S. et al. Early development of new cardiovascular risk factors in the systemic vasculitides. Clin. Exp. Rheumatol. 38, 126–134 (2020).
Evans, J. M., O’Fallon, W. M. & Hunder, G. G. Increased incidence of aortic aneurysm and dissection in giant cell (temporal) arteritis. A population-based study. Ann. Intern. Med. 122, 502–507 (1995).
Robson, J. C. et al. The relative risk of aortic aneurysm in patients with giant cell arteritis compared with the general population of the UK. Ann. Rheum. Dis. 74, 129–135 (2015).
Lareyre, F. et al. High neutrophil to lymphocyte ratio is associated with symptomatic and ruptured thoracic aortic aneurysm. Angiology 69, 686–691 (2018).
Hatipoglu, O. F. et al. Deficiency of CD44 prevents thoracic aortic dissection in a murine model. Sci. Rep. 10, 6869 (2020).
O’Neil, L. J. & Kaplan, M. J. Neutrophils in rheumatoid arthritis: breaking immune tolerance and fueling disease. Trends Mol. Med. 25, 215–227 (2019).
van Delft, M. A. M. & Huizinga, T. W. J. An overview of autoantibodies in rheumatoid arthritis. J. Autoimmun. 110, 102392 (2020).
Fawthrop, F. et al. A comparison of normal and pathological synovial fluid. Br. J. Rheumatol. 24, 61–69 (1985).
Freemont, A. J. & Denton, J. Disease distribution of synovial fluid mast cells and cytophagocytic mononuclear cells in inflammatory arthritis. Ann. Rheum. Dis. 44, 312–315 (1985).
Carmona-Rivera, C. et al. Synovial fibroblast-neutrophil interactions promote pathogenic adaptive immunity in rheumatoid arthritis. Sci. Immunol. 2, eaag3358 (2017).
O’Neil, L. J. et al. Neutrophil-mediated carbamylation promotes articular damage in rheumatoid arthritis. Sci. Adv. 6, eabd2688 (2020).
Wright, H. L., Moots, R. J. & Edwards, S. W. The multifactorial role of neutrophils in rheumatoid arthritis. Nat. Rev. Rheumatol. 10, 593–601 (2014).
Assi, L. K. et al. Tumor necrosis factor α activates release of B lymphocyte stimulator by neutrophils infiltrating the rheumatoid joint. Arthritis Rheum. 56, 1776–1786 (2007).
de Cerqueira, D. P. A., Pedreira, A. L. S., de Cerqueira, M. G. & Santiago, M. B. Biological therapy in rheumatoid vasculitis: a systematic review. Clin. Rheumatol. 40, 1717–1724 (2021).
Solomon, D. H. et al. Patterns of cardiovascular risk in rheumatoid arthritis. Ann. Rheum. Dis. 65, 1608–1612 (2006).
Sandoo, A., Veldhuijzen van Zanten, J. J., Metsios, G. S., Carroll, D. & Kitas, G. D. Vascular function and morphology in rheumatoid arthritis: a systematic review. Rheumatology 50, 2125–2139 (2011).
McInnes, I. B. & Schett, G. The pathogenesis of rheumatoid arthritis. N. Engl. J. Med. 365, 2205–2219 (2011).
Sitia, S. et al. From endothelial dysfunction to atherosclerosis. Autoimmun. Rev. 9, 830–834 (2010).
Perez-Sanchez, C. et al. Diagnostic potential of NETosis-derived products for disease activity, atherosclerosis and therapeutic effectiveness in rheumatoid arthritis patients. J. Autoimmun. 82, 31–40 (2017).
Pieterse, E. et al. Cleaved N-terminal histone tails distinguish between NADPH oxidase (NOX)-dependent and NOX-independent pathways of neutrophil extracellular trap formation. Ann. Rheum. Dis. 77, 1790–1798 (2018).
Ruiz-Limon, P. et al. Tocilizumab improves the proatherothrombotic profile of rheumatoid arthritis patients modulating endothelial dysfunction, NETosis, and inflammation. Transl. Res. 183, 87–103 (2017).
Maria, N. I. & Davidson, A. Emerging areas for therapeutic discovery in SLE. Curr. Opin. Immunol. 55, 1–8 (2018).
Barrat, F. J. et al. Nucleic acids of mammalian origin can act as endogenous ligands for Toll-like receptors and may promote systemic lupus erythematosus. J. Exp. Med. 202, 1131–1139 (2005).
Means, T. K. et al. Human lupus autoantibody-DNA complexes activate DCs through cooperation of CD32 and TLR9. J. Clin. Invest. 115, 407–417 (2005).
Vollmer, J. et al. Immune stimulation mediated by autoantigen binding sites within small nuclear RNAs involves Toll-like receptors 7 and 8. J. Exp. Med. 202, 1575–1585 (2005).
Garcia-Romo, G. S. et al. Netting neutrophils are major inducers of type I IFN production in pediatric systemic lupus erythematosus. Sci. Transl. Med. 3, 73ra20 (2011).
Banchereau, R. et al. Personalized immunomonitoring uncovers molecular networks that stratify lupus patients. Cell 165, 551–565 (2016).
Haynes, W. A. et al. Integrated, multicohort analysis reveals unified signature of systemic lupus erythematosus. JCI Insight 5, e122312 (2020).
Gupta, S. & Kaplan, M. J. Bite of the wolf: innate immune responses propagate autoimmunity in lupus. J. Clin. Invest. 131, e144918 (2021).
Gestermann, N. et al. Netting neutrophils activate autoreactive B cells in lupus. J. Immunol. 200, 3364–3371 (2018).
Villanueva, E. et al. Netting neutrophils induce endothelial damage, infiltrate tissues, and expose immunostimulatory molecules in systemic lupus erythematosus. J. Immunol. 187, 538–552 (2011).
Kahlenberg, J. M., Carmona-Rivera, C., Smith, C. K. & Kaplan, M. J. Neutrophil extracellular trap-associated protein activation of the NLRP3 inflammasome is enhanced in lupus macrophages. J. Immunol. 190, 1217–1226 (2013).
Caielli, S. et al. Oxidized mitochondrial nucleoids released by neutrophils drive type I interferon production in human lupus. J. Exp. Med. 213, 697–713 (2016).
Barile-Fabris, L., Hernandez-Cabrera, M. F. & Barragan-Garfias, J. A. Vasculitis in systemic lupus erythematosus. Curr. Rheumatol. Rep. 16, 440 (2014).
Baragetti, A. et al. Disease trends over time and CD4(+)CCR5(+) T-cells expansion predict carotid atherosclerosis development in patients with systemic lupus erythematosus. Nutr. Metab. Cardiovasc. Dis. 28, 53–63 (2018).
O’Neil, L. J., Kaplan, M. J. & Carmona-Rivera, C. The role of neutrophils and neutrophil extracellular traps in vascular damage in systemic lupus erythematosus. J. Clin. Med. 8, 1325 (2019).
Ding, X., Xiang, W. & He, X. IFN-I mediates dysfunction of endothelial progenitor cells in atherosclerosis of systemic lupus erythematosus. Front. Immunol. 11, 581385 (2020).
Pieterse, E. et al. Neutrophil extracellular traps drive endothelial-to-mesenchymal transition. Arterioscler. Thromb. Vasc. Biol. 37, 1371–1379 (2017).
Moore, S. et al. Role of neutrophil extracellular traps regarding patients at risk of increased disease activity and cardiovascular comorbidity in systemic lupus erythematosus. J. Rheumatol. 47, 1652–1660 (2020).
Carlucci, P. M. et al. Neutrophil subsets and their gene signature associate with vascular inflammation and coronary atherosclerosis in lupus. JCI Insight 3, e99276 (2018).
Smith, C. K. et al. Neutrophil extracellular trap-derived enzymes oxidize high-density lipoprotein: an additional proatherogenic mechanism in systemic lupus erythematosus. Arthritis Rheumatol. 66, 2532–2544 (2014).
Carmona-Rivera, C., Zhao, W., Yalavarthi, S. & Kaplan, M. J. Neutrophil extracellular traps induce endothelial dysfunction in systemic lupus erythematosus through the activation of matrix metalloproteinase-2. Ann. Rheum. Dis. 74, 1417–1424 (2015).
Bashant, K. R. et al. Proteomic, biomechanical and functional analyses define neutrophil heterogeneity in systemic lupus erythematosus. Ann. Rheum. Dis. 80, 209–218 (2021).
Miralda, I., Uriarte, S. M. & McLeish, K. R. Multiple phenotypic changes define neutrophil priming. Front. Cell Infect. Microbiol. 7, 217 (2017).
Mayadas, T. N., Tsokos, G. C. & Tsuboi, N. Mechanisms of immune complex-mediated neutrophil recruitment and tissue injury. Circulation 120, 2012–2024 (2009).
Silvestre-Roig, C., Braster, Q., Ortega-Gomez, A. & Soehnlein, O. Neutrophils as regulators of cardiovascular inflammation. Nat. Rev. Cardiol. 17, 327–340 (2020).
Brinkmann, V. Neutrophil extracellular traps in the second decade. J. Innate Immun. 10, 414–421 (2018).
Santocki, M. & Kolaczkowska, E. On neutrophil extracellular trap (NET) removal: what we know thus far and why so little. Cells 9, 2079 (2020).
Jimenez-Alcazar, M. et al. Host DNases prevent vascular occlusion by neutrophil extracellular traps. Science 358, 1202–1206 (2017).
Hakkim, A. et al. Impairment of neutrophil extracellular trap degradation is associated with lupus nephritis. Proc. Natl Acad. Sci. USA 107, 9813–9818 (2010).
Hardy, R. S., Raza, K. & Cooper, M. S. Therapeutic glucocorticoids: mechanisms of actions in rheumatic diseases. Nat. Rev. Rheumatol. 16, 133–144 (2020).
Jayne, D. R. W., Merkel, P. A., Schall, T. J., Bekker, P. & Group, A. S. Avacopan for the treatment of ANCA-associated vasculitis. N. Engl. J. Med. 384, 599–609 (2021).
U.S. Food & Drug Administration. FDA approves add-on drug for adults with rare form of blood vessel inflammation. FDA.gov https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-add-drug-adults-rare-form-blood-vessel-inflammation (2021).
Bechman, K. Yates, M. & Galloway, J. B. The new entries in the therapeutic armamentarium: the small molecule JAK inhibitors. Pharmacol. Res. 147,104392 (2019).
Hasni, S. A. et al. Phase 1 double-blind randomized safety trial of the Janus kinase inhibitor tofacitinib in systemic lupus erythematosus. Nat. Commun. 12, 3391 (2021).
Yousefi, S., Mihalache, C., Kozlowski, E., Schmid, I. & Simon, H. U. Viable neutrophils release mitochondrial DNA to form neutrophil extracellular traps. Cell Death Differ. 16, 1438–1444 (2009).
Buckley, C. D. et al. Efficacy, patient-reported outcomes, and safety of the anti-granulocyte macrophage colony-stimulating factor antibody otilimab (GSK3196165) in patients with rheumatoid arthritis: a randomised, phase 2b, dose-ranging study. Lancet Rheumatol. 2, e677–e688 (2021).
Cid, M. et al. Mavrilimumab (anti GM-CSF receptor A monoclonal antibody) reduces riks of flare and increases sustained remission in a phase 2 trial of patients with giant cell arteritis [abstract OP0059]. Ann. Rheum. Dis. 82, 31–32 (2021).
Lewis, H. D. et al. Inhibition of PAD4 activity is sufficient to disrupt mouse and human NET formation. Nat. Chem. Biol. 11, 189–191 (2015).
Willis, V. C. et al. Protein arginine deiminase 4 inhibition is sufficient for the amelioration of collagen-induced arthritis. Clin. Exp. Immunol. 188, 263–274 (2017).
Knight, J. S. et al. Peptidylarginine deiminase inhibition is immunomodulatory and vasculoprotective in murine lupus. J. Clin. Invest. 123, 2981–2993 (2013).
Knight, J. S. et al. Peptidylarginine deiminase inhibition reduces vascular damage and modulates innate immune responses in murine models of atherosclerosis. Circ. Res. 114, 947–956 (2014).
Smallwood, M. J. et al. Oxidative stress in autoimmune rheumatic diseases. Free. Radic. Biol. Med. 125, 3–14 (2018).
van der Veen, B. S. et al. Effects of p38 mitogen-activated protein kinase inhibition on anti-neutrophil cytoplasmic autoantibody pathogenicity in vitro and in vivo. Ann. Rheum. Dis. 70, 356–365 (2011).
Clemente-Casares, X. & Santamaria, P. Nanomedicine in autoimmunity. Immunol. Lett. 158, 167–174 (2014).
Su, Y., Gao, J., Kaur, P. & Wang, Z. Neutrophils and macrophages as targets for development of nanotherapeutics in inflammatory diseases. Pharmaceutics 12, 1222 (2020).
Bornhofft, K. F., Viergutz, T., Kuhnle, A. & Galuska, S. P. Nanoparticles equipped with α2,8-linked sialic acid chains inhibit the release of neutrophil extracellular traps. Nanomaterials 9, 610 (2019).
Gorabi, A. M. et al. The therapeutic potential of nanoparticles to reduce inflammation in atherosclerosis. Biomolecules 9, 416 (2019).
Liu, T. et al. Ultrasmall copper-based nanoparticles for reactive oxygen species scavenging and alleviation of inflammation related diseases. Nat. Commun. 11, 2788 (2020).
Mestas, J. & Hughes, C. C. Of mice and not men: differences between mouse and human immunology. J. Immunol. 172, 2731–2738 (2004).
Moses, S. R., Adorno, J. J., Palmer, A. F. & Song, J. W. Vessel-on-a-chip models for studying microvascular physiology, transport, and function in vitro. Am. J. Physiol. Cell Physiol. 320, C92–C105 (2021).
Wimmer, R. A. et al. Human blood vessel organoids as a model of diabetic vasculopathy. Nature 565, 505–510 (2019).
Wimmer, R. A., Leopoldi, A., Aichinger, M., Kerjaschki, D. & Penninger, J. M. Generation of blood vessel organoids from human pluripotent stem cells. Nat. Protoc. 14, 3082–3100 (2019).
Regnier, P. et al. Targeting JAK/STAT pathway in Takayasu’s arteritis. Ann. Rheum. Dis. 79, 951–959 (2020).
Kuo, D. et al. HBEGF+ macrophages in rheumatoid arthritis induce fibroblast invasiveness. Sci. Transl. Med. 11, eaau8587 (2019).
Zhang, F. et al. Defining inflammatory cell states in rheumatoid arthritis joint synovial tissues by integrating single-cell transcriptomics and mass cytometry. Nat. Immunol. 20, 928–942 (2019).
Arazi, A. et al. The immune cell landscape in kidneys of patients with lupus nephritis. Nat. Immunol. 20, 902–914 (2019).
Der, E. et al. Tubular cell and keratinocyte single-cell transcriptomics applied to lupus nephritis reveal type I IFN and fibrosis relevant pathways. Nat. Immunol. 20, 915–927 (2019).
Stahl, P. L. et al. Visualization and analysis of gene expression in tissue sections by spatial transcriptomics. Science 353, 78–82 (2016).
Hartmann, F. J. & Bendall, S. C. Immune monitoring using mass cytometry and related high-dimensional imaging approaches. Nat. Rev. Rheumatol. 16, 87–99 (2020).
Francisco-Cruz, A., Parra, E. R., Tetzlaff, M. T. & Wistuba, I. I. Multiplex immunofluorescence assays. Methods Mol. Biol. 2055, 467–495 (2020).
Angelo, M. et al. Multiplexed ion beam imaging of human breast tumors. Nat. Med. 20, 436–442 (2014).
Giesen, C. et al. Highly multiplexed imaging of tumor tissues with subcellular resolution by mass cytometry. Nat. Methods 11, 417–422 (2014).
Wharton, K. A. Jr. et al. Tissue multiplex analyte detection in anatomic pathology–pathways to clinical implementation. Front. Mol. Biosci. 8, 672531 (2021).
Winterbourn, C. C., Hampton, M. B., Livesey, J. H. & Kettle, A. J. Modeling the reactions of superoxide and myeloperoxidase in the neutrophil phagosome: implications for microbial killing. J. Biol. Chem. 281, 39860–39869 (2006).
Narasaraju, T. et al. Excessive neutrophils and neutrophil extracellular traps contribute to acute lung injury of influenza pneumonitis. Am. J. Pathol. 179, 199–210 (2011).
Saitoh, T. et al. Neutrophil extracellular traps mediate a host defense response to human immunodeficiency virus-1. Cell Host Microbe 12, 109–116 (2012).
Branzk, N. et al. Neutrophils sense microbe size and selectively release neutrophil extracellular traps in response to large pathogens. Nat. Immunol. 15, 1017–1025 (2014).
Kho, S. et al. Circulating neutrophil extracellular traps and neutrophil activation are increased in proportion to disease severity in human malaria. J. Infect. Dis. 219, 1994–2004 (2019).
Papayannopoulos, V. Neutrophil extracellular traps in immunity and disease. Nat. Rev. Immunol. 18, 134–147 (2018).
Metzler, K. D., Goosmann, C., Lubojemska, A., Zychlinsky, A. & Papayannopoulos, V. A myeloperoxidase-containing complex regulates neutrophil elastase release and actin dynamics during NETosis. Cell Rep. 8, 883–896 (2014).
Papayannopoulos, V., Metzler, K. D., Hakkim, A. & Zychlinsky, A. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J. Cell Biol. 191, 677–691 (2010).
Boeltz, S. et al. To NET or not to NET: current opinions and state of the science regarding the formation of neutrophil extracellular traps. Cell Death Differ. 26, 395–408 (2019).
Opasawatchai, A. et al. Neutrophil activation and early features of NET formation are associated with dengue virus infection in human. Front. Immunol. 9, 3007 (2018).
Zhou, Y. et al. Evidence for a direct link between PAD4-mediated citrullination and the oxidative burst in human neutrophils. Sci. Rep. 8, 15228 (2018).
Shah, P. K. Inflammation, infection and atherosclerosis. Trends Cardiovasc. Med. 29, 468–472 (2019).
Gupta, A. K. et al. Activated endothelial cells induce neutrophil extracellular traps and are susceptible to NETosis-mediated cell death. FEBS Lett. 584, 3193–3197 (2010).
Quillard, T. et al. TLR2 and neutrophils potentiate endothelial stress, apoptosis and detachment: implications for superficial erosion. Eur. Heart J. 36, 1394–1404 (2015).
Moore, K. J., Sheedy, F. J. & Fisher, E. A. Macrophages in atherosclerosis: a dynamic balance. Nat. Rev. Immunol. 13, 709–721 (2013).
Liu, Y. et al. Myeloid-specific deletion of peptidylarginine deiminase 4 mitigates atherosclerosis. Front. Immunol. 9, 1680 (2018).
Josefs, T. et al. Neutrophil extracellular traps promote macrophage inflammation and impair atherosclerosis resolution in diabetic mice. JCI Insight 5, e134796 (2020).
Maugeri, N. et al. Activated platelets present high mobility group Box 1 to neutrophils, inducing autophagy and promoting the extrusion of neutrophil extracellular traps. J. Thromb. Haemost. 12, 2074–2088 (2014).
Pircher, J., Engelmann, B., Massberg, S. & Schulz, C. Platelet-neutrophil crosstalk in atherothrombosis. Thromb. Haemost. 119, 1274–1282 (2019).
Author information
Authors and Affiliations
Contributions
L.W. researched data for this article. R.L. and I.A.U. provided substantial contributions to discussions of content. L.W. and I.A.U. wrote the article. All authors reviewed and/or edited the manuscript before submission.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Rheumatology thanks C. Carmon-Rivera, C. Lood and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, L., Luqmani, R. & Udalova, I.A. The role of neutrophils in rheumatic disease-associated vascular inflammation. Nat Rev Rheumatol 18, 158–170 (2022). https://doi.org/10.1038/s41584-021-00738-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41584-021-00738-4
This article is cited by
-
Neutrophils as emerging protagonists and targets in chronic inflammatory diseases
Inflammation Research (2022)