Abstract
Systemic lupus erythematosus (SLE) is an autoimmune disease with heterogeneous clinical manifestations that can potentially affect every organ and system. SLE is usually identified on the basis of clinical or serological manifestations; however, some individuals can present with signs and symptoms that are consistent with SLE but are not sufficient for a definite diagnosis. Disease in these individuals can either progress over time to definite SLE or remain stable, in which case their disease is often described as intermediate, possible or probable SLE. Alternatively, such individuals might have undifferentiated connective tissue disease (UCTD). Being able to differentiate between those with stable UCTD and those with SLE at an early stage is important to avoid irreversible target-organ damage from occurring. This Review provides insight into existing and evolving perceptions of the early stages of SLE, including clinical and mechanistic considerations, as well as potential paths towards early identification and intervention. Further research into the earliest phases of SLE will be important for the development of targeted diagnostic approaches and biomarkers for the identification of individuals with early disease who are likely to progress to definite SLE.
Key points
-
Systemic lupus erythematosus (SLE) is a complex autoimmune condition characterized by autoantibody production that can precede disease by several years and heterogeneous clinical manifestations.
-
A considerable proportion of individuals have clinical and serological features that are suggestive of a systemic autoimmune disorder, but cannot be diagnosed as having a defined connective tissue disease.
-
Nomenclature used to define such individuals has been inconsistent, with terms such as latent, incomplete, possible and probable SLE being used, as well as undifferentiated connective tissue disease (UCTD).
-
Although discriminating between UCTD and early SLE can be challenging, the presence of some features (such as a malar rash or specific autoantibodies) would tip the diagnosis in favour of SLE.
-
The absence of new clinical and laboratory manifestations over a 3-year period and the lack of a connective tissue disease-specific serological profile could support a diagnosis of UCTD.
-
The specificity of definitions of SLE-spectrum disorders should improve in the future as more molecular data become available with which disease subgroups can be defined.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Wandstrat, A. E. et al. Autoantibody profiling to identify individuals at risk for systemic lupus erythematosus. J. Autoimmun. 27, 153–160 (2006).
Antunes, M. et al. Undifferentiated connective tissue disease: state of the art on clinical practice guidelines. RMD Open. 4 (Suppl. 1), e000786 (2019).
Combe, B. et al. 2016 update of the EULAR recommendations for the management of early arthritis. Ann. Rheum. Dis. 76, 948–959 (2017).
Kamataki, N. et al. Complement genes contribute sex-biased vulnerability in diverse disorders. Nature 582, 577–581 (2020).
Zhao, Z. et al. Nature of T cell epitopes in lupus antigens and HLA-DR determines autoantibody initiation and diversification. Ann. Rheum. Dis. 78, 380–390 (2019).
Jog, N. R. et al. Association of Epstein-Barr virus serological reactivation with transitioning to systemic lupus erythematosus in at-risk individuals. Ann. Rheum. Dis. 78, 1235–1241 (2019).
Mosca, M., Tani, C. & Bombardieri, S. Undifferentiated connective tissue diseases (UCTD): a new frontier for rheumatology. Best. Pract. Res. Clin. Rheumatol. 21, 1011–1023 (2007).
Carneiro, A. C., Ruiz, M. M., Freitas, S. & Isenberg, D. A comparison of three classification criteria sets for systemic lupus erythematosus–a study looking at links to outcome and mortality. Arthritis Care Res. 72, 1611–1614 (2019).
Adamichou, C. et al. In an early SLE cohort the ACR-1997, SLICC-2012 and EULAR/ACR-2019 criteria classify non-overlapping groups of patients: use of all three criteria ensures optimal capture for clinical studies while their modification earlier classification and treatment. Ann. Rheum. Dis. 79, 232–241 (2020).
Aringer, M. et al. 2019 European League Against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Ann. Rheum. Dis. 78, 1151–1159 (2019).
Mosca, M., Neri, R. & Bombardieri, S. Undifferentiated connective tissue diseases (UCTD): a review of the literature and a proposal for preliminary classification criteria. Clin. Exp. Rheumatol. 17, 615–620 (1999).
Mosca, M., Tani, C., Carli, L. & Bombardieri, S. Undifferentiated CTD: a wide spectrum of autoimmune diseases. Best. Pract. Res. Clin. Rheumatol. 26, 73–77 (2012).
Mosca, M., Tani, C., Talarico, R. & Bombardieri, S. Undifferentiated connective tissue diseases (UCTD): simplified systemic autoimmune diseases. Autoimmun. Rev. 10, 256–258 (2011).
Radin, M. et al. A multicentre study of 244 pregnancies in undifferentiated connective tissue disease: maternal/foetal outcomes and disease evolution. Rheumatology 59, 2412–2418 (2020).
Bortoluzzi, A., Furini, F., Campanaro, F. & Govoni, M. Application of SLICC classification criteria in undifferentiated connective tissue disease and evolution in systemic lupus erythematosus: analysis of a large monocentric cohort with a long-term follow-up. Lupus 26, 616–622 (2017).
Vaz, C. C. et al. Undifferentiated connective tissue disease: a seven-center cross-sectional study of 184 patients. Clin. Rheumatol. 28, 915–921 (2009).
Mosca, M., Tani, C., Vagnani, S., Carli, L. & Bombardieri, S. The diagnosis and classification of undifferentiated connective tissue diseases. J. Autoimmun. 48–49, 50–52 (2014).
Bourn, R. & James, J. A. Preclinical lupus. Curr. Opin. Rheumatol. 27, 433–439 (2015).
Radin, M. et al. Impact of the 2019 European Alliance of Associations for Rheumatology/American College of Rheumatology classification criteria for systemic lupus erythematosus in a multicenter cohort study of 133 women with undifferentiated connective tissue disease. Arthritis Care Res. https://doi.org/10.1002/acr.24391 (2020).
Hallengren, C. S., Nived, O. & Sturfelt, G. Outcome of incomplete systemic lupus erythematosus after 10 years. Lupus 13, 85–88 (2004).
Md Yusof, M. Y. et al. Prediction of autoimmune connective tissue disease in an at-risk cohort: prognostic value of a novel two-score system for interferon status. Ann. Rheum. Dis. 77, 1432–1439 (2018).
Mosca, M., Tavoni, A., Neri, R., Bencivelli, W. & Bombardieri, S. Undifferentiated connective tissue diseases: the clinical and serological profiles of 91 patients followed for at least 1 year. Lupus 7, 95–100 (1998).
Rúa-Figueroa, Í. et al. Comprehensive description of clinical characteristics of a large systemic lupus erythematosus cohort from the Spanish Rheumatology Society Lupus Registry (RELESSER) with emphasis on complete versus incomplete lupus differences. Medicine 94, e267 (2015).
Rasmussen, A. et al. The lupus family registry and repository. Rheumatology 50, 47–59 (2011).
Tan, E. M. et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 25, 1271–1277 (1982).
Petri, M. et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 64, 2677–2686 (2012).
Aberle, T. et al. Use of SLICC criteria in a large, diverse lupus registry enables SLE classification of a subset of ACR-designated subjects with incomplete lupus. Lupus Sci. Med. 4, e000176 (2017).
Chen, Z. et al. Organ damage in patients with incomplete lupus syndromes: from a Chinese academic center. Clin. Rheumatol. 34, 1383–1389 (2015).
Rees, F. et al. Early clinical features in systemic lupus erythematosus: can they be used to achieve earlier diagnosis? A risk prediction model. Arthritis Care Res. 69, 833–841 (2017).
García-González, M., Rodríguez-Lozano, B., Bustabad, S. & Ferraz-Amaro, I. Undifferentiated connective tissue disease: predictors of evolution into definite disease. Clin. Exp. Rheumatol. 35, 739–745 (2017).
Drehmel, K. R. et al. Applying SLICC and ACR/EULAR systemic lupus erythematosus classification criteria in a cohort of patients with undifferentiated connective tissue disease. Lupus 30, 280–284 (2021).
Mosca, M. et al. Brief report: how do patients with newly diagnosed systemic lupus erythematosus present? A multicenter cohort of early systemic lupus erythematosus to inform the development of new classification criteria. Arthritis Rheumatol. 71, 91–98 (2019).
Nightingale, A. L., Davidson, J. E., Molta, C. T., Kan, H. J. & McHugh, N. J. Presentation of SLE in UK primary care using the Clinical Practice Research Datalink. Lupus Sci. Med. 4, e000172 (2017).
Negrini, S. et al. Sjögren’s syndrome: a systemic autoimmune disease. Clin. Exp. Med. https://doi.org/10.1007/S10238-021-00728-6 (2021).
Sebastiani, M. et al. Interstitial pneumonia with autoimmune features: why rheumatologist-pulmonologist collaboration is essential. Biomedicines 9, 17 (2020).
Fischer, A. Interstitial pneumonia with autoimmune features. Clin. Chest Med. 40, 609–616 (2019).
Satoh, M. et al. Clinical implication of autoantibodies in patients with systemic rheumatic diseases. Expert Rev. Clin. Immunol. 3, 721–738 (2007).
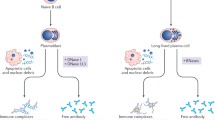
Arbuckle, M. R. et al. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N. Engl. J. Med. 349, 1526–1533 (2003).
Lu, R. et al. Dysregulation of innate and adaptive serum mediators precedes systemic lupus erythematosus classification and improves prognostic accuracy of autoantibodies. J. Autoimmun. 74, 182–193 (2016).
Landolt-Marticorena, C. The need for preclinical biomarkers in systemic autoimmune rheumatic diseases. J. Rheumatol. 42, 152–154 (2015).
Cavazzana, I. et al. Undifferentiated connective tissue disease with antibodies to Ro/SSa: clinical features and follow-up of 148 patients. Clin. Exp. Rheumatol. 19, 403–409 (2001).
Belfiore, N. et al. Anti-Ro(SS-A) 52 kDa and 60 kDa specificities in undifferentiated connective tissue disease. Jt. Bone Spine 67, 183–187 (2000).
Murng, S. H. K. & Thomas, M. Clinical associations of the positive anti Ro52 without Ro60 autoantibodies: undifferentiated connective tissue diseases. J. Clin. Pathol. 71, 12–19 (2018).
Alarcón, G. S. et al. Early undifferentiated connective tissue disease. I. Early clinical manifestation in a large cohort of patients with undifferentiated connective tissue diseases compared with cohorts of well established connective tissue disease. J. Rheumatol. 18, 1332–1339 (1991).
McClain, M. T. et al. The prevalence, onset, and clinical significance of antiphospholipid antibodies prior to diagnosis of systemic lupus erythematosus. Arthritis Rheum. 50, 1226–1232 (2004).
Van Gaalen, F. A. et al. Autoantibodies to cyclic citrullinated peptides predict progression to rheumatoid arthritis in patients with undifferentiated arthritis: a prospective cohort study. Arthritis Rheum. 50, 709–715 (2004).
Allenbach, Y., Benveniste, O., Goebel, H. H. & Stenzel, W. Integrated classification of inflammatory myopathies. Neuropathol. Appl. Neurobiol. 43, 62–81 (2017).
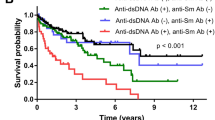
Andrejevic, S. et al. Immunoserological parameters in SLE: high-avidity anti-dsDNA detected by ELISA are the most closely associated with the disease activity. Clin. Rheumatol. 32, 1619–1626 (2013).
Aletaha, D. et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann. Rheum. Dis. 69, 1580–1588 (2010).
Biliavska, I. et al. Application of the 2010 ACR/EULAR classification criteria in patients with very early inflammatory arthritis: analysis of sensitivity, specificity and predictive values in the SAVE study cohort. Ann. Rheum. Dis. 72, 1335–1341 (2013).
Avouac, J. et al. Preliminary criteria for the very early diagnosis of systemic sclerosis: results of a Delphi consensus study from EULAR scleroderma trials and research group. Ann. Rheum. Dis. 70, 476–481 (2011).
Low, E. S. H., Krishnaswamy, G. & Thumboo, J. Comparing the 1997 update of the 1982 American College of Rheumatology (ACR-97) and the 2012 Systemic Lupus International Collaborating Clinics (SLICC-12) criteria for systemic lupus erythematosus (SLE) classification: which enables earlier classification. Lupus 28, 11–18 (2019).
Gatto, M., Saccon, F., Zen, M., Iaccarino, L. & Doria, A. Preclinical and early systemic lupus erythematosus. Best. Pract. Res. Clin. Rheumatol. 33, 101422 (2019).
Robertson, J. M. & James, J. A. Preclinical systemic lupus erythematosus. Rheum. Dis. Clin. North. Am. 40, 621–635 (2014).
Trapiella-Martínez, L. et al. Very early and early systemic sclerosis in the Spanish scleroderma Registry (RESCLE) cohort. Autoimmu. Rev. 16, 796–802 (2017).
Oglesby, A. et al. Impact of early versus late systemic lupus erythematosus diagnosis on clinical and economic outcomes. Appl. Health Econ. Health Policy 12, 179–190 (2014).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03543839 (2020).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03804723 (2019).
James, J. A. et al. Hydroxychloroquine sulfate treatment is associated with later onset of systemic lupus erythematosus. Lupus 16, 401–409 (2007).
Yan, Q. et al. Prevention of immune nephritis by the small molecular weight immunomodulator iguratimod in MRL/lpr mice. PLoS ONE 9, e108273 (2014).
Perper, S. J. et al. Treatment with a CD40 antagonist antibody reverses severe proteinuria and loss of saliva production and restores glomerular morphology in murine systemic lupus erythematosus. J. Immunol. 203, 58–75 (2019).
Durcan, L. & Petri, M. Immunomodulators in SLE: clinical evidence and immunologic actions. J. Autoimmun. 74, 73–84 (2016).
Doria, A. et al. SLE diagnosis and treatment: when early is early. Autoimmun. Rev. 10, 55–60 (2010).
Pengo, V. et al. What have we learned about antiphospholipid syndrome from patients and antiphospholipid carrier cohorts? Semin. Thromb. Hemost. 38, 322–327 (2012).
El-Sherbiny, Y. M. et al. A novel two-score system for interferon status segregates autoimmune diseases and correlates with clinical features. Sci. Rep. 8, 5793 (2018).
Pisetsky, D. S., Rovin, B. H. & Lipsky, P. E. New perspectives in rheumatology: biomarkers as entry criteria for clinical trials of new therapies for systemic lupus erythematosus: the example of antinuclear antibodies and anti-DNA. Arthritis Rheumatol. 69, 487–493 (2017).
Pérez, D. et al. Antinuclear antibodies: is the indirect immunofluorescence still the gold standard or should be replaced by solid phase assays? Autoimmun. Rev. 17, 548–552 (2018).
van Vollenhoven, R. F. et al. Efficacy and safety of ustekinumab, an IL-12 and IL-23 inhibitor, in patients with active systemic lupus erythematosus: results of a multicentre, double-blind, phase 2, randomised, controlled study. Lancet 392, 1330–1339 (2018).
Banchereau, R. et al. Personalized immunomonitoring uncovers molecular networks that stratify lupus patients. Cell 165, 551–565 (2016).
Agarwal, A., Ressler, D. & Snyder, G. The current and future state of companion diagnostics. Pharmacogenomics Pers. Med. 8, 99–110 (2015).
Jørgensen, J. T. & Hersom, M. Companion diagnostics — a tool to improve pharmacotherapy. Ann. Transl. Med. 4, 482 (2016).
Kraus, V. B. Biomarkers as drug development tools: discovery, validation, qualification and use. Nat. Rev. Rheumatol. 14, 354–362 (2018).
Munroe, M. E. et al. Altered type II interferon precedes autoantibody accrual and elevated type I interferon activity prior to systemic lupus erythematosus classification. Ann. Rheum. Dis. 75, 2014–2021 (2016).
Chyuan, I.-T., Tzeng, H.-T. & Chen, J.-Y. Signaling pathways of type I and type III interferons and targeted therapies in systemic lupus erythematosus. Cells 8, 963 (2019).
Gallucci, S., Meka, S. & Gamero, A. M. Abnormalities of the type I interferon signaling pathway in lupus autoimmunity. Cytokine 146, 155633 (2021).
Slight-Webb, S. et al. Autoantibody-positive healthy individuals display unique immune profiles that may regulate autoimmunity. Arthritis Rheumatol. 68, 2492–2502 (2016).
Luo, S., Wang, Y., Zhao, M. & Lu, Q. The important roles of type I interferon and interferon-inducible genes in systemic lupus erythematosus. Int. Immunopharmacol. 40, 542–549 (2016).
Wither, J. et al. Presence of an interferon signature in individuals who are anti-nuclear antibody positive lacking a systemic autoimmune rheumatic disease diagnosis. Arthritis Res. Ther. 19, 41 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02234388 (2021).
US National Library of Medicine. Clinicaltrials.gov https://clinicaltrials.gov/ct2/show/NCT03671174 (2019).
US National Library of Medicine. Clinicaltrials.gov https://clinicaltrials.gov/ct2/show/NCT04402086 (2021).
Greer, J. M. Incomplete lupus erythematosus. Arch. Intern. Med. 149, 2473 (1989).
Swaak, A. J. et al. Incomplete lupus erythematosus: results of a multicentre study under the supervision of the EULAR Standing Committee on International Clinical Studies Including Therapeutic Trials (ESCISIT). Rheumatology 40, 89–94 (2001).
Laustrup, H., Voss, A., Green, A. & Junker, P. SLE disease patterns in a Danish population-based lupus cohort: an 8-year prospective study. Lupus 19, 239–246 (2010).
Olsen, N. J. et al. Autoantibody profiling to follow evolution of lupus syndromes. Arthritis Res. Ther. 14, R174 (2012).
Calvo-Alen, J. et al. Systemic lupus erythematosus: predictors of its occurrence among a cohort of patients with early undifferentiated connective tissue disease: multivariate analyses and identification of risk factors. J. Rheumatol. 23, 469–475 (1996).
Danieli, M. G., Fraticelli, P., Salvi, A., Gabrielli, A. & Danieli, G. Undifferentiated connective tissue disease: natural history and evolution into definite CTD assessed in 84 patients initially diagnosed as early UCTD. Clin. Rheumatol. 17, 195–201 (1998).
Danieli, M. G. et al. Five-year follow-up of 165 Italian patients with undifferentiated connective tissue diseases. Clin. Exp. Rheumatol. 17, 585–591 (1999).
Williams, H. J. et al. Early undifferentiated connective tissue disease (CTD). VI. An inception cohort after 10 years: disease remissions and changes in diagnoses in well established and undifferentiated CTD. J. Rheumatol. 26, 816–825 (1999).
Bodolay, E. et al. Five-year follow-up of 665 Hungarian patients with undifferentiated connective tissue disease (UCTD). Clin. Exp. Rheumatol. 21, 313–230 (2003).
Guerrero, L. F., Rueda, J. C., Arciniegas, R. & Rueda, J. M. Undifferentiated connective tissue disease in a rheumatology center in Cali, Colombia: clinical features of 94 patients followed for a year. Rheumatol. Int. 33, 1085–1088 (2013).
Zucchi, D. et al. Pregnancy and undifferentiated connective tissue disease: outcome and risk of flare in 100 pregnancies. Rheumatology 59, 1335–1339 (2020).
Ganczarczyk, L., Urowitz, M. B. & Gladman, D. D. “Latent lupus”. J. Rheumatol. 4, 475–478 (1989).
Al Daabil, M. et al. Development of SLE among ‘potential SLE’ patients seen in consultation: long-term follow-up. Int. J. Clin. Pract. 68, 1508–1513 (2014).
Tiao, J., Feng, R., Carr, K., Okawa, J. & Werth, V. P. Using the American College of Rheumatology (ACR) and Systemic Lupus International Collaborating Clinics (SLICC) criteria to determine the diagnosis of systemic lupus erythematosus (SLE) in patients with subacute cutaneous lupus erythematosus (SCLE). J. Am. Acad. Dermatol. 74, 862–869 (2016).
Ramsey-Goldman, R. et al. Complement activation in patients with probable systemic lupus erythematosus and ability to predict progression to American College of Rheumatology-classified systemic lupus erythematosus. Arthritis Rheumatol. 72, 78–88 (2020).
Spinillo, A. et al. Prevalence of undiagnosed autoimmune rheumatic diseases in the first trimester of pregnancy. Results of a two-steps strategy using a self-administered questionnaire and autoantibody testing. BJOG 115, 51–57 (2008).
Mosca, M. et al. Pregnancy outcome in patients with undifferentiated connective tissue disease: a preliminary study on 25 pregnancies. Lupus 11, 304–307 (2002).
Spinillo, A. et al. The effect of newly diagnosed undifferentiated connective tissue disease on pregnancy outcome. Am. J. Obstet. Gynecol. 199, 632.e1–632.e6 (2008).
Grava, C. et al. Isolated congenital heart block in undifferentiated connective tissue disease and in primary Sjögren’s syndrome: a clinical study of 81 pregnancies in 41 patients [Italian]. Reumatismo 57, 180–186 (2005).
Castellino, G. et al. Pregnancy in patients with undifferentiated connective tissue disease: a prospective case-control study. Lupus 20, 1305–1311 (2011).
Spinillo, A. et al. The impact of unrecognized autoimmune rheumatic diseases on the incidence of preeclampsia and fetal growth restriction: a longitudinal cohort study. BMC Pregnancy Childbirth 16, 313 (2016).
Fredi, M. et al. First report of the Italian registry on immune-mediated congenital heart block (Lu.Ne Registry). Front. Cardiovasc. Med. 6, 11 (2019).
Brito-Zerón, P., Izmirly, P. M., Ramos-Casals, M., Buyon, J. P. & Khamashta, M. A. The clinical spectrum of autoimmune congenital heart block. Nat. Rev. Rheumatol. 11, 301–312 (2015).
Schreiber, K., Radin, M. & Sciascia, S. Current insights in obstetric antiphospholipid syndrome. Curr. Opin. Obstet. Gynecol. 29, 397–403 (2017).
Andreoli, L. et al. EULAR recommendations for women’s health and the management of family planning, assisted reproduction, pregnancy and menopause in patients with systemic lupus erythematosus and/or antiphospholipid syndrome. Ann. Rheum. Dis. 76, 476–485 (2017).
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Rheumatology thanks A. Rahman and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sciascia, S., Roccatello, D., Radin, M. et al. Differentiating between UCTD and early-stage SLE: from definitions to clinical approach. Nat Rev Rheumatol 18, 9–21 (2022). https://doi.org/10.1038/s41584-021-00710-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41584-021-00710-2
This article is cited by
-
Phosphopeptides P140 cause oxidative burst responses of pulmonary macrophages in an imiquimod-induced lupus model
Molecular Biomedicine (2023)
-
Undifferentiated Connective Tissue Disease: Comprehensive Review
Current Rheumatology Reports (2023)