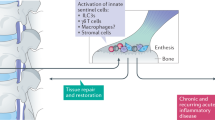
Abstract
Most rheumatic and musculoskeletal diseases (RMDs) can be placed along a spectrum of disorders, with autoinflammatory diseases (including monogenic systemic autoinflammatory diseases) and autoimmune diseases (such as systemic lupus erythematosus and antiphospholipid syndrome) representing the two ends of this spectrum. However, although most autoinflammatory diseases are characterized by the activation of innate immunity and inflammasomes and classical autoimmunity typically involves adaptive immune responses, there is some overlap in the features of autoimmunity and autoinflammation in RMDs. Indeed, some ‘mixed-pattern’ diseases such as spondyloarthritis and some forms of rheumatoid arthritis can also be delineated. A better understanding of the pathogenic pathways of autoinflammation and autoimmunity in RMDs, as well as the preferential cytokine patterns observed in these diseases, could help us to design targeted treatment strategies.
Key points
-
Rheumatic and musculoskeletal diseases (RMDs) form a continuum between classical autoimmune and autoinflammatory conditions.
-
Classical autoinflammatory and autoimmune diseases are associated with the activation of innate immunity and adaptive immune responses, respectively.
-
There are some ‘mixed-pattern’ disorders that carry the features of both autoimmune and autoinflammatory conditions, and one disorder might have autoimmune and autoinflammatory characteristics at different stages of disease development.
-
The autoimmune, autoinflammatory or mixed phenotype of RMDs might help us to develop and administer therapies targeted to specific disease phenotypes.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Hedrich, C. M. & Tsokos, G. C. Bridging the gap between autoinflammation and autoimmunity. Clin. Immunol. 147, 151–154 (2013).
Abbas, A., Lichtman A. H., Pillai S. Cellular and Molecular Immunology 9th edn (Elsevier, 2017).
Hedrich, C. M. Shaping the spectrum — from autoinflammation to autoimmunity. Clin. Immunol. 165, 21–28 (2016).
McGonagle, D. & McDermott, M. F. A proposed classification of the immunological diseases. PLoS Med. 3, e297 (2006).
Davidson, A. & Diamond, B. Autoimmune diseases. N. Engl. J. Med. 345, 340–350 (2001).
Masters, S. L. Broadening the definition of autoinflammation. Semin. Immunopathol. 37, 311–312 (2015).
Michels, A. W. & Gottlieb, P. A. Autoimmune polyglandular syndromes. Nat. Rev. Endocrinol. 6, 270–277 (2010).
McGonagle, D., Watad, A. & Savic, S. Mechanistic immunological based classification of rheumatoid arthritis. Autoimmun. Rev. 17, 1115–1123 (2018).
Savic, S. et al. Autoimmune-autoinflammatory rheumatoid arthritis overlaps: a rare but potentially important subgroup of diseases. RMD Open 3, e000550 (2017).
Smolen, J. S. et al. Rheumatoid arthritis. Nat. Rev. Dis. Prim. 4, 18001 (2018).
Kuek, A., Hazleman, B. L. & Ostor, A. J. Immune-mediated inflammatory diseases (IMIDs) and biologic therapy: a medical revolution. Postgrad. Med. J. 83, 251–260 (2007).
Szekanecz, Z., Szamosi, S., Kovacs, G. E., Kocsis, E. & Benko, S. The NLRP3 inflammasome–interleukin 1 pathway as a therapeutic target in gout. Arch. Biochem. Biophys. 670, 82–93 (2019).
Frizinsky, S. et al. The innate immune perspective of autoimmune and autoinflammatory conditions. Rheumatology 58, vi1–vi8 (2019).
Melki, I. & Fremond, M. L. Type I Interferonopathies: from a novel concept to targeted therapeutics. Curr. Rheumatol. Rep. 22, 32 (2020).
Tsokos, G. C. Systemic lupus erythematosus. N. Engl. J. Med. 365, 2110–2121 (2011).
Cook, H. T. & Botto, M. Mechanisms of disease: the complement system and the pathogenesis of systemic lupus erythematosus. Nat. Clin. Pract. Rheumatol. 2, 330–337 (2006).
David, T., Ling, S. F. & Barton, A. Genetics of immune-mediated inflammatory diseases. Clin. Exp. Immunol. 193, 3–12 (2018).
Crow, M. K. Advances in understanding the role of type I interferons in systemic lupus erythematosus. Curr. Opin. Rheumatol. 26, 467–474 (2014).
Crispin, J. C., Hedrich, C. M. & Tsokos, G. C. Gene-function studies in systemic lupus erythematosus. Nat. Rev. Rheumatol. 9, 476–484 (2013).
Brown, E. E., Edberg, J. C. & Kimberly, R. P. Fc receptor genes and the systemic lupus erythematosus diathesis. Autoimmunity 40, 567–581 (2007).
Trinchieri, G. Type I interferon: friend or foe? J. Exp. Med. 207, 2053–2063 (2010).
Brkic, Z. et al. The interferon type I signature is present in systemic sclerosis before overt fibrosis and might contribute to its pathogenesis through high BAFF gene expression and high collagen synthesis. Ann. Rheum. Dis. 75, 1567–1573 (2016).
Wu, M. & Assassi, S. The role of type 1 interferon in systemic sclerosis. Front. Immunol. 4, 266 (2013).
Greenberg, S. A. et al. Relationship between disease activity and type 1 interferon- and other cytokine-inducible gene expression in blood in dermatomyositis and polymyositis. Genes. Immun. 13, 207–213 (2012).
Wong, D. et al. Interferon and biologic signatures in dermatomyositis skin: specificity and heterogeneity across diseases. PLoS ONE 7, e29161 (2012).
Chiche, L. & Cornec, D. Mysterious uncoupled clinical symptoms and interferon signature in Sjogren’s syndrome: limitations of current approaches for unravelling complexity? Rheumatology 59, 5–6 (2019).
Brkic, Z. et al. Prevalence of interferon type I signature in CD14 monocytes of patients with Sjogren’s syndrome and association with disease activity and BAFF gene expression. Ann. Rheum. Dis. 72, 728–735 (2013).
Klareskog, L., Malmstrom, V., Lundberg, K., Padyukov, L. & Alfredsson, L. Smoking, citrullination and genetic variability in the immunopathogenesis of rheumatoid arthritis. Semin. Immunol. 23, 92–98 (2011).
Gordon, R. A., Grigoriev, G., Lee, A., Kalliolias, G. D. & Ivashkiv, L. B. The interferon signature and STAT1 expression in rheumatoid arthritis synovial fluid macrophages are induced by tumor necrosis factor α and counter-regulated by the synovial fluid microenvironment. Arthritis Rheum. 64, 3119–3128 (2012).
McInnes, I. B. & O’Dell, J. R. State-of-the-art: rheumatoid arthritis. Ann. Rheum. Dis. 69, 1898–1906 (2010).
Kahlenberg, J. M. & Kang, I. Advances in disease mechanisms and translational technologies: clinicopathologic significance of inflammasome activation in autoimmune diseases. Arthritis Rheumatol. 72, 386–395 (2020).
Kraetsch, H. G., Antoni, C., Kalden, J. R. & Manger, B. Successful treatment of a small cohort of patients with adult onset of Still’s disease with infliximab: first experiences. Ann. Rheum. Dis. 60 (Suppl. 3), iii55–iii57 (2001).
Betrains, A. et al. Systemic autoinflammatory disease in adults. Autoimmun. Rev. 20, 102774 (2021).
Krainer, J., Siebenhandl, S. & Weinhausel, A. Systemic autoinflammatory diseases. J. Autoimmun. 109, 102421 (2020).
Nirmala, N. et al. Gene-expression analysis of adult-onset Still’s disease and systemic juvenile idiopathic arthritis is consistent with a continuum of a single disease entity. Pediatr. Rheumatol. Online J. 13, 50 (2015).
Rowczenio, D. M. et al. Molecular genetic investigation, clinical features, and response to treatment in 21 patients with Schnitzler syndrome. Blood 131, 974–981 (2018).
Georgin-Lavialle, S. et al. Systemic autoinflammatory diseases: clinical state of the art. Best Pract. Res. Clin. Rheumatol. 34, 101529 (2020).
Ter Haar, N. M. et al. Development of the autoinflammatory disease damage index (ADDI). Ann. Rheum. Dis. 76, 821–830 (2017).
ter Haar, N. M. et al. Recommendations for the management of autoinflammatory diseases. Ann. Rheum. Dis. 74, 1636–1644 (2015).
Martinon, F. & Aksentijevich, I. New players driving inflammation in monogenic autoinflammatory diseases. Nat. Rev. Rheumatol. 11, 11–20 (2015).
Holzinger, D., Kessel, C., Omenetti, A. & Gattorno, M. From bench to bedside and back again: translational research in autoinflammation. Nat. Rev. Rheumatol. 11, 573–585 (2015).
Savic, S., Caseley, E. A. & McDermott, M. F. Moving towards a systems-based classification of innate immune-mediated diseases. Nat. Rev. Rheumatol. 16, 222–237 (2020).
Dinarello, C. A. The IL-1 family of cytokines and receptors in rheumatic diseases. Nat. Rev. Rheumatol. 15, 612–632 (2019).
Swanson, K. V., Deng, M. & Ting, J. P. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 19, 477–489 (2019).
Cudrici, C., Deuitch, N. & Aksentijevich, I. Revisiting TNF receptor-associated periodic syndrome (TRAPS): current perspectives. Int. J. Mol. Sci. 21, 3263 (2020).
McDermott, M. F. et al. Germline mutations in the extracellular domains of the 55 kDa TNF receptor, TNFR1, define a family of dominantly inherited autoinflammatory syndromes. Cell 97, 133–144 (1999).
Bruck, N., Schnabel, A. & Hedrich, C. M. Current understanding of the pathophysiology of systemic juvenile idiopathic arthritis (sJIA) and target-directed therapeutic approaches. Clin. Immunol. 159, 72–83 (2015).
Kessel, C., Hedrich, C. M. & Foell, D. Innately adaptive or truly autoimmune: is there something unique about systemic juvenile idiopathic arthritis? Arthritis Rheumatol. 72, 210–219 (2020).
Ter Haar, N. M., Jansen, M. H. A., Frenkel, J. F. & Vastert, S. J. How autoinflammation may turn into autoimmune inflammation: insights from monogenetic and complex IL-1 mediated auto-inflammatory diseases. Clin. Immunol. 219, 108538 (2020).
Nigrovic, P. A. Review: is there a window of opportunity for treatment of systemic juvenile idiopathic arthritis? Arthritis Rheumatol. 66, 1405–1413 (2014).
Rock, K. L., Kataoka, H. & Lai, J. J. Uric acid as a danger signal in gout and its comorbidities. Nat. Rev. Rheumatol. 9, 13–23 (2013).
Martinon, F., Petrilli, V., Mayor, A., Tardivel, A. & Tschopp, J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 440, 237–241 (2006).
Gerfaud-Valentin, M., Jamilloux, Y., Iwaz, J. & Seve, P. Adult-onset Still’s disease. Autoimmun. Rev. 13, 708–722 (2014).
Sighart, R. et al. Evidence for genetic overlap between adult onset Still’s disease and hereditary periodic fever syndromes. Rheumatol. Int. 38, 111–120 (2018).
Marshall, S. E. Behcet’s disease. Best Pract. Res. Clin. Rheumatol. 18, 291–311 (2004).
McGonagle, D., Aydin, S. Z., Gul, A., Mahr, A. & Direskeneli, H. ‘MHC-I-opathy’-unified concept for spondyloarthritis and Behcet disease. Nat. Rev. Rheumatol. 11, 731–740 (2015).
Gul, A. Behcet’s disease as an autoinflammatory disorder. Curr. Drug Targets Inflamm. Allergy 4, 81–83 (2005).
Bonnekoh, H. et al. Skin and systemic inflammation in Schnitzler’s syndrome are associated with neutrophil extracellular trap formation. Front. Immunol. 10, 546 (2019).
Simon, A. et al. Schnitzler’s syndrome: diagnosis, treatment, and follow-up. Allergy 68, 562–568 (2013).
Pathak, S. et al. Exploratory study of MYD88 L265P, rare NLRP3 variants, and clonal hematopoiesis prevalence in patients with Schnitzler syndrome. Arthritis Rheumatol. 71, 2121–2125 (2019).
Generali, E., Bose, T., Selmi, C., Voncken, J. W. & Damoiseaux, J. Nature versus nurture in the spectrum of rheumatic diseases: classification of spondyloarthritis as autoimmune or autoinflammatory. Autoimmun. Rev. 17, 935–941 (2018).
Deodhar, A., Miossec, P. & Baraliakos, X. Is undifferentiated spondyloarthritis a discrete entity? A debate. Autoimmun. Rev. 17, 29–32 (2018).
Chimenti, M. S. et al. Auto-reactions, autoimmunity and psoriatic arthritis. Autoimmun. Rev. 14, 1142–1146 (2015).
Bodnar, N. et al. Anti-mutated citrullinated vimentin (anti-MCV) and anti-65 kDa heat shock protein (anti-hsp65): new biomarkers in ankylosing spondylitis. Jt. Bone Spine 79, 63–66 (2012).
Liu, Y., Liao, X. & Shi, G. Autoantibodies in spondyloarthritis, focusing on anti-CD74 antibodies. Front. Immunol. 10, 5 (2019).
Schett, G., Elewaut, D., McInnes, I. B., Dayer, J. M. & Neurath, M. F. How cytokine networks fuel inflammation: toward a cytokine-based disease taxonomy. Nat. Med. 19, 822–824 (2013).
Pazar, B. et al. Association of ARTS1 gene polymorphisms with ankylosing spondylitis in the Hungarian population: the rs27044 variant is associated with HLA-B*2705 subtype in Hungarian patients with ankylosing spondylitis. J. Rheumatol. 37, 379–384 (2010).
Alippe, Y. & Mbalaviele, G. Omnipresence of inflammasome activities in inflammatory bone diseases. Semin. Immunopathol. 41, 607–618 (2019).
Szekanecz, Z. & Koch, A. E. Macrophages and their products in rheumatoid arthritis. Curr. Opin. Rheumatol. 19, 289–295 (2007).
Yang, C. A., Huang, S. T. & Chiang, B. L. Association of NLRP3 and CARD8 genetic polymorphisms with juvenile idiopathic arthritis in a Taiwanese population. Scand. J. Rheumatol. 43, 146–152 (2014).
Maria, A. T. et al. Adult onset Still’s disease (AOSD) in the era of biologic therapies: dichotomous view for cytokine and clinical expressions. Autoimmun. Rev. 13, 1149–1159 (2014).
Zhou, Q. et al. Loss-of-function mutations in TNFAIP3 leading to A20 haploinsufficiency cause an early-onset autoinflammatory disease. Nat. Genet. 48, 67–73 (2016).
Guo, C. et al. NLRP3 inflammasome activation contributes to the pathogenesis of rheumatoid arthritis. Clin. Exp. Immunol. 194, 231–243 (2018).
Mathews, R. J. et al. Evidence of NLRP3-inflammasome activation in rheumatoid arthritis (RA); genetic variants within the NLRP3-inflammasome complex in relation to susceptibility to RA and response to anti-TNF treatment. Ann. Rheum. Dis. 73, 1202–1210 (2014).
Dong, X. et al. ACPAs promote IL-1β production in rheumatoid arthritis by activating the NLRP3 inflammasome. Cell Mol. Immunol. 17, 261–271 (2019).
Kahlenberg, J. M. & Kaplan, M. J. The inflammasome and lupus: another innate immune mechanism contributing to disease pathogenesis? Curr. Opin. Rheumatol. 26, 475–481 (2014).
Kim, S. K., Cho, Y. J. & Choe, J. Y. NLRP3 inflammasomes and NLRP3 inflammasome-derived proinflammatory cytokines in peripheral blood mononuclear cells of patients with ankylosing spondylitis. Clin. Chim. Acta 486, 269–274 (2018).
Kastbom, A. et al. Genetic variants in CARD8 but not in NLRP3 are associated with ankylosing spondylitis. Scand. J. Rheumatol. 42, 465–468 (2013).
Strand, V. & Kavanaugh, A. F. The role of interleukin-1 in bone resorption in rheumatoid arthritis. Rheumatology 43 (Suppl. 3), iii10–iii16 (2004).
Watt, I. & Cobby, M. Treatment of rheumatoid arthritis patients with interleukin-1 receptor antagonist: radiologic assessment. Semin. Arthritis Rheum. 30, 21–25 (2001).
Guo, C. et al. Pathogenesis of lupus nephritis: RIP3 dependent necroptosis and NLRP3 inflammasome activation. J. Autoimmun. 103, 102286 (2019).
Deuteraiou, K., Kitas, G., Garyfallos, A. & Dimitroulas, T. Novel insights into the role of inflammasomes in autoimmune and metabolic rheumatic diseases. Rheumatol. Int. 38, 1345–1354 (2018).
Henderson, J. & O’Reilly, S. Inflammasome lights up in systemic sclerosis. Arthritis Res. Ther. 19, 205 (2017).
Martinez-Godinez, M. A. et al. Expression of NLRP3 inflammasome, cytokines and vascular mediators in the skin of systemic sclerosis patients. Isr. Med. Assoc. J. 17, 5–10 (2015).
Vakrakou, A. G. et al. Systemic activation of NLRP3 inflammasome in patients with severe primary Sjogren’s syndrome fueled by inflammagenic DNA accumulations. J. Autoimmun. 91, 23–33 (2018).
Yin, X., Han, G. C., Jiang, X. W., Shi, Q. & Pu, C. Q. Increased expression of the NOD-like receptor family, pyrin domain containing 3 inflammasome in dermatomyositis and polymyositis is a potential contributor to their pathogenesis. Chin. Med. J. 129, 1047–1052 (2016).
Barrat, F. J., Crow, M. K. & Ivashkiv, L. B. Interferon target-gene expression and epigenomic signatures in health and disease. Nat. Immunol. 20, 1574–1583 (2019).
Brinkmann, V. et al. Neutrophil extracellular traps kill bacteria. Science 303, 1532–1535 (2004).
Goel, R. R. & Kaplan, M. J. Deadliest catch: neutrophil extracellular traps in autoimmunity. Curr. Opin. Rheumatol. 32, 64–70 (2020).
Mutua, V. & Gershwin, L. J. A review of neutrophil extracellular traps (NETs) in disease: potential anti-NETs therapeutics. Clin. Rev. Allergy Immunol. https://doi.org/10.1007/s12016-020-08804-7 (2020).
Schett, G., Schauer, C., Hoffmann, M. & Herrmann, M. Why does the gout attack stop? A roadmap for the immune pathogenesis of gout. RMD Open 1, e000046 (2015).
Delgado-Rizo, V. et al. Neutrophil extracellular traps and its implications in inflammation: an overview. Front. Immunol. 8, 81 (2017).
Villanueva, E. et al. Netting neutrophils induce endothelial damage, infiltrate tissues, and expose immunostimulatory molecules in systemic lupus erythematosus. J. Immunol. 187, 538–552 (2011).
Gul, A. Dynamics of inflammatory response in autoinflammatory disorders: autonomous and hyperinflammatory states. Front. Immunol. 9, 2422 (2018).
Ikeda, S. et al. Excess IL-1 signaling enhances the development of TH17 cells by downregulating TGF-β-induced Foxp3 expression. J. Immunol. 192, 1449–1458 (2014).
Netea, M. G. et al. Trained immunity: a program of innate immune memory in health and disease. Science 352, aaf1098 (2016).
Dougados, M. et al. Prevalence of comorbidities in rheumatoid arthritis and evaluation of their monitoring: results of an international, cross-sectional study (COMORA). Ann. Rheum. Dis. 73, 62–68 (2014).
Radner, H., Yoshida, K., Smolen, J. S. & Solomon, D. H. Multimorbidity and rheumatic conditions — enhancing the concept of comorbidity. Nat. Rev. Rheumatol. 10, 252–256 (2014).
Szekanecz, Z. et al. Autoimmune atherosclerosis in 3D: how it develops, how to diagnose and what to do. Autoimmun. Rev. 15, 756–769 (2016).
Szekanecz, Z., Kerekes, G., Kardos, Z., Baráth, Z. & Tamási, L. Mechanisms of inflammatory atherosclerosis in rheumatoid arthritis. Curr. Immunol. Rev. 12, 35–46 (2016).
Agca, R. et al. EULAR recommendations for cardiovascular disease risk management in patients with rheumatoid arthritis and other forms of inflammatory joint disorders: 2015/2016 update. Ann. Rheum. Dis. 76, 17–28 (2017).
Geraldino-Pardilla, L. et al. Association of anti-citrullinated peptide antibodies with coronary artery calcification in rheumatoid arthritis. Arthritis Care Res. 69, 1276–1281 (2017).
Sokolove, J. et al. Brief report: citrullination within the atherosclerotic plaque: a potential target for the anti-citrullinated protein antibody response in rheumatoid arthritis. Arthritis Rheum. 65, 1719–1724 (2013).
Spinelli, F. R. et al. Association between antibodies to carbamylated proteins and subclinical atherosclerosis in rheumatoid arthritis patients. BMC Musculoskelet. Disord. 18, 214 (2017).
Lopez-Mejias, R. et al. Influence of elevated-CRP level-related polymorphisms in non-rheumatic Caucasians on the risk of subclinical atherosclerosis and cardiovascular disease in rheumatoid arthritis. Sci. Rep. 6, 31979 (2016).
Kastbom, A., Arlestig, L. & Rantapaa-Dahlqvist, S. Genetic variants of the NLRP3 inflammasome are associated with stroke in patients with rheumatoid arthritis. J. Rheumatol. 42, 1740–1745 (2015).
Paramel Varghese, G. et al. NLRP3 inflammasome expression and activation in human atherosclerosis. J. Am. Heart Assoc. 5, e003031 (2016).
Ahmed, A. et al. Brief report: proatherogenic cytokine microenvironment in the aortic adventitia of patients with rheumatoid arthritis. Arthritis Rheumatol. 68, 1361–1366 (2016).
Blankenberg, S. et al. Interleukin-18 is a strong predictor of cardiovascular death in stable and unstable angina. Circulation 106, 24–30 (2002).
Cacoub, P. & Marques, C. Acute recurrent pericarditis: from pathophysiology towards new treatment strategy. Heart 106, 1046–1051 (2020).
Kontzias, A., Barkhodari, A. & Yao, Q. Pericarditis in systemic rheumatologic diseases. Curr. Cardiol. Rep. 22, 142 (2020).
Cantarini, L. et al. Autoimmunity and autoinflammation as the yin and yang of idiopathic recurrent acute pericarditis. Autoimmun. Rev. 14, 90–97 (2015).
Mecoli, C. A. & Christopher-Stine, L. Management of interstitial lung disease in patients with myositis specific autoantibodies. Curr. Rheumatol. Rep. 20, 27 (2018).
Castillo-Tandazo, W., Gonzalez, J. & Flores-Fortty, A. Pathogenesis and therapeutics of interstitial lung disease in systemic sclerosis. Curr. Rheumatol. Rev. 9, 105–112 (2013).
Lasithiotaki, I. et al. NLRP3 inflammasome expression in idiopathic pulmonary fibrosis and rheumatoid lung. Eur. Respir. J. 47, 910–918 (2016).
Duarte-Delgado, N. P., Vasquez, G. & Ortiz-Reyes, B. L. Blood–brain barrier disruption and neuroinflammation as pathophysiological mechanisms of the diffuse manifestations of neuropsychiatric systemic lupus erythematosus. Autoimmun. Rev. 18, 426–432 (2019).
Masson, C. et al. Adult Still’s disease: part I. Manifestations and complications in sixty-five cases in France. Rev. Rhum. Engl. Ed. 62, 748–757 (1995).
Szentpetery, A. et al. Effects of targeted therapies on the bone in arthritides. Autoimmun. Rev. 16, 313–320 (2017).
Dinarello, C. A. & van der Meer, J. W. Treating inflammation by blocking interleukin-1 in humans. Semin. Immunol. 25, 469–484 (2013).
Cantarini, L. et al. Interleukin-1: Ariadne’s thread in autoinflammatory and autoimmune disorders. Isr. Med. Assoc. J. 17, 93–97 (2015).
Schett, G., Dayer, J. M. & Manger, B. Interleukin-1 function and role in rheumatic disease. Nat. Rev. Rheumatol. 12, 14–24 (2016).
European Commission. Ilaris alkalmazási előírás. https://ec.europa.eu/health/documents/community-register/2016/20160801135455/anx_135455_hu.pdf (2016).
Kuemmerle-Deschner, J. B. et al. Canakinumab (ACZ885, a fully human IgG1 anti-IL-1β mAb) induces sustained remission in pediatric patients with cryopyrin-associated periodic syndrome (CAPS). Arthritis Res. Ther. 13, R34 (2011).
Hoffman, H. M. et al. Long-term efficacy and safety profile of rilonacept in the treatment of cryopryin-associated periodic syndromes: results of a 72-week open-label extension study. Clin. Ther. 34, 2091–2103 (2012).
Hoffman, H. M. et al. Efficacy and safety of rilonacept (interleukin-1 Trap) in patients with cryopyrin-associated periodic syndromes: results from two sequential placebo-controlled studies. Arthritis Rheum. 58, 2443–2452 (2008).
Kuemmerle-Deschner, J. B. et al. Efficacy and safety of anakinra therapy in pediatric and adult patients with the autoinflammatory Muckle–Wells syndrome. Arthritis Rheum. 63, 840–849 (2011).
Schlesinger, N. Canakinumab in gout. Expert Opin. Biol. Ther. 12, 1265–1275 (2012).
Terkeltaub, R. et al. The interleukin 1 inhibitor rilonacept in treatment of chronic gouty arthritis: results of a placebo-controlled, monosequence crossover, non-randomised, single-blind pilot study. Ann. Rheum. Dis. 68, 1613–1617 (2009).
So, A., De Smedt, T., Revaz, S. & Tschopp, J. A pilot study of IL-1 inhibition by anakinra in acute gout. Arthritis Res. Ther. 9, R28 (2007).
Vitale, A., Cantarini, L., Rigante, D., Bardelli, M. & Galeazzi, M. Anakinra treatment in patients with gout and type 2 diabetes. Clin. Rheumatol. 34, 981–984 (2015).
Ruperto, N. et al. Two randomized trials of canakinumab in systemic juvenile idiopathic arthritis. N. Engl. J. Med. 367, 2396–2406 (2012).
Ruperto, N. et al. A phase II, multicenter, open-label study evaluating dosing and preliminary safety and efficacy of canakinumab in systemic juvenile idiopathic arthritis with active systemic features. Arthritis Rheum. 64, 557–567 (2012).
Yokota, S. et al. Efficacy and safety of tocilizumab in patients with systemic-onset juvenile idiopathic arthritis: a randomised, double-blind, placebo-controlled, withdrawal phase III trial. Lancet 371, 998–1006 (2008).
Quartier, P. et al. A multicentre, randomised, double-blind, placebo-controlled trial with the interleukin-1 receptor antagonist anakinra in patients with systemic-onset juvenile idiopathic arthritis (ANAJIS trial). Ann. Rheum. Dis. 70, 747–754 (2011).
Ilowite, N. T. et al. Randomized, double-blind, placebo-controlled trial of the efficacy and safety of rilonacept in the treatment of systemic juvenile idiopathic arthritis. Arthritis Rheumatol. 66, 2570–2579 (2014).
Kedor, C. et al. Canakinumab for Treatment of Adult-Onset Still’s Disease to Achieve Reduction of Arthritic Manifestation (CONSIDER): phase II, randomised, double-blind, placebo-controlled, multicentre, investigator-initiated trial. Ann. Rheum. Dis. 79, 1090–1097 (2020).
Junge, G., Mason, J. & Feist, E. Adult onset Still’s disease — the evidence that anti-interleukin-1 treatment is effective and well-tolerated (a comprehensive literature review). Semin. Arthritis Rheum. 47, 295–302 (2017).
Castaneda, S. et al. Tocilizumab for the treatment of adult-onset Still’s disease. Expert. Opin. Biol. Ther. 19, 273–286 (2019).
Vitale, A. et al. Interleukin-1 inhibition in Behcet’s disease. Isr. Med. Assoc. J. 18, 171–176 (2016).
de Koning, H. D. et al. Sustained efficacy of the monoclonal anti-interleukin-1β antibody canakinumab in a 9-month trial in Schnitzler’s syndrome. Ann. Rheum. Dis. 72, 1634–1638 (2013).
Garcia-Carrasco, M. et al. Use of rituximab in patients with systemic lupus erythematosus: an update. Autoimmun. Rev. 8, 343–348 (2009).
McQueen, F. M. & Solanki, K. Rituximab in diffuse cutaneous systemic sclerosis: should we be using it today? Rheumatology 54, 757–767 (2015).
Rios Fernandez, R., Callejas Rubio, J. L., Sanchez Cano, D., Saez Moreno, J. A. & Ortego Centeno, N. Rituximab in the treatment of dermatomyositis and other inflammatory myopathies. A report of 4 cases and review of the literature. Clin. Exp. Rheumatol. 27, 1009–1016 (2009).
Grigoriadou, S. et al. B cell depletion with rituximab in the treatment of primary Sjogren’s syndrome: what have we learnt? Clin. Exp. Rheumatol. 37 (Suppl 118), 217–224 (2019).
Navarra, S. V. et al. Efficacy and safety of belimumab in patients with active systemic lupus erythematosus: a randomised, placebo-controlled, phase 3 trial. Lancet 377, 721–731 (2011).
Pimentel-Quiroz, V. R., Ugarte-Gil, M. F. & Alarcon, G. S. Abatacept for the treatment of systemic lupus erythematosus. Expert Opin. Invest. Drugs 25, 493–499 (2016).
Boleto, G., Allanore, Y. & Avouac, J. Targeting costimulatory pathways in systemic sclerosis. Front. Immunol. 9, 2998 (2018).
Machado, A. C. et al. Effectiveness and safety of abatacept for the treatment of patients with primary Sjogren’s syndrome. Clin. Rheumatol. 39, 243–248 (2019).
Khanna, D. et al. Safety and efficacy of subcutaneous tocilizumab in systemic sclerosis: results from the open-label period of a phase II randomised controlled trial (faSScinate). Ann. Rheum. Dis. 77, 212–220 (2018).
Alten, R. & Maleitzke, T. Tocilizumab: a novel humanized anti-interleukin 6 (IL-6) receptor antibody for the treatment of patients with non-RA systemic, inflammatory rheumatic diseases. Ann. Med. 45, 357–363 (2013).
Illei, G. G. et al. Tocilizumab in systemic lupus erythematosus: data on safety, preliminary efficacy, and impact on circulating plasma cells from an open-label phase I dosage-escalation study. Arthritis Rheum. 62, 542–552 (2010).
Jamilloux, Y. et al. JAK inhibitors for the treatment of autoimmune and inflammatory diseases. Autoimmun. Rev. 18, 102390 (2019).
Chauhan, D., Vande Walle, L. & Lamkanfi, M. Therapeutic modulation of inflammasome pathways. Immunol. Rev. 297, 123–138 (2020).
Acknowledgements
This work was supported by the European Union Social Fund TAMOP-4.2.4.A/2-11/1-2012-0001 ‘National Excellence Program’ and the European Union GINOP-2.3.2-15-2016-00015 and GINOP-2.3.2-15-2016-00050 grants (to Z.S.). It was also supported by the Hungarian National Scientific Research Fund (NKFIH-OTKA Grant No. K131844 to S.B.) and the Faculty of Medicine of the University of Debrecen (1G3DBKD0TUDF 247 to S.B.).
Author information
Authors and Affiliations
Contributions
All authors contributed to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Rheumatology thanks A. Doria, S. Savic and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Szekanecz, Z., McInnes, I.B., Schett, G. et al. Autoinflammation and autoimmunity across rheumatic and musculoskeletal diseases. Nat Rev Rheumatol 17, 585–595 (2021). https://doi.org/10.1038/s41584-021-00652-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41584-021-00652-9
This article is cited by
-
Efficacy and safety of gut microbiota-based therapies in autoimmune and rheumatic diseases: a systematic review and meta-analysis of 80 randomized controlled trials
BMC Medicine (2024)
-
The diagnostic role of the systemic inflammation index in patients with immunological diseases: a systematic review and meta-analysis
Clinical and Experimental Medicine (2024)
-
Renal manifestations in adult-onset Still’s disease: a systematic review
Rheumatology International (2024)
-
Disease stratification in GCA and PMR: state of the art and future perspectives
Nature Reviews Rheumatology (2023)
-
Post-transcriptional checkpoints in autoimmunity
Nature Reviews Rheumatology (2023)