Abstract
In autoimmune rheumatic diseases, oestrogens can stimulate certain immune responses (including effects on B cells and innate immunity), but can also have dose-related anti-inflammatory effects on T cells, macrophages and other immune cells. By contrast, androgens and progesterone have predominantly immunosuppressive and anti-inflammatory effects. Hormone replacement therapies and oral contraception (and also pregnancy) enhance or decrease the severity of autoimmune rheumatic diseases at a genetic or epigenetic level. Serum androgen concentrations are often low in men and in women with autoimmune rheumatic diseases, suggesting that androgen-like compounds might be a promising therapeutic approach. However, androgen-to-oestrogen conversion (known as intracrinology) is enhanced in inflamed tissues, such as those present in patients with autoimmune rheumatic diseases. In addition, it is becoming evident that the gut microbiota differs between the sexes (known as the microgenderome) and leads to sex-dependent genetic and epigenetic changes in gastrointestinal inflammation, systemic immunity and, potentially, susceptibility to autoimmune or inflammatory rheumatic diseases. Future clinical research needs to focus on the therapeutic use of androgens and progestins or their downstream signalling cascades and on new oestrogenic compounds such as tissue-selective oestrogen complex to modulate altered immune responses.
Key points
-
Oestrogens have both pro-inflammatory and anti-inflammatory effects, acting as stimulators of B cell-mediated immune responses but inhibitors of pro-inflammatory macrophages and some T cells.
-
In contrast to oestrogens, androgens and progesterone have immunosuppressive and anti-inflammatory effects.
-
In men and postmenopausal women with rheumatic diseases, increased androgen-to-oestrogen conversion in inflamed tissues and local oestrogen metabolite synthesis support disease.
-
Pregnancy, sex hormone replacement therapies and oral contraceptives can negatively or positively affect the severity of autoimmune rheumatic diseases, depending on the respective predominance of oestrogens or androgens (and progesterone).
-
Sex-dependent differences in gut microbiota may lead to genetic or epigenetic changes in local gastrointestinal inflammation, systemic immunity and susceptibility to a range of rheumatic diseases.
-
Therapies with androgens and progestins, selective oestrogen receptor modulators and tissue-selective oestrogen complex need to be tested more rigorously in autoimmune rheumatic diseases.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Hench, P. S. The ameliorating effect of pregnancy on chronic atrophic (infectious, rheumatoid) arthritis, fibrositis, and intermittent hydrarthrosis. Proc. Staff. Meet. Mayo Clin. 13, 161–167 (1938).
Whitacre, C. C. Sex differences in autoimmune disease. Nat. Immunol. 2, 777–780 (2001).
Benagiano, M., Bianchi, P., D’Elios, M. M., Brosens, I. & Benagiano, G. Autoimmune diseases: role of steroid hormones. Best Pract. Res. Clin. Obstet. Gynaecol. 60, 24–34 (2019).
Lockshin, M. D. Nonhormonal explanations for sex discrepancy in human illness. Ann. N. Y. Acad. Sci. 1193, 22–24 (2010).
Christou, E. A. A., Banos, A., Kosmara, D., Bertsias, G. K. & Boumpas, D. T. Sexual dimorphism in SLE: above and beyond sex hormones. Lupus 28, 3–10 (2019).
Lambert, N. C. Nonendocrine mechanisms of sex bias in rheumatic diseases. Nat. Rev. Rheumatol. 15, 673–686 (2019).
Cutolo, M. & Wilder, R. L. Different roles for androgens and estrogens in the susceptibility to autoimmune rheumatic diseases. Rheum. Dis. Clin. North Am. 26, 825–839 (2000).
Straub, R. H. The complex role of estrogens in inflammation. Endocr. Rev. 28, 521–574 (2007).
Cohen-Solal, J. F. et al. Hormonal regulation of B-cell function and systemic lupus erythematosus. Lupus 17, 528–532 (2008).
Cutolo, M. Androgens in rheumatoid arthritis: when are they effectors? Arthritis Res. Ther. 11, 126 (2009).
Islander, U., Jochems, C., Lagerquist, M. K., Forsblad-d’Elia, H. & Carlsten, H. Estrogens in rheumatoid arthritis; the immune system and bone. Mol. Cell. Endocrinol. 335, 14–29 (2011).
Cutolo, M., Sulli, A. & Straub, R. H. Estrogen metabolism and autoimmunity. Autoimmun. Rev. 11, A460–A464 (2012).
Konttinen, Y. T. et al. Sex steroids in Sjögren’s syndrome. J. Autoimmun. 39, 49–56 (2012).
Hughes, G. C. Progesterone and autoimmune disease. Autoimmun. Rev. 11, A502–A514 (2012).
Hughes, G. C. & Choubey, D. Modulation of autoimmune rheumatic diseases by oestrogen and progesterone. Nat. Rev. Rheumatol. 10, 740–751 (2014).
Trigunaite, A., Dimo, J. & Jorgensen, T. N. Suppressive effects of androgens on the immune system. Cell Immunol. 294, 87–94 (2015).
Gubbels Bupp, M. R. Sex, the aging immune system, and chronic disease. Cell. Immunol. 294, 102–110 (2015).
Lahita, R. G. The immunoendocrinology of systemic lupus erythematosus. Clin. Immunol. 172, 98–100 (2016).
Traish, A., Bolanos, J., Nair, S., Saad, F. & Morgentaler, A. Do androgens modulate the pathophysiological pathways of inflammation? Appraising the contemporary evidence. J. Clin. Med. 7, 549 (2018).
Gubbels Bupp, M. R. & Jorgensen, T. N. Androgen-induced immunosuppression. Front. Immunol. 9, 794 (2018).
Szekeres-Bartho, J. & Schindler, A. E. Progestogens and immunology. Best Pract. Res. Clin. Obstet. Gynaecol. 60, 17–23 (2019).
Castagnetta, L. A. et al. Increased estrogen formation and estrogen to androgen ratio in the synovial fluid of patients with rheumatoid arthritis. J. Rheumatol. 30, 2597–2605 (2003).
Olsen, N. J. & Kovacs, W. J. Gonadal steroids and immunity. Endocr. Rev. 17, 369–384 (1996).
Sanchez-Maldonado, J. M. et al. Steroid hormone-related polymorphisms associate with the development of bone erosions in rheumatoid arthritis and help to predict disease progression: results from the REPAIR consortium. Sci. Rep. 9, 14812 (2019).
Xie, Q. M. et al. Association of oestrogen receptor alpha gene polymorphisms with systemic lupus erythematosus risk: an updated meta-analysis. Microb. Pathog. 127, 352–358 (2019).
Lewis, M. J. et al. UBE2L3 polymorphism amplifies NF-κB activation and promotes plasma cell development, linking linear ubiquitination to multiple autoimmune diseases. Am. J. Hum. Genet. 96, 221–234 (2015).
Verma, S. et al. The ubiquitin-conjugating enzyme UBCH7 acts as a coactivator for steroid hormone receptors. Mol. Cell Biol. 24, 8716–8726 (2004).
Canet, L. M. et al. Polymorphisms at phase I-metabolizing enzyme and hormone receptor loci influence the response to anti-TNF therapy in rheumatoid arthritis patients. Pharmacogenomics J. 19, 83–96 (2019).
Stark, K. et al. CYB5A polymorphism increases androgens and reduces risk of rheumatoid arthritis in women. Arthritis Res. Ther. 17, 56 (2015).
Plenge, R. M. et al. TRAF1-C5 as a risk locus for rheumatoid arthritis — a genomewide study. N. Engl. J. Med. 357, 1199–1209 (2007).
Wellcome Trust Case Control Consortium. Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature 447, 661–678 (2007).
Jaaskelainen, J. Molecular biology of androgen insensitivity. Mol. Cell. Endocrinol. 352, 4–12 (2012).
Yu, S. F. et al. Association of tri-nucleotide (CAG and GGC) repeat polymorphism of androgen receptor gene in Taiwanese women with refractory or remission rheumatoid arthritis. Clin. Rheumatol. 26, 2051 (2007).
Karlson, E. W. et al. A prospective study of androgen levels, hormone-related genes and risk of rheumatoid arthritis. Arthritis Res. Ther. 11, R97 (2009).
Dziedziejko, V. et al. CAG repeat polymorphism in the androgen receptor gene in women with rheumatoid arthritis. J. Rheumatol. 39, 10–17 (2012).
Lo, S. F. et al. Androgen receptor gene polymorphism and rheumatoid arthritis in Taiwan. Clin. Exp. Rheumatol. 24, 209–210 (2006).
Kawasaki, T. et al. Polymorphic CAG repeats of the androgen receptor gene and rheumatoid arthritis. Ann. Rheum. Dis. 58, 500–502 (1999).
Tessnow, A. H., Olsen, N. J. & Kovacs, W. J. Expression of humoral autoimmunity is related to androgen receptor CAG repeat length in men with systemic lupus erythematosus. J. Clin. Immunol. 31, 567–573 (2011).
Olsen, N. J., Benko, A. L. & Kovacs, W. J. Variation in the androgen receptor gene exon 1 CAG repeat correlates with manifestations of autoimmunity in women with lupus. Endocr. Connect. 3, 99–109 (2014).
Robeva, R. et al. Androgen receptor (CAG)n polymorphism and androgen levels in women with systemic lupus erythematosus and healthy controls. Rheumatol. Int. 33, 2031–2038 (2013).
Yu, S. F. et al. Androgen receptor genetic variants in male patients with ankylosing spondylitis in Taiwan. Int. J. Rheum. Dis. 16, 81–87 (2013).
Mori, K., Ushiyama, T., Inoue, K. & Hukuda, S. Polymorphic CAG repeats of the androgen receptor gene in Japanese male patients with ankylosing spondylitis. Rheumatology 39, 530–532 (2000).
Barlow, D. P. Genomic imprinting: a mammalian epigenetic discovery model. Annu. Rev. Genet. 45, 379–403 (2011).
Liu, H. W. et al. Demethylation within the proximal promoter region of human estrogen receptor alpha gene correlates with its enhanced expression: implications for female bias in lupus. Mol. Immunol. 61, 28–37 (2014).
Wu, Z. et al. 17β-oestradiol enhances global DNA hypomethylation in CD4-positive T cells from female patients with lupus, through overexpression of oestrogen receptor-α-mediated downregulation of DNMT1. Clin. Exp. Dermatol. 39, 525–532 (2014).
Alivernini, S. et al. MicroRNA-155-at the critical interface of innate and adaptive immunity in arthritis. Front. Immunol. 8, 1932 (2017).
Dai, R. et al. Suppression of LPS-induced Interferon-γ and nitric oxide in splenic lymphocytes by select estrogen-regulated microRNAs: a novel mechanism of immune modulation. Blood 112, 4591–4597 (2008).
Dupuis, M. L. et al. The natural agonist of estrogen receptor β silibinin plays an immunosuppressive role representing a potential therapeutic tool in rheumatoid arthritis. Front. Immunol. 9, 1903 (2018).
Tong, W. W. et al. Silibinin alleviates inflammation and induces apoptosis in human rheumatoid arthritis fibroblast-like synoviocytes and has a therapeutic effect on arthritis in rats. Sci. Rep. 8, 3241 (2018).
Khan, D., Dai, R. & Ansar Ahmed, S. Sex differences and estrogen regulation of miRNAs in lupus, a prototypical autoimmune disease. Cell Immunol. 294, 70–79 (2015).
Zan, H., Tat, C. & Casali, P. MicroRNAs in lupus. Autoimmunity 47, 272–285 (2014).
Perniola, R. Twenty years of AIRE. Front. Immunol. 9, 98 (2018).
Dragin, N. et al. Estrogen-mediated downregulation of AIRE influences sexual dimorphism in autoimmune diseases. J. Clin. Invest. 126, 1525–1537 (2016).
Pauklin, S., Sernandez, I. V., Bachmann, G., Ramiro, A. R. & Petersen-Mahrt, S. K. Estrogen directly activates AID transcription and function. J. Exp. Med. 206, 99–111 (2009).
Jones, B. G. et al. Binding of estrogen receptors to switch sites and regulatory elements in the immunoglobulin heavy chain locus of activated B cells suggests a direct influence of estrogen on antibody expression. Mol. Immunol. 77, 97–102 (2016).
Baker, K. F. & Isaacs, J. D. Novel therapies for immune-mediated inflammatory diseases: what can we learn from their use in rheumatoid arthritis, spondyloarthritis, systemic lupus erythematosus, psoriasis, Crohn’s disease and ulcerative colitis? Ann. Rheum. Dis. 77, 175–187 (2018).
Joosten, L. A., Abdollahi-Roodsaz, S., Dinarello, C. A., O’Neill, L. & Netea, M. G. Toll-like receptors and chronic inflammation in rheumatic diseases: new developments. Nat. Rev. Rheumatol. 12, 344–357 (2016).
Young, N. A. et al. Estrogen modulation of endosome-associated toll-like receptor 8: an IFNα-independent mechanism of sex-bias in systemic lupus erythematosus. Clin. Immunol. 151, 66–77 (2014).
Lee, J. et al. Oestrogen up-regulates interleukin-21 production by CD4+ T lymphocytes in patients with systemic lupus erythematosus. Immunology 142, 573–580 (2014).
Smith, S. et al. Estrogen receptor alpha regulates tripartite motif-containing protein 21 expression, contributing to dysregulated cytokine production in systemic lupus erythematosus. Arthritis Rheumatol. 66, 163–172 (2014).
Lee, S. et al. Interleukin-23 drives expansion of Thelper 17 cells through epigenetic regulation by signal transducer and activators of transcription 3 in lupus patients. Rheumatology https://doi.org/10.1093/rheumatology/keaa176 (2020).
Scott, J. L. et al. Estrogen receptor alpha deficiency modulates TLR ligand-mediated PDC-TREM expression in plasmacytoid dendritic cells in lupus-prone mice. J. Immunol. 195, 5561–5571 (2015).
Xue, L. et al. Estrogen-induced expression of tumor necrosis factor-like weak inducer of apoptosis through ERα accelerates the progression of lupus nephritis. Rheumatology 55, 1880–1888 (2016).
Lu, J. et al. Gene expression of TWEAK/Fn14 and IP-10/CXCR3 in glomerulus and tubulointerstitium of patients with lupus nephritis. Nephrology 16, 426–432 (2011).
Schwartz, N. et al. Urinary TWEAK and the activity of lupus nephritis. J. Autoimmun. 27, 242–250 (2006).
Lubberts, E. The IL-23-IL-17 axis in inflammatory arthritis. Nat. Rev. Rheumatol. 11, 415–429 (2015).
Andersson, A. et al. Estrogen regulates T helper 17 phenotype and localization in experimental autoimmune arthritis. Arthritis Res. Ther. 17, 32 (2015).
Chen, R. Y. et al. Estradiol inhibits Th17 cell differentiation through inhibition of RORγT transcription by recruiting the ERα/REA complex to estrogen response elements of the RORγT promoter. J. Immunol. 194, 4019–4028 (2015).
Newcomb, D. C. et al. Estrogen and progesterone decrease let-7f microRNA expression and increase IL-23/IL-23 receptor signaling and IL-17A production in patients with severe asthma. J. Allergy Clin. Immunol. 136, 1025–1034 (2015).
Qin, J. et al. Estrogen receptor β activation stimulates the development of experimental autoimmune thyroiditis through up-regulation of Th17-type responses. Clin. Immunol. 190, 41–52 (2018).
Engdahl, C. et al. Estrogen induces St6gal1 expression and increases IgG sialylation in mice and patients with rheumatoid arthritis: a potential explanation for the increased risk of rheumatoid arthritis in postmenopausal women. Arthritis Res. Ther. 20, 84 (2018).
Zhu, M. L. et al. Sex bias in CNS autoimmune disease mediated by androgen control of autoimmune regulator. Nat. Commun. 7, 11350 (2016).
Lu, L., Barbi, J. & Pan, F. The regulation of immune tolerance by FOXP3. Nat. Rev. Immunol. 17, 703–717 (2017).
Walecki, M. et al. Androgen receptor modulates Foxp3 expression in CD4+CD25+Foxp3+regulatory T-cells. Mol. Biol. Cell 26, 2845–2857 (2015).
Olsen, N. J., Gu, X. & Kovacs, W. J. Bone marrow stromal cells mediate androgenic suppression of B lymphocyte development. J. Clin. Invest. 108, 1697–1704 (2001).
Pongratz, G., Straub, R. H., Scholmerich, J., Fleck, M. & Harle, P. Serum BAFF strongly correlates with PsA activity in male patients only — is there a role for sex hormones? Clin. Exp. Rheumatol. 28, 813–819 (2010).
Wilhelmson, A. S. et al. Testosterone is an endogenous regulator of BAFF and splenic B cell number. Nat. Commun. 9, 2067 (2018).
Hill, L., Jeganathan, V., Chinnasamy, P., Grimaldi, C. & Diamond, B. Differential roles of estrogen receptors alpha and beta in control of B-cell maturation and selection. Mol. Med. 17, 211–220 (2011).
Drehmer, M. N., Suterio, D. G., Muniz, Y. C., de Souza, I. R. & Lofgren, S. E. BAFF expression is modulated by female hormones in human immune cells. Biochem. Genet. 54, 722–730 (2016).
U.S. National Library of Medicine. Entrez Gene https://www.ncbi.nlm.nih.gov/gene/5770 (2019).
Kissick, H. T. et al. Androgens alter T-cell immunity by inhibiting T-helper 1 differentiation. Proc. Natl Acad. Sci. USA 111, 9887–9892 (2014).
Wong, A. H., Agrawal, N. & Hughes, G. C. Altered IgG autoantibody levels and CD4+ T cell subsets in lupus-prone Nba2 mice lacking the nuclear progesterone receptor. Autoimmunity 48, 389–401 (2015).
Pauklin, S. & Petersen-Mahrt, S. K. Progesterone inhibits activation-induced deaminase by binding to the promoter. J. Immunol. 183, 1238–1244 (2009).
Giaglis, S. et al. Multimodal regulation of NET formation in pregnancy: progesterone antagonizes the pro-NETotic effect of estrogen and G-CSF. Front. Immunol. 7, 565 (2016).
Taurog, J. D. et al. The germfree state prevents development of gut and joint inflammatory disease in HLA-B27 transgenic rats. J. Exp. Med. 180, 2359–2364 (1994).
Manfredo Vieira, S. et al. Translocation of a gut pathobiont drives autoimmunity in mice and humans. Science 359, 1156–1161 (2018).
Shamriz, O. et al. Microbiota at the crossroads of autoimmunity. Autoimmun. Rev. 15, 859–869 (2016).
Tanoue, T., Atarashi, K. & Honda, K. Development and maintenance of intestinal regulatory T cells. Nat. Rev. Immunol. 16, 295–309 (2016).
Rizzetto, L., Fava, F., Tuohy, K. M. & Selmi, C. Connecting the immune system, systemic chronic inflammation and the gut microbiome: the role of sex. J. Autoimmun. 92, 12–34 (2018).
Vemuri, R. et al. The microgenderome revealed: sex differences in bidirectional interactions between the microbiota, hormones, immunity and disease susceptibility. Semin. Immunopathol. 41, 265–275 (2019).
Ding, T. & Schloss, P. D. Dynamics and associations of microbial community types across the human body. Nature 509, 357–360 (2014).
Jaggar, M., Rea, K., Spicak, S., Dinan, T. G. & Cryan, J. F. You’ve got male: sex and the microbiota-gut brain axis across the lifespan. Front. Neuroendocrinol. 56, 100815 (2019).
Menon, R. et al. Diet complexity and estrogen receptor beta status affect the composition of the murine intestinal microbiota. Appl. Env. Microbiol. 79, 5763–5773 (2013).
Andoh, A. Physiological role of gut microbiota for maintaining human health. Digestion 93, 176–181 (2016).
Benedek, G. et al. Estrogen protection against EAE modulates the microbiota and mucosal-associated regulatory cells. J. Neuroimmunol. 310, 51–59 (2017).
Markle, J. G. et al. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science 339, 1084–1088 (2013).
Yurkovetskiy, L. et al. Gender bias in autoimmunity is influenced by microbiota. Immunity 39, 400–412 (2013).
Gomez, A. et al. Loss of sex and age driven differences in the gut microbiome characterize arthritis-susceptible 0401 mice but not arthritis-resistant 0402 mice. PLoS ONE 7, e36095 (2012).
Caprioli, M., Carrara, G., Sakellariou, G., Silvagni, E. & Scire, C. A. Influence of aromatase inhibitors therapy on the occurrence of rheumatoid arthritis in women with breast cancer: results from a large population-based study of the Italian Society for Rheumatology. RMD Open 3, e000523 (2017).
Tenti, S., Giordano, N., Cutolo, M., Giannini, F. & Fioravanti, A. Primary antiphospholipid syndrome during aromatase inhibitors therapy: a case report and review of the literature. Medicine 98, e15052 (2019).
Masi, A. T. et al. Lower serum androstenedione levels in pre-rheumatoid arthritis versus normal control women: correlations with lower serum cortisol levels. Autoimmune. Dis. 2013, 593493 (2013).
Heikkila, R. et al. Serum androgen-anabolic hormones and the risk of rheumatoid arthritis. Ann. Rheum. Dis. 57, 281–285 (1998).
Pikwer, M. et al. Association between testosterone levels and risk of future rheumatoid arthritis in men: a population-based case-control study. Ann. Rheum. Dis. 73, 573–579 (2014).
Ciaffi, J., van Leeuwen, N. M., Schoones, J. W., Huizinga, T. W. J. & de Vries-Bouwstra, J. K. Sex hormones and sex hormone-targeting therapies in systemic sclerosis: a systematic literature review. Semin. Arthritis Rheum. 50, 140–148 (2020).
Campochiaro, C., Host, L. V., Ong, V. H. & Denton, C. P. Development of systemic sclerosis in transgender females: a case series and review of the literature. Clin. Exp. Rheumatol. 36, 50–52 (2018).
Baillargeon, J. et al. Hypogonadism and the risk of rheumatic autoimmune disease. Clin. Rheumatol. 35, 2983–2987 (2016).
Seminog, O. O., Seminog, A. B., Yeates, D. & Goldacre, M. J. Associations between Klinefelter’s syndrome and autoimmune diseases: English national record linkage studies. Autoimmunity 48, 125–128 (2015).
Pakpoor, J., Goldacre, R. & Goldacre, M. J. Associations between clinically diagnosed testicular hypofunction and systemic lupus erythematosus: a record linkage study. Clin. Rheumatol. 37, 559–562 (2018).
Chighizola, C. & Meroni, P. L. The role of environmental estrogens and autoimmunity. Autoimmun. Rev. 11, A493–A501 (2012).
Warner, G. R., Mourikes, V. E., Neff, A. M., Brehm, E. & Flaws, J. A. Mechanisms of action of agrochemicals acting as endocrine disrupting chemicals. Mol. Cell Endocrinol. 502, 110680 (2019).
Aljadeff, G., Longhi, E. & Shoenfeld, Y. Bisphenol A: a notorious player in the mosaic of autoimmunity. Autoimmunity 51, 370–377 (2018).
Jayasuriya, N. A. et al. A lower maternal cortisol-to-cortisone ratio precedes clinical diagnosis of preterm and term preeclampsia by many weeks. J. Clin. Endocrinol. Metab. 104, 2355–2366 (2019).
Borba, V. V., Zandman-Goddard, G. & Shoenfeld, Y. Exacerbations of autoimmune diseases during pregnancy and postpartum. Best Pract. Res. Clin. Endocrinol. Metab. 33, 101321 (2019).
Ursin, K., Lydersen, S., Skomsvoll, J. F. & Wallenius, M. Disease activity during and after pregnancy in women with axial spondyloarthritis: a prospective multicentre study. Rheumatology 57, 1064–1071 (2018).
Ince-Askan, H., Hazes, J. M. W. & Dolhain, R. Identifying clinical factors associated with low disease activity and remission of rheumatoid arthritis during pregnancy. Arthritis Care Res. 69, 1297–1303 (2017).
Zbinden, A., van den Brandt, S., Ostensen, M., Villiger, P. M. & Forger, F. Risk for adverse pregnancy outcome in axial spondyloarthritis and rheumatoid arthritis: disease activity matters. Rheumatology 57, 1235–1242 (2018).
Davis-Porada, J. et al. Low frequency of flares during pregnancy and post-partum in stable lupus patients. Arthritis Res. Ther. 22, 52 (2020).
Chen, D. et al. Fetal and maternal outcomes of planned pregnancy in patients with systemic lupus erythematosus: a retrospective multicenter study. J. Immunol. Res. 2018, 2413637 (2018).
Costenbader, K. H., Feskanich, D., Stampfer, M. J. & Karlson, E. W. Reproductive and menopausal factors and risk of systemic lupus erythematosus in women. Arthritis Rheum. 56, 1251–1262 (2007).
Sammaritano, L. R. Menopause in patients with autoimmune diseases. Autoimmun. Rev. 11, A430–A436 (2012).
Pfeifer, E. C., Crowson, C. S., Amin, S., Gabriel, S. E. & Matteson, E. L. The influence of early menopause on cardiovascular risk in women with rheumatoid arthritis. J. Rheumatol. 41, 1270–1275 (2014).
Wong, L. E. et al. Effect of age at menopause on disease presentation in early rheumatoid arthritis: results from the Canadian Early Arthritis Cohort. Arthritis Care Res. 67, 616–623 (2015).
Benagiano, G., Benagiano, M., Bianchi, P., D’Elios, M. M. & Brosens, I. Contraception in autoimmune diseases. Best Pract. Res. Clin. Obstet. Gynaecol. 60, 111–123 (2019).
Buyon, J. P. et al. The effect of combined estrogen and progesterone hormone replacement therapy on disease activity in systemic lupus erythematosus: a randomized trial. Ann. Intern. Med. 142, 953–962 (2005).
Roman-Blas, J. A., Castaneda, S., Cutolo, M. & Herrero-Beaumont, G. Efficacy and safety of a selective estrogen receptor β agonist, ERB-041, in patients with rheumatoid arthritis: a 12-week, randomized, placebo-controlled, phase II study. Arthritis Care Res. 62, 1588–1593 (2010).
van Vollenhoven, R. F. et al. The selective estrogen receptor alpha agonist Org 37663 induces estrogenic effects but lacks antirheumatic activity: a phase IIa trial investigating efficacy and safety of Org 37663 in postmenopausal female rheumatoid arthritis patients receiving stable background methotrexate or sulfasalazine. Arthritis Rheum. 62, 351–358 (2010).
Abdou, N. I., Rider, V., Greenwell, C., Li, X. & Kimler, B. F. Fulvestrant (Faslodex), an estrogen selective receptor downregulator, in therapy of women with systemic lupus erythematosus. clinical, serologic, bone density, and T cell activation marker studies: a double-blind placebo-controlled trial. J. Rheumatol. 35, 797 (2008).
Adami, G. et al. Osteoporosis in rheumatic diseases. Int. J. Mol. Sci. 20, 5867 (2019).
Schett, G., Saag, K. G. & Bijlsma, J. W. From bone biology to clinical outcome: state of the art and future perspectives. Ann. Rheum. Dis. 69, 1415–1419 (2010).
Ellis, A. J., Hendrick, V. M., Williams, R. & Komm, B. S. Selective estrogen receptor modulators in clinical practice: a safety overview. Expert Opin. Drug Saf. 14, 921–934 (2015).
Andersson, A. et al. Roles of activating functions 1 and 2 of estrogen receptor alpha in lymphopoiesis. J. Endocrinol. 236, 99–109 (2018).
Borjesson, A. E. et al. SERMs have substance-specific effects on bone, and these effects are mediated via ERαAF-1 in female mice. Am. J. Physiol. Endocrinol. Metab. 310, E912–E918 (2016).
Börjesson, A. E. et al. Roles of transactivating functions 1 and 2 of estrogen receptor-α in bone. Proc. Natl Acad. Sci. USA 108, 6288–6293 (2011).
Bernardi, A. I. et al. Effects of lasofoxifene and bazedoxifene on B cell development and function. Immun. Inflamm. Dis. 2, 214–225 (2014).
Mirkin, S. & Komm, B. S. Tissue-selective estrogen complexes for postmenopausal women. Maturitas 76, 213–220 (2013).
Nordqvist, J., Bernardi, A., Islander, U. & Carlsten, H. Effects of a tissue-selective estrogen complex on B lymphopoiesis and B cell function. Immunobiology 222, 918–923 (2017).
Andersson, A. et al. Suppression of experimental arthritis and associated bone loss by a tissue-selective estrogen complex. Endocrinology 157, 1013–1020 (2016).
Straub, R. H., Bijlsma, J. W., Masi, A. & Cutolo, M. Role of neuroendocrine and neuroimmune mechanisms in chronic inflammatory rheumatic diseases — the 10-year update. Semin. Arthritis Rheum. 43, 392–404 (2013).
Arver, S. & Lehtihet, M. Current guidelines for the diagnosis of testosterone deficiency. Front. Horm. Res. 37, 5–20 (2009).
Cutolo, M., Balleari, E., Giusti, M., Intra, E. & Accardo, S. Androgen replacement therapy in male patients with rheumatoid arthritis. Arthritis Rheum. 34, 1–5 (1991).
Booji, A. et al. Androgens as adjuvant treatment in postmenopausal female patients with rheumatoid arthritis. Ann. Rheum. Dis. 55, 811–815 (1996).
Saad, F., Haider, A. & Gooren, L. Hypogonadal men with psoriasis benefit from long-term testosterone replacement therapy — a series of 15 case reports. Andrologia 48, 341–346 (2016).
Porola, P., Straub, R. H., Virkki, L. M., Konttinen, Y. T. & Nordstrom, D. C. Failure of oral DHEA treatment to increase local salivary androgen outputs of female patients with Sjogren’s syndrome. Scand. J. Rheumatol. 40, 387–390 (2011).
Hazelton, R. A., McCruden, A. B., Sturrock, R. D. & Stimson, W. H. Hormonal manipulation of the immune response in systemic lupus erythematosus: a drug trial of an anabolic steroid, 19-nortestosterone. Ann. Rheum. Dis. 42, 155–157 (1983).
Lahita, R. G., Cheng, C. Y., Monder, C. & Bardin, C. W. Experience with 19-nortestosterone in the therapy of systemic lupus erythematosus: worsened disease after treatment with 19-nortestosterone in men and lack of improvement in women. J. Rheumatol. 19, 547–555 (1992).
Olsen, N. J. & Kovacs, W. J. Case report: testosterone treatment of systemic lupus erythematosus in a patient with Klinefelter’s syndrome. Am. J. Med. Sci. 310, 158–160 (1995).
Crosbie, D., Black, C., McIntyre, L., Royle, P. L. & Thomas, S. Dehydroepiandrosterone for systemic lupus erythematosus. Cochrane Database Syst. Rev. 4, CD005114 (2007).
Letchumanan, P. & Thumboo, J. Danazol in the treatment of systemic lupus erythematosus: a qualitative systematic review. Semin. Arthritis Rheum. 40, 298–306 (2011).
Jungers, P. et al. Influence of oral contraceptive therapy on the activity of systemic lupus erythematosus. Arthritis Rheum. 25, 618–623 (1982).
Chabbert-Buffet, N. et al. Pregnane progestin contraception in systemic lupus erythematosus: a longitudinal study of 187 patients. Contraception 83, 229–237 (2011).
Rivier, C. Neuroendocrine effects of cytokines in the rat. Rev. Neurosci. 4, 223–237 (1993).
Rivier, C. & Vale, W. In the rat, interleukin-1 alpha acts at the level of the brain and the gonads to interfere with gonadotropin and sex steroid secretion. Endocrinology 124, 2105–2109 (1989).
Masi, A. T., Josipovic, D. B. & Jefferson, W. E. Low adrenal androgenic-anabolic steroids in women with rheumatoid arthritis (RA): gas-liquid chromatographic studies of RA patients and matched normal control women indicating decreased 11-deoxy-17-ketosteroid excretion. Semin. Arthritis Rheum. 14, 1–23 (1984).
Lahita, R. G., Bradlow, H. L., Ginzler, E., Pang, S. & New, M. Low plasma androgens in women with systemic lupus erythematosus. Arthritis Rheum. 30, 241–248 (1987).
Cutolo, M., Balleari, E., Giusti, M., Monachesi, M. & Accardo, S. Sex hormone status of male patients with rheumatoid arthritis: evidence of low serum concentrations of testosterone at baseline and after human chorionic gonadotropin stimulation. Arthritis Rheum. 31, 1314–1317 (1988).
Bijlsma, J. W., Cutolo, M., Masi, A. T. & Chikanza, I. C. The neuroendocrine immune basis of rheumatic diseases. Immunol. Today 20, 298–301 (1999).
Straub, R. H. & Cutolo, M. Involvement of the hypothalamic–pituitary–adrenal/gonadal axis and the peripheral nervous system in rheumatoid arthritis: viewpoint based on a systemic pathogenetic role. Arthritis Rheum. 44, 493–507 (2001).
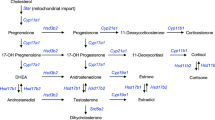
Schmidt, M., Kreutz, M., Löffler, G., Schölmerich, J. & Straub, R. H. Conversion of dehydroepiandrosterone to downstream steroid hormones in macrophages. J. Endocrinol. 164, 161–169 (2000).
Lahita, R. G., Bradlow, H. L., Kunkel, H. G. & Fishman, J. Alterations of estrogen metabolism in systemic lupus erythematosus. Arthritis Rheum. 22, 1195–1198 (1979).
Weidler, C. et al. Patients with rheumatoid arthritis and systemic lupus erythematosus have increased renal excretion of mitogenic estrogens in relation to endogenous antiestrogens. J. Rheumatol. 31, 489–494 (2004).
Herrmann, M., Schölmerich, J. & Straub, R. H. Influence of cytokines and growth factors on distinct steroidogenic enzymes in vitro: a short tabular data collection. Ann. N. Y. Acad. Sci. 966, 166–186 (2002).
Straub, R. H. et al. Anti-interleukin-6 receptor antibody therapy favors adrenal androgen secretion in patients with rheumatoid arthritis: a randomized, double-blind, placebo-controlled study. Arthritis Rheum. 54, 1778–1785 (2006).
Straub, R. H., Cutolo, M., Buttgereit, F. & Pongratz, G. Energy regulation and neuroendocrine-immune control in chronic inflammatory diseases. J. Intern. Med. 267, 543–560 (2010).
Straub, R. H. The brain and immune system prompt energy shortage in chronic inflammation and ageing. Nat. Rev. Rheumatol. 13, 743–751 (2017).
Browne, H. et al. Assessment of ovarian function with anti-Müllerian hormone in systemic lupus erythematosus patients undergoing hematopoietic stem cell transplant. Fertil. Steril. 91, 1529–1532 (2009).
Brouwer, J., Laven, J. S., Hazes, J. M., Schipper, I. & Dolhain, R. J. Levels of serum anti-Müllerian hormone, a marker for ovarian reserve, in women with rheumatoid arthritis. Arthritis Care Res. 65, 1534–1538 (2013).
Clowse, M. E. et al. Ovarian reserve diminished by oral cyclophosphamide therapy for granulomatosis with polyangiitis (Wegener’s). Arthritis Care Res. 63, 1777–1781 (2011).
Mont’Alverne, A. R. et al. Diminished ovarian reserve in Behçet’s disease patients. Clin. Rheumatol. 34, 179–183 (2015).
de Souza, F. H. et al. Reduction of ovarian reserve in adult patients with dermatomyositis. Clin. Exp. Rheumatol. 33, 44–49 (2015).
Henes, M. et al. Ovarian reserve alterations in premenopausal women with chronic inflammatory rheumatic diseases: impact of rheumatoid arthritis, Behçet’s disease and spondyloarthritis on anti-Müllerian hormone levels. Rheumatology 54, 1709–1712 (2015).
de Souza, F. H. et al. Reduced ovarian reserve in patients with adult polymyositis. Clin. Rheumatol. 34, 1795–1799 (2015).
Nelson, J. L. et al. Fecundity before disease onset in women with rheumatoid arthritis. Arthritis Rheum. 36, 7–14 (1993).
Silva, C. A., Bonfa, E. & Ostensen, M. Maintenance of fertility in patients with rheumatic diseases needing antiinflammatory and immunosuppressive drugs. Arthritis Care Res. 62, 1682–1690 (2010).
Provost, M., Eaton, J. L. & Clowse, M. E. Fertility and infertility in rheumatoid arthritis. Curr. Opin. Rheumatol. 26, 308–314 (2014).
Ostensen, M. Rheumatoid arthritis: the effect of RA and medication on female fertility. Nat. Rev. Rheumatol. 10, 518–519 (2014).
Brouwer, J., Hazes, J. M., Laven, J. S. & Dolhain, R. J. Fertility in women with rheumatoid arthritis: influence of disease activity and medication. Ann. Rheum. Dis. 74, 1836–1841 (2015).
Chatzimeletiou, K. et al. Fertility potential in a man with ankylosing spondylitis as revealed by semen analysis by light, electron and fluorescence microscopy. SAGE Open Med. Case Rep. https://doi.org/10.1177/2050313X18759898 (2018).
Fan, D. et al. Male sexual dysfunction and ankylosing spondylitis: a systematic review and metaanalysis. J. Rheumatol. 42, 252–257 (2015).
Jaeger, V. K. & Walker, U. A. Erectile dysfunction in systemic sclerosis. Curr. Rheumatol. Rep. 18, 49 (2016).
Luo, L. et al. Gout is associated with elevated risk of erectile dysfunction: a systematic review and meta-analysis. Rheumatol. Int. 39, 1527–1535 (2019).
Moraes, A. J. et al. Minor sperm abnormalities in young male post-pubertal patients with juvenile dermatomyositis. Braz. J. Med. Biol. Res. 41, 1142–1147 (2008).
Rabelo-Junior, C. N. et al. Penile alterations with severe sperm abnormalities in antiphospholipid syndrome associated with systemic lupus erythematosus. Clin. Rheumatol. 32, 109–113 (2013).
Soares, P. M. et al. Gonad evaluation in male systemic lupus erythematosus. Arthritis Rheum. 56, 2352–2361 (2007).
Taylan, A. & Birlik, M. Parenchymal neuro-Behçet disease with erectile dysfunction and micturition disturbances: case report and literature review. Rheumatol. Int. 38, 149–152 (2018).
Villiger, P. M. et al. Effects of TNF antagonists on sperm characteristics in patients with spondyloarthritis. Ann. Rheum. Dis. 69, 1842–1844 (2010).
Puchner, R., Danninger, K., Puchner, A. & Pieringer, H. Impact of TNF-blocking agents on male sperm characteristics and pregnancy outcomes in fathers exposed to TNF-blocking agents at time of conception. Clin. Exp. Rheumatol. 30, 765–767 (2012).
Straub, R. H. & Schradin, C. Chronic inflammatory systemic diseases: an evolutionary trade-off between acutely beneficial but chronically harmful programs. Evol. Med. Public. Health 2016, 37–51 (2016).
Simard, J. F. et al. Perinatal factors and adult-onset lupus. Arthritis Rheum. 59, 1155–1161 (2008).
Ulff-Moller, C. J., Jorgensen, K. T., Pedersen, B. V., Nielsen, N. M. & Frisch, M. Reproductive factors and risk of systemic lupus erythematosus: nationwide cohort study in Denmark. J. Rheumatol. 36, 1903–1909 (2009).
Rojas-Villarraga, A., Torres-Gonzalez, J. V. & Ruiz-Sternberg, A. M. Safety of hormonal replacement therapy and oral contraceptives in systemic lupus erythematosus: a systematic review and meta-analysis. PLoS ONE 9, e104303 (2014).
Washio, M. et al. Risk factors for development of systemic lupus erythematosus among Japanese females: medical history and reproductive factors. Int. J. Rheum. Dis. 20, 76–83 (2017).
Tarvin, S. E. & O’Neil, K. M. Systemic lupus erythematosus, Sjogren syndrome, and mixed connective tissue disease in children and adolescents. Pediatr. Clin. North. Am. 65, 711–737 (2018).
Lateef, A. & Petri, M. Hormone replacement and contraceptive therapy in autoimmune diseases. J. Autoimmun. 38, J170–J176 (2012).
Petri, M. et al. Combined oral contraceptives in women with systemic lupus erythematosus. N. Engl. J. Med. 353, 2550–2558 (2005).
Mostafavi, B. et al. Perinatal characteristics and risk of developing primary Sjogren’s syndrome: a case-control study. J. Rheumatol. 32, 665–668 (2005).
Harris, V. M. et al. Klinefelter’s syndrome (47,XXY) is in excess among men with Sjogren’s syndrome. Clin. Immunol. 168, 25–29 (2016).
Ramirez Sepulveda, J. I., Kvarnstrom, M., Brauner, S., Baldini, C. & Wahren-Herlenius, M. Difference in clinical presentation between women and men in incident primary Sjogren’s syndrome. Biol. Sex Differ. 8, 16 (2017).
Ballester, C. et al. Pregnancy and primary Sjögren’s syndrome: management and outcomes in a multicentre retrospective study of 54 pregnancies. Scand. J. Rheumatol. 46, 56–63 (2017).
Gupta, S. & Gupta, N. Sjögren syndrome and pregnancy: a literature review. Perm. J. 21, 16–047 (2017).
McCoy, S. S., Sampene, E. & Baer, A. N. Sjögren’s syndrome is associated with reduced lifetime sex hormone exposure: a case-control study. Arthritis Care Res. 72, 1315–1322 (2019).
d’Elia, H. F. & Carlsten, H. The impact of hormone replacement therapy on humoral and cell-mediated immune responses in vivo in post-menopausal women with rheumatoid arthritis. Scand. J. Immunol. 68, 661–667 (2008).
Walitt, B. et al. Effects of postmenopausal hormone therapy on rheumatoid arthritis: the Women’s Health Initiative randomized controlled trials. Arthritis Rheum. 59, 302–310 (2008).
Holroyd, C. R. & Edwards, C. J. The effects of hormone replacement therapy on autoimmune disease: rheumatoid arthritis and systemic lupus erythematosus. Climacteric 12, 378–386 (2009).
Berglin, E., Kokkonen, H., Einarsdottir, E., Agren, A. & Rantapaa Dahlqvist, S. Influence of female hormonal factors, in relation to autoantibodies and genetic markers, on the development of rheumatoid arthritis in northern Sweden: a case-control study. Scand. J. Rheumatol. 39, 454–460 (2010).
Salliot, C., Bombardier, C., Saraux, A., Combe, B. & Dougados, M. Hormonal replacement therapy may reduce the risk for RA in women with early arthritis who carry HLA-DRB1 *01 and/or *04 alleles by protecting against the production of anti-CCP: results from the ESPOIR cohort. Ann. Rheum. Dis. 69, 1683–1686 (2010).
Orellana, C. et al. Postmenopausal hormone therapy and the risk of rheumatoid arthritis: results from the Swedish EIRA population-based case-control study. Eur. J. Epidemiol. 30, 449–457 (2015).
Desai, M. K. & Brinton, R. D. Autoimmune disease in women: endocrine transition and risk across the lifespan. Front. Endocrinol. 10, 265 (2019).
Chen, W. M. Y. et al. The association between gravidity, parity and the risk of developing rheumatoid arthritis: a systematic review and meta-analysis. Semin. Arthritis Rheum. 50, 252–260 (2020).
Alpizar-Rodriguez, D., Forger, F., Courvoisier, D. S., Gabay, C. & Finckh, A. Role of reproductive and menopausal factors in functional and structural progression of rheumatoid arthritis: results from the SCQM cohort. Rheumatology 58, 432–440 (2019).
Masi, A. T. & Medsger, T. A. Jr. A new look at the epidemiology of ankylosing spondylitis and related syndromes. Clin. Orthop. Relat. Res. 143, 15–29 (1979).
Ostensen, M. & Husby, G. Ankylosing spondylitis and pregnancy. Rheum. Dis. Clin. North Am. 15, 241–254 (1989).
van der Linden, S. & van der Heijde, D. M. Clinical and epidemiologic aspects of ankylosing spondylitis and spondyloarthropathies. Curr. Opin. Rheumatol. 8, 269–274 (1996).
Gran, J. T. & Husby, G. Clinical, epidemiologic, and therapeutic aspects of ankylosing spondylitis. Curr. Opin. Rheumatol. 10, 292–298 (1998).
Ostensen, M. et al. Pregnancy in patients with rheumatic disease: anti-inflammatory cytokines increase in pregnancy and decrease post partum. Ann. Rheum. Dis. 64, 839–844 (2005).
Lee, W., Reveille, J. D. & Weisman, M. H. Women with ankylosing spondylitis: a review. Arthritis Rheum. 59, 449–454 (2008).
Rovensky, J., Imrich, R., Lazurova, I. & Payer, J. Rheumatic diseases and Klinefelter’s syndrome. Ann. N. Y. Acad. Sci. 1193, 1–9 (2010).
Mahendira, D. et al. Analysis of the effect of the oral contraceptive pill on clinical outcomes in women with ankylosing spondylitis. J. Rheumatol. 41, 1344–1348 (2014).
Ostensen, M. et al. State of the art: reproduction and pregnancy in rheumatic diseases. Autoimmun. Rev. 14, 376–386 (2015).
Andreoli, L. et al. EULAR recommendations for women’s health and the management of family planning, assisted reproduction, pregnancy and menopause in patients with systemic lupus erythematosus and/or antiphospholipid syndrome. Ann. Rheum. Dis. 76, 476–485 (2017).
Carlsten, H. et al. Estrogen accelerates immune complex glomerulonephritis but ameliorates T cell-mediated vasculitis and sialadenitis in autoimmune MRL lpr/lpr mice. Cell Immunol. 144, 190–202 (1992).
Bernardi, A. I. et al. Selective estrogen receptor modulators in T cell development and T cell dependent inflammation. Immunobiology 220, 1122–1128 (2015).
Capellino, S., Straub, R. H. & Cutolo, M. Aromatase and regulation of the estrogen-to-androgen ratio in synovial tissue inflammation: common pathway in both sexes. Ann. N. Y. Acad. Sci. 1317, 24–31 (2014).
Tengstrand, B., Carlstrom, K. & Hafstrom, I. Gonadal hormones in men with rheumatoid arthritis — from onset through 2 years. J. Rheumatol. 36, 887–892 (2009).
Weidler, C. et al. Tumor necrosis factor inhibits conversion of dehydroepiandrosterone sulfate (DHEAS) to DHEA in rheumatoid arthritis synovial cells: a prerequisite for local androgen deficiency. Arthritis Rheum. 52, 1721–1729 (2005).
Schmidt, M., Weidler, C., Naumann, H., Schölmerich, J. & Straub, R. H. Androgen conversion in osteoarthritis and rheumatoid arthritis synoviocytes — androstenedione and testosterone inhibit estrogen formation and favor production of more potent 5α-reduced androgens. Arthritis Res. Ther. 7, R938–R948 (2005).
Straub, R. H., Härle, P., Sarzi-Puttini, P. & Cutolo, M. Tumor necrosis factor-neutralizing therapies improve altered hormone axes: an alternative mode of antiinflammatory action. Arthritis Rheum. 54, 2039–2046 (2006).
Ernestam, S., Hafstrom, I., Werner, S., Carlstrom, K. & Tengstrand, B. Increased DHEAS levels in patients with rheumatoid arthritis after treatment with tumor necrosis factor antagonists: evidence for improved adrenal function. J. Rheumatol. 34, 1451–1458 (2007).
Genest, G., Spitzer, K. A. & Laskin, C. A. Maternal and fetal outcomes in a cohort of patients exposed to tumor necrosis factor inhibitors throughout pregnancy. J. Rheumatol. 45, 1109–1115 (2018).
Jawaheer, D., Olsen, J. & Hetland, M. L. Sex differences in response to anti-tumor necrosis factor therapy in early and established rheumatoid arthritis — results from the DANBIO registry. J. Rheumatol. 39, 46–53 (2012).
Cutolo, M. et al. Sex hormones modulate the effects of leflunomide on cytokine production by cultures of differentiated monocyte/macrophages and synovial macrophages from rheumatoid arthritis patients. J. Autoimmun. 32, 254–260 (2009).
Acknowledgements
M.C. and R.H.S. are members of the EULAR Study Group on Neuroendocrine Immunology of Rheumatic Diseases (NEIRD).
Review criteria
A search for articles published between 2006 and 2020 was performed in PubMed, Embase and the Cochrane library using the following search terms alone and in combination: “oestrogens”, “androgens”, “progesterone”, “steroid hormone”, “immun*”, “inflam*”, “rheum*”, “SLE”, “vasculitis”, “autoimmun*” and “systemic sclerosis”.
Author information
Authors and Affiliations
Contributions
R.H.S. researched data for this article and wrote the draft. M.C. contributed substantially to discussions of content. Both authors wrote and reviewed or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cutolo, M., Straub, R.H. Sex steroids and autoimmune rheumatic diseases: state of the art. Nat Rev Rheumatol 16, 628–644 (2020). https://doi.org/10.1038/s41584-020-0503-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41584-020-0503-4
This article is cited by
-
The conneXion between sex and immune responses
Nature Reviews Immunology (2024)
-
Heat of the night: sleep disturbance activates inflammatory mechanisms and induces pain in rheumatoid arthritis
Nature Reviews Rheumatology (2023)
-
Involvement of the secosteroid vitamin D in autoimmune rheumatic diseases and COVID-19
Nature Reviews Rheumatology (2023)
-
Sex bias in immune response: it is time to include the sex variable in studies of autoimmune rheumatic diseases
Rheumatology International (2023)
-
No evidence of genetic causal association between sex hormone-related traits and systemic lupus erythematosus: A two-sample Mendelian randomization study
Clinical Rheumatology (2023)